Abstract
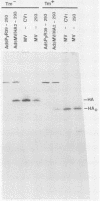
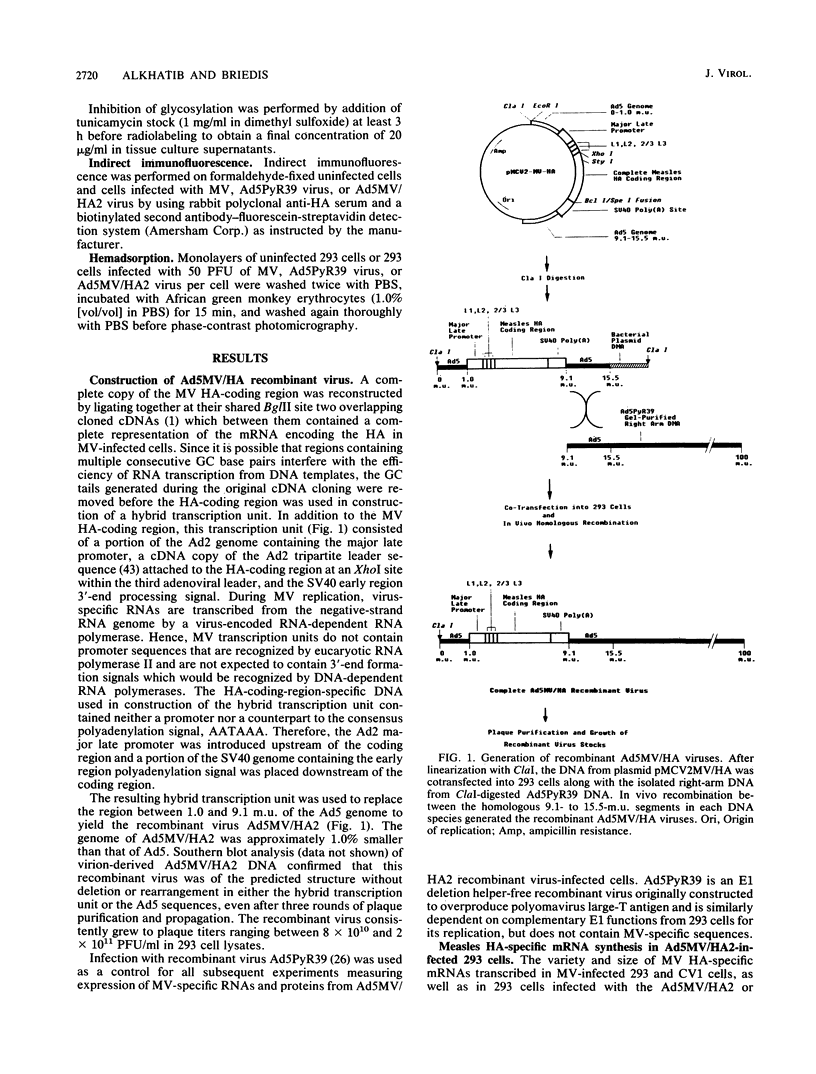
The entire measles virus (MV) hemagglutinin (HA)-coding region was reconstructed from cloned cDNAs and used as part of a hybrid transcription unit to replace a region of the adenovirus type 5 genome corresponding to the entire E1a transcription unit and most of the E1b transcription unit. The resulting recombinant virus was stable and able to replicate to high titers in 293 cells (which constitutively express the complementary E1a-E1b functions) in the absence of helper virus. During infection of 293 cells, the hybrid virus expressed MV HA protein which was indistinguishable from that expressed in MV-infected cells in terms of immunoreactivity, gel mobility, glycosylation, subcellular localization, and biologic activity. Infection of 293 cells with the hybrid virus led to high-level synthesis of the MV HA protein (equivalent to 65 to 130% of the level seen in MV-infected cells). At late times after high-multiplicity hybrid virus infection of HeLa and Vero cells (which do not express E1 functions), the level of HA protein synthesis was at least 35% of that seen in 293 cells. This MV-adenovirus recombinant will be useful in the study of the biologic properties of the MV HA protein and in assessment of the potential usefulness of hybrid adenoviruses as live-virus vaccine vectors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkhatib G., Briedis D. J. The predicted primary structure of the measles virus hemagglutinin. Virology. 1986 Apr 30;150(2):479–490. doi: 10.1016/0042-6822(86)90312-0. [DOI] [PubMed] [Google Scholar]
- Ballay A., Levrero M., Buendia M. A., Tiollais P., Perricaudet M. In vitro and in vivo synthesis of the hepatitis B virus surface antigen and of the receptor for polymerized human serum albumin from recombinant human adenoviruses. EMBO J. 1985 Dec 30;4(13B):3861–3865. doi: 10.1002/j.1460-2075.1985.tb04158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini W. J., Englund G., Rozenblatt S., Arnheiter H., Richardson C. D. Measles virus P gene codes for two proteins. J Virol. 1985 Mar;53(3):908–919. doi: 10.1128/jvi.53.3.908-919.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkner K. L., Schaffhausen B. S., Roberts T. M., Sharp P. A. Abundant expression of polyomavirus middle T antigen and dihydrofolate reductase in an adenovirus recombinant. J Virol. 1987 Apr;61(4):1213–1220. doi: 10.1128/jvi.61.4.1213-1220.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkner K. L., Sharp P. A. Generation of adenovirus by transfection of plasmids. Nucleic Acids Res. 1983 Sep 10;11(17):6003–6020. doi: 10.1093/nar/11.17.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blok J., Air G. M., Laver W. G., Ward C. W., Lilley G. G., Woods E. F., Roxburgh C. M., Inglis A. S. Studies on the size, chemical composition, and partial sequence of the neuraminidase (NA) from type A influenza viruses show that the N-terminal region of the NA is not processed and serves to anchor the NA in the viral membrane. Virology. 1982 May;119(1):109–121. doi: 10.1016/0042-6822(82)90069-1. [DOI] [PubMed] [Google Scholar]
- Blumberg B., Giorgi C., Roux L., Raju R., Dowling P., Chollet A., Kolakofsky D. Sequence determination of the Sendai virus HN gene and its comparison to the influenza virus glycoproteins. Cell. 1985 May;41(1):269–278. doi: 10.1016/0092-8674(85)90080-7. [DOI] [PubMed] [Google Scholar]
- Briedis D. J., Lamb R. A., Choppin P. W. Influenza B virus RNA segment 8 codes for two nonstructural proteins. Virology. 1981 Jul 30;112(2):417–425. doi: 10.1016/0042-6822(81)90289-0. [DOI] [PubMed] [Google Scholar]
- Carter M. J., Willcocks M. M., Löffler S., ter Meulen V. Relationships between monoclonal antibody-binding sites on the measles virus haemagglutinin. J Gen Virol. 1982 Nov;63(Pt 1):113–120. doi: 10.1099/0022-1317-63-1-113. [DOI] [PubMed] [Google Scholar]
- Davidson D., Hassell J. A. Overproduction of polyomavirus middle T antigen in mammalian cells through the use of an adenovirus vector. J Virol. 1987 Apr;61(4):1226–1239. doi: 10.1128/jvi.61.4.1226-1239.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A. R., Kostek B., Mason B. B., Hsiao C. L., Morin J., Dheer S. K., Hung P. P. Expression of hepatitis B surface antigen with a recombinant adenovirus. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7560–7564. doi: 10.1073/pnas.82.22.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elango N., Coligan J. E., Jambou R. C., Venkatesan S. Human parainfluenza type 3 virus hemagglutinin-neuraminidase glycoprotein: nucleotide sequence of mRNA and limited amino acid sequence of the purified protein. J Virol. 1986 Feb;57(2):481–489. doi: 10.1128/jvi.57.2.481-489.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gaynor R. B., Berk A. J. Cis-acting induction of adenovirus transcription. Cell. 1983 Jul;33(3):683–693. doi: 10.1016/0092-8674(83)90011-9. [DOI] [PubMed] [Google Scholar]
- Gaynor R. B., Hillman D., Berk A. J. Adenovirus early region 1A protein activates transcription of a nonviral gene introduced into mammalian cells by infection or transfection. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1193–1197. doi: 10.1073/pnas.81.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hiebert S. W., Paterson R. G., Lamb R. A. Hemagglutinin-neuraminidase protein of the paramyxovirus simian virus 5: nucleotide sequence of the mRNA predicts an N-terminal membrane anchor. J Virol. 1985 Apr;54(1):1–6. doi: 10.1128/jvi.54.1.1-6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979 Jul;17(3):683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Logan J., Shenk T. Adenovirus tripartite leader sequence enhances translation of mRNAs late after infection. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3655–3659. doi: 10.1073/pnas.81.12.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massie B., Gluzman Y., Hassell J. A. Construction of a helper-free recombinant adenovirus that expresses polyomavirus large T antigen. Mol Cell Biol. 1986 Aug;6(8):2872–2883. doi: 10.1128/mcb.6.8.2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. Mechanism of activation of early viral transcription by the adenovirus E1A gene product. Cell. 1981 Oct;26(2 Pt 2):213–220. doi: 10.1016/0092-8674(81)90304-4. [DOI] [PubMed] [Google Scholar]
- Norrby E., Chen S. N., Togashi T., Shesberadaran H., Johnson K. P. Five measles virus antigens demonstrated by use of mouse hybridoma antibodies in productively infected tissue culture cells. Arch Virol. 1982;71(1):1–11. doi: 10.1007/BF01315171. [DOI] [PubMed] [Google Scholar]
- Paterson R. G., Hiebert S. W., Lamb R. A. Expression at the cell surface of biologically active fusion and hemagglutinin/neuraminidase proteins of the paramyxovirus simian virus 5 from cloned cDNA. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7520–7524. doi: 10.1073/pnas.82.22.7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R. G., Lamb R. A., Moss B., Murphy B. R. Comparison of the relative roles of the F and HN surface glycoproteins of the paramyxovirus simian virus 5 in inducing protective immunity. J Virol. 1987 Jun;61(6):1972–1977. doi: 10.1128/jvi.61.6.1972-1977.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C. D., Berkovich A., Rozenblatt S., Bellini W. J. Use of antibodies directed against synthetic peptides for identifying cDNA clones, establishing reading frames, and deducing the gene order of measles virus. J Virol. 1985 Apr;54(1):186–193. doi: 10.1128/jvi.54.1.186-193.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux L., Beffy P., Portner A. Restriction of cell surface expression of Sendai virus hemagglutinin-neuraminidase glycoprotein correlates with its higher instability in persistently and standard plus defective interfering virus infected BHK-21 cells. Virology. 1984 Oct 15;138(1):118–128. doi: 10.1016/0042-6822(84)90152-1. [DOI] [PubMed] [Google Scholar]
- Roux L., Waldvogel F. A. Defective interfering particles of Sendai virus modulate HN expression at the surface of infected BHK cells. Virology. 1983 Oct 15;130(1):91–104. doi: 10.1016/0042-6822(83)90120-4. [DOI] [PubMed] [Google Scholar]
- Schuy W., Garten W., Linder D., Klenk H. D. The carboxyterminus of the hemagglutinin-neuraminidase of Newcastle disease virus is exposed at the surface of the viral envelope. Virus Res. 1984;1(5):415–426. doi: 10.1016/0168-1702(84)90027-3. [DOI] [PubMed] [Google Scholar]
- Shaw M. W., Lamb R. A., Erickson B. W., Briedis D. J., Choppin P. W. Complete nucleotide sequence of the neuraminidase gene of influenza B virus. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6817–6821. doi: 10.1073/pnas.79.22.6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spriggs M. K., Murphy B. R., Prince G. A., Olmsted R. A., Collins P. L. Expression of the F and HN glycoproteins of human parainfluenza virus type 3 by recombinant vaccinia viruses: contributions of the individual proteins to host immunity. J Virol. 1987 Nov;61(11):3416–3423. doi: 10.1128/jvi.61.11.3416-3423.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel C., Tjian R., Grodzicker T. Expression of SV40 T antigen under control of adenovirus promoters. Cell. 1981 Mar;23(3):825–836. doi: 10.1016/0092-8674(81)90447-5. [DOI] [PubMed] [Google Scholar]
- Togashi T., Orvell C., Vartdal F., Norrby E. Production of antibodies against measles virions by use of the mouse hybridoma technique. Arch Virol. 1981;67(2):149–157. doi: 10.1007/BF01318598. [DOI] [PubMed] [Google Scholar]
- Zain S., Sambrook J., Roberts R. J., Keller W., Fried M., Dunn A. R. Nucleotide sequence analysis of the leader segments in a cloned copy of adenovirus 2 fiber mRNA. Cell. 1979 Apr;16(4):851–861. doi: 10.1016/0092-8674(79)90100-4. [DOI] [PubMed] [Google Scholar]