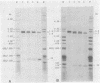
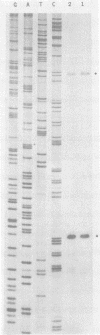
Abstract
The structure and replication of the single-stranded circular RNA genome of hepatitis delta virus (HDV) are unique relative to those of known animal viruses, and yet there are real similarities between HDV and certain infectious RNAs of plants. Therefore, since some of the latter RNAs have been shown to undergo in vitro site-specific cleavage and even ligation, we tested the hypothesis that similar events might also occur for HDV RNA. In partial confirmation of this hypothesis, we found that in vitro the RNA complementary to the HDV genome, the antigenomic RNA, could undergo a self-cleavage that was not only more than 90% efficient but also occurred only at a single location. This cleavage was found to produce junction fragments consistent with a 5'-hydroxyl and a cyclic 2',3'-monophosphate. Since the observed cleavage was both site-specific and occurred only once per genome length, we propose that the site may be relevant to the normal intracellular replication of the HDV genome. Because the site is located almost adjacent to the 3' end of the delta antigen-coding region, the only known functional open reading frame of HDV, we suggest that the cleavage may have a role not only in genome replication but also in RNA processing, helping to produce a functional mRNA for the translation of delta antigen.
Full text
PDF
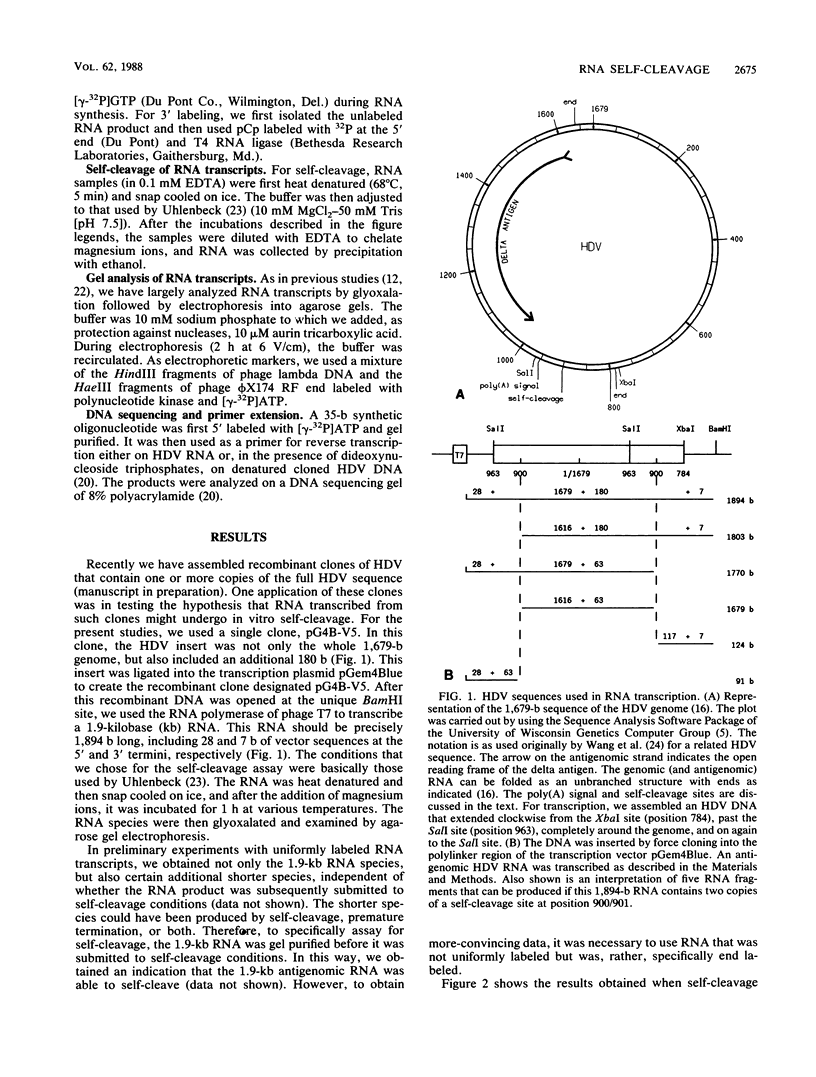
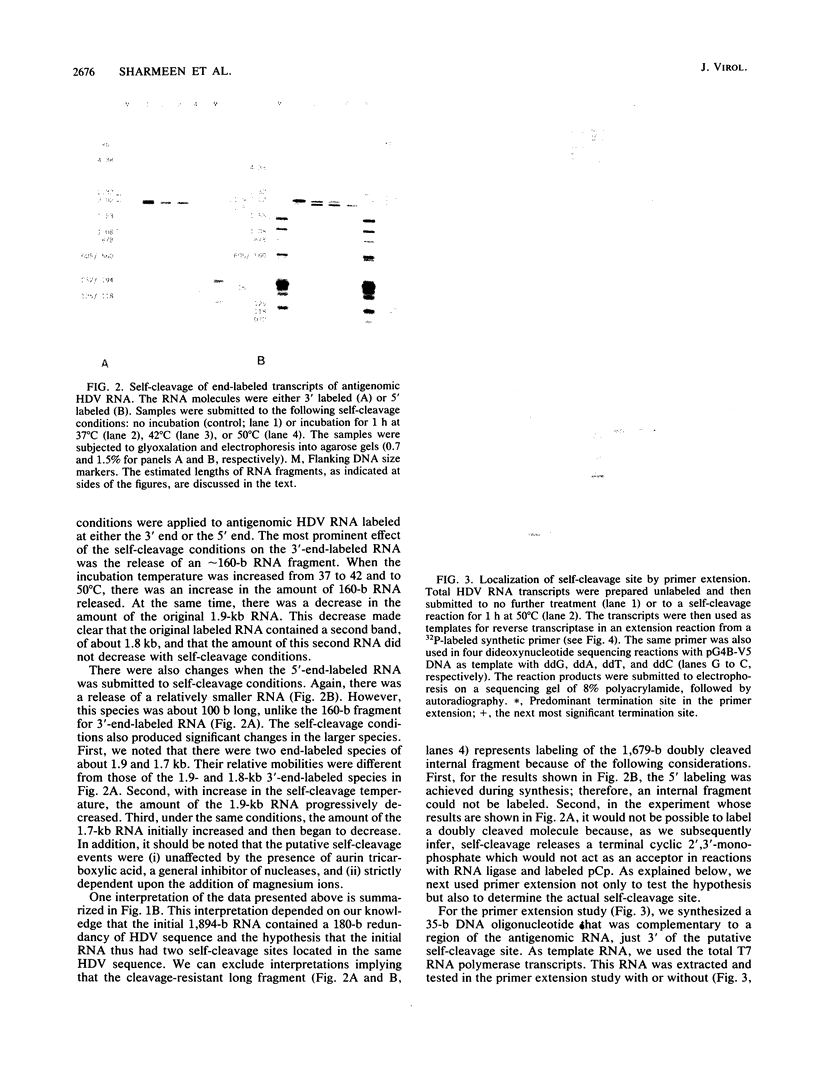



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Branch A. D., Robertson H. D. A replication cycle for viroids and other small infectious RNA's. Science. 1984 Feb 3;223(4635):450–455. doi: 10.1126/science.6197756. [DOI] [PubMed] [Google Scholar]
- Cech T. R., Bass B. L. Biological catalysis by RNA. Annu Rev Biochem. 1986;55:599–629. doi: 10.1146/annurev.bi.55.070186.003123. [DOI] [PubMed] [Google Scholar]
- Chen P. J., Kalpana G., Goldberg J., Mason W., Werner B., Gerin J., Taylor J. Structure and replication of the genome of the hepatitis delta virus. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8774–8778. doi: 10.1073/pnas.83.22.8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinter-Gottlieb G. Viroids and virusoids are related to group I introns. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6250–6254. doi: 10.1073/pnas.83.17.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein L. M., Gall J. G. Self-cleaving transcripts of satellite DNA from the newt. Cell. 1987 Feb 13;48(3):535–543. doi: 10.1016/0092-8674(87)90204-2. [DOI] [PubMed] [Google Scholar]
- Forster A. C., Symons R. H. Self-cleavage of plus and minus RNAs of a virusoid and a structural model for the active sites. Cell. 1987 Apr 24;49(2):211–220. doi: 10.1016/0092-8674(87)90562-9. [DOI] [PubMed] [Google Scholar]
- Forster A. C., Symons R. H. Self-cleavage of virusoid RNA is performed by the proposed 55-nucleotide active site. Cell. 1987 Jul 3;50(1):9–16. doi: 10.1016/0092-8674(87)90657-x. [DOI] [PubMed] [Google Scholar]
- Hoyer B., Bonino F., Ponzetto A., Denniston K., Nelson J., Purcell R., Gerin J. L. Properties of delta-associated ribonucleic acid. Prog Clin Biol Res. 1983;143:91–97. [PubMed] [Google Scholar]
- Hsu T. W., Taylor J. M., Aldrich C., Townsend J. B., Seal G., Mason W. S. Tandem duplication of the proviral DNA in an avian sarcoma virus-transformed quail clone. J Virol. 1981 Apr;38(1):219–223. doi: 10.1128/jvi.38.1.219-223.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins C. J., Rathjen P. D., Forster A. C., Symons R. H. Self-cleavage of plus and minus RNA transcripts of avocado sunblotch viroid. Nucleic Acids Res. 1986 May 12;14(9):3627–3640. doi: 10.1093/nar/14.9.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos A., Dijkema R., Arnberg A. C., van der Meide P. H., Schellekens H. The hepatitis delta (delta) virus possesses a circular RNA. Nature. 1986 Oct 9;323(6088):558–560. doi: 10.1038/323558a0. [DOI] [PubMed] [Google Scholar]
- Kuo M. Y., Goldberg J., Coates L., Mason W., Gerin J., Taylor J. Molecular cloning of hepatitis delta virus RNA from an infected woodchuck liver: sequence, structure, and applications. J Virol. 1988 Jun;62(6):1855–1861. doi: 10.1128/jvi.62.6.1855-1861.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prody G. A., Bakos J. T., Buzayan J. M., Schneider I. R., Bruening G. Autolytic processing of dimeric plant virus satellite RNA. Science. 1986 Mar 28;231(4745):1577–1580. doi: 10.1126/science.231.4745.1577. [DOI] [PubMed] [Google Scholar]
- Riesner D., Gross H. J. Viroids. Annu Rev Biochem. 1985;54:531–564. doi: 10.1146/annurev.bi.54.070185.002531. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J., Mason W., Summers J., Goldberg J., Aldrich C., Coates L., Gerin J., Gowans E. Replication of human hepatitis delta virus in primary cultures of woodchuck hepatocytes. J Virol. 1987 Sep;61(9):2891–2895. doi: 10.1128/jvi.61.9.2891-2895.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenbeck O. C. A small catalytic oligoribonucleotide. Nature. 1987 Aug 13;328(6131):596–600. doi: 10.1038/328596a0. [DOI] [PubMed] [Google Scholar]
- Wang K. S., Choo Q. L., Weiner A. J., Ou J. H., Najarian R. C., Thayer R. M., Mullenbach G. T., Denniston K. J., Gerin J. L., Houghton M. Structure, sequence and expression of the hepatitis delta (delta) viral genome. Nature. 1986 Oct 9;323(6088):508–514. doi: 10.1038/323508a0. [DOI] [PubMed] [Google Scholar]
- Williamson C. L., Tierney W. M., Kerker B. J., Burke J. M. Site-directed mutagenesis of core sequence elements 9R', 9L, 9R, and 2 in self-splicing Tetrahymena pre-rRNA. J Biol Chem. 1987 Oct 25;262(30):14672–14682. [PubMed] [Google Scholar]
- Zaug A. J., Grabowski P. J., Cech T. R. Autocatalytic cyclization of an excised intervening sequence RNA is a cleavage-ligation reaction. Nature. 1983 Feb 17;301(5901):578–583. doi: 10.1038/301578a0. [DOI] [PubMed] [Google Scholar]