Abstract
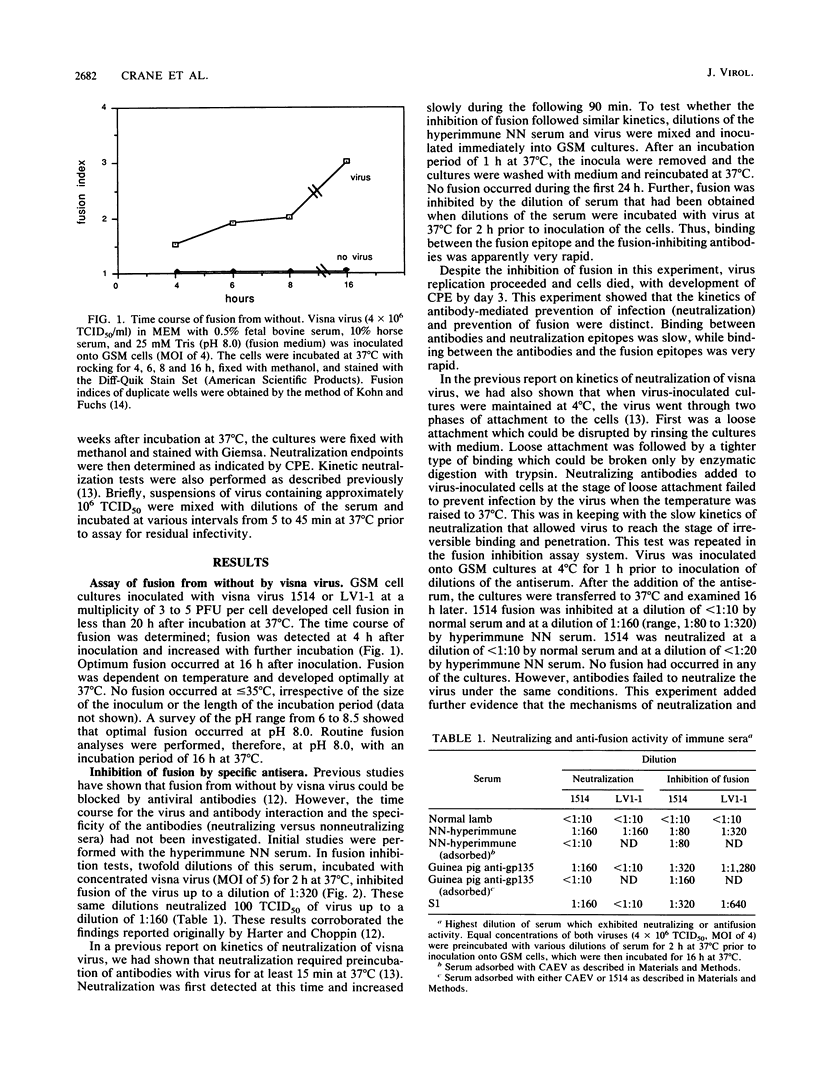
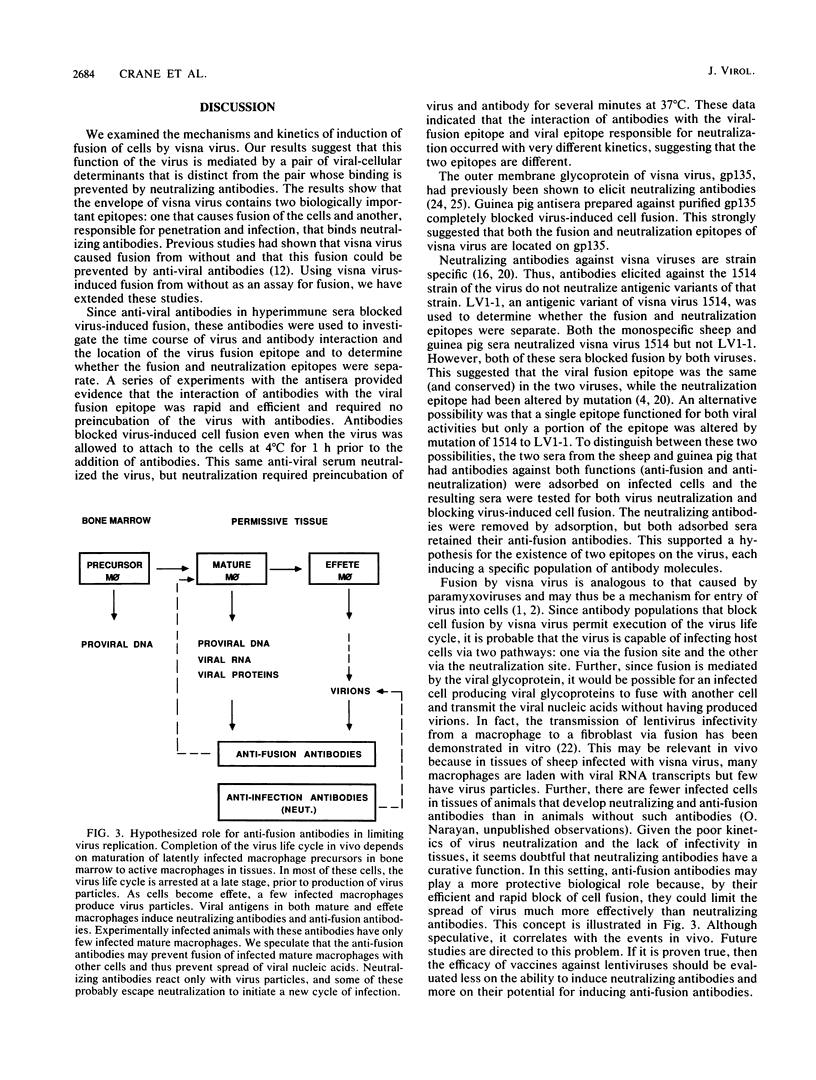
Visna virus is a lentivirus which causes fusion of infected cells in vitro. Two types of fusion occur. Fusion from without requires no viral replication and a relatively high multiplicity of infection; fusion from within results from the replication of virus in cells. By using fusion from without as an assay, the mechanism of fusion by visna virus was investigated. Immune sera which contained both anti-fusion and neutralizing antibodies interacted with the virus with rapid kinetics in blocking fusion but relatively slow kinetics in the virus neutralization assay. By using visna virus and an antigenic variant, the epitopes responsible for fusion and virus neutralization were shown to be different. Antigenic variation of visna virus resulted in alteration of the neutralization epitope and conservation of the fusion epitope. This suggested that there were two populations of antibodies and that the viral epitopes for fusion and neutralization were separate. These data suggest that visna virus is capable of infecting cells via two pathways: one via the fusion site and the other via the viral epitope which mediates neutralization.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abenes G., Kida H., Yanagawa R. Antigenic mapping and functional analysis of the F protein of Newcastle disease virus using monoclonal antibodies. Arch Virol. 1986;90(1-2):97–110. doi: 10.1007/BF01314148. [DOI] [PubMed] [Google Scholar]
- Bratt M. A., Gallaher W. R. Preliminary analysis of the requirements for fusion from within and fusion from without by Newcastle disease virus. Proc Natl Acad Sci U S A. 1969 Oct;64(2):536–543. doi: 10.1073/pnas.64.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu I. M., Yaniv A., Dahlberg J. E., Gazit A., Skuntz S. F., Tronick S. R., Aaronson S. A. Nucleotide sequence evidence for relationship of AIDS retrovirus to lentiviruses. 1985 Sep 26-Oct 2Nature. 317(6035):366–368. doi: 10.1038/317366a0. [DOI] [PubMed] [Google Scholar]
- Clements J. E., D'Antonio N., Narayan O. Genomic changes associated with antigenic variation of visna virus. II. Common nucleotide sequence changes detected in variants from independent isolations. J Mol Biol. 1982 Jul 5;158(3):415–434. doi: 10.1016/0022-2836(82)90207-8. [DOI] [PubMed] [Google Scholar]
- Clements J. E., Narayan O., Cork L. C. Biochemical characterization of the virus causing leukoencephalitis and arthritis in goats. J Gen Virol. 1980 Oct;50(2):423–427. doi: 10.1099/0022-1317-50-2-423. [DOI] [PubMed] [Google Scholar]
- Cork L. C., Hadlow W. J., Crawford T. B., Gorham J. R., Piper R. C. Infectious leukoencephalomyelitis of young goats. J Infect Dis. 1974 Feb;129(2):134–141. doi: 10.1093/infdis/129.2.134. [DOI] [PubMed] [Google Scholar]
- Florkiewicz R. Z., Rose J. K. A cell line expressing vesicular stomatitis virus glycoprotein fuses at low pH. Science. 1984 Aug 17;225(4663):721–723. doi: 10.1126/science.6087454. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Narayan O., Kennedy-Stoskopf S., Kennedy P. G., Ghotbi Z., Clements J. E., Stanley J., Pezeshkpour G. Tropism of sheep lentiviruses for monocytes: susceptibility to infection and virus gene expression increase during maturation of monocytes to macrophages. J Virol. 1986 Apr;58(1):67–74. doi: 10.1128/jvi.58.1.67-74.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman H. E., Narayan O., Molineaux S., Clements J. E., Ghotbi Z. Slow, persistent replication of lentiviruses: role of tissue macrophages and macrophage precursors in bone marrow. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7086–7090. doi: 10.1073/pnas.82.20.7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda M. A., Braun M. J., Clements J. E., Pyper J. M., Wong-Staal F., Gallo R. C., Gilden R. V. Human T-cell lymphotropic virus type III shares sequence homology with a family of pathogenic lentiviruses. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4007–4011. doi: 10.1073/pnas.83.11.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda M. A., Wong-Staal F., Gallo R. C., Clements J. E., Narayan O., Gilden R. V. Sequence homology and morphologic similarity of HTLV-III and visna virus, a pathogenic lentivirus. Science. 1985 Jan 11;227(4683):173–177. doi: 10.1126/science.2981428. [DOI] [PubMed] [Google Scholar]
- Harter D. H., Choppin P. W. Cell-fusing activity of visna virus particles. Virology. 1967 Feb;31(2):279–288. doi: 10.1016/0042-6822(67)90172-9. [DOI] [PubMed] [Google Scholar]
- Kennedy-Stoskopf S., Narayan O. Neutralizing antibodies to visna lentivirus: mechanism of action and possible role in virus persistence. J Virol. 1986 Jul;59(1):37–44. doi: 10.1128/jvi.59.1.37-44.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn A., Fuchs P. Cell fusion by various strains of Newcastle disease virus and their virulence. J Virol. 1969 May;3(5):539–540. doi: 10.1128/jvi.3.5.539-540.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. H., Thormar H. Ribonucleic acid-dependent deoxyribonucleic acid polymerase in visna virus. J Virol. 1970 Nov;6(5):702–704. doi: 10.1128/jvi.6.5.702-704.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan O., Clements J. E., Griffin D. E., Wolinsky J. S. Neutralizing antibody spectrum determines the antigenic profiles of emerging mutants of visna virus. Infect Immun. 1981 Jun;32(3):1045–1050. doi: 10.1128/iai.32.3.1045-1050.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan O., Clements J. E., Strandberg J. D., Cork L. C., Griffin D. E. Biological characterization of the virus causing leukoencephalitis and arthritis in goats. J Gen Virol. 1980 Sep;50(1):69–79. doi: 10.1099/0022-1317-50-1-69. [DOI] [PubMed] [Google Scholar]
- Narayan O., Cork L. C. Lentiviral diseases of sheep and goats: chronic pneumonia leukoencephalomyelitis and arthritis. Rev Infect Dis. 1985 Jan-Feb;7(1):89–98. doi: 10.1093/clinids/7.1.89. [DOI] [PubMed] [Google Scholar]
- Narayan O., Griffin D. E., Chase J. Antigenic shift of visna virus in persistently infected sheep. Science. 1977 Jul 22;197(4301):376–378. doi: 10.1126/science.195339. [DOI] [PubMed] [Google Scholar]
- Narayan O., Griffin D. E., Clements J. E. Virus mutation during 'slow infection': temporal development and characterization of mutants of visna virus recovered from sheep. J Gen Virol. 1978 Nov;41(2):343–352. doi: 10.1099/0022-1317-41-2-343. [DOI] [PubMed] [Google Scholar]
- Narayan O., Wolinsky J. S., Clements J. E., Strandberg J. D., Griffin D. E., Cork L. C. Slow virus replication: the role of macrophages in the persistence and expression of visna viruses of sheep and goats. J Gen Virol. 1982 Apr;59(Pt 2):345–356. doi: 10.1099/0022-1317-59-2-345. [DOI] [PubMed] [Google Scholar]
- Pyper J. M., Clements J. E., Gonda M. A., Narayan O. Sequence homology between cloned caprine arthritis encephalitis virus and visna virus, two neurotropic lentiviruses. J Virol. 1986 May;58(2):665–670. doi: 10.1128/jvi.58.2.665-670.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyper J. M., Clements J. E., Molineaux S. M., Narayan O. Genetic variation among lentiviruses: homology between visna virus and caprine arthritis-encephalitis virus is confined to the 5' gag-pol region and a small portion of the env gene. J Virol. 1984 Sep;51(3):713–721. doi: 10.1128/jvi.51.3.713-721.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. V., Stowring L., Haase A. T., Narayan O., Vigne R. Antigenic variation in visna virus. Cell. 1979 Oct;18(2):321–327. doi: 10.1016/0092-8674(79)90051-5. [DOI] [PubMed] [Google Scholar]
- Stephens R. M., Casey J. W., Rice N. R. Equine infectious anemia virus gag and pol genes: relatedness to visna and AIDS virus. Science. 1986 Feb 7;231(4738):589–594. doi: 10.1126/science.3003905. [DOI] [PubMed] [Google Scholar]
- THORMAR H. The growth cycle of visna virus in monolayer cultures of sheep cells. Virology. 1963 Mar;19:273–278. doi: 10.1016/0042-6822(63)90064-3. [DOI] [PubMed] [Google Scholar]
- Yaniv A., Dahlberg J. E., Tronick S. R., Chiu I. M., Aaronson S. A. Molecular cloning of integrated caprine arthritis-encephalitis virus. Virology. 1985 Sep;145(2):340–345. doi: 10.1016/0042-6822(85)90169-2. [DOI] [PubMed] [Google Scholar]



