Abstract
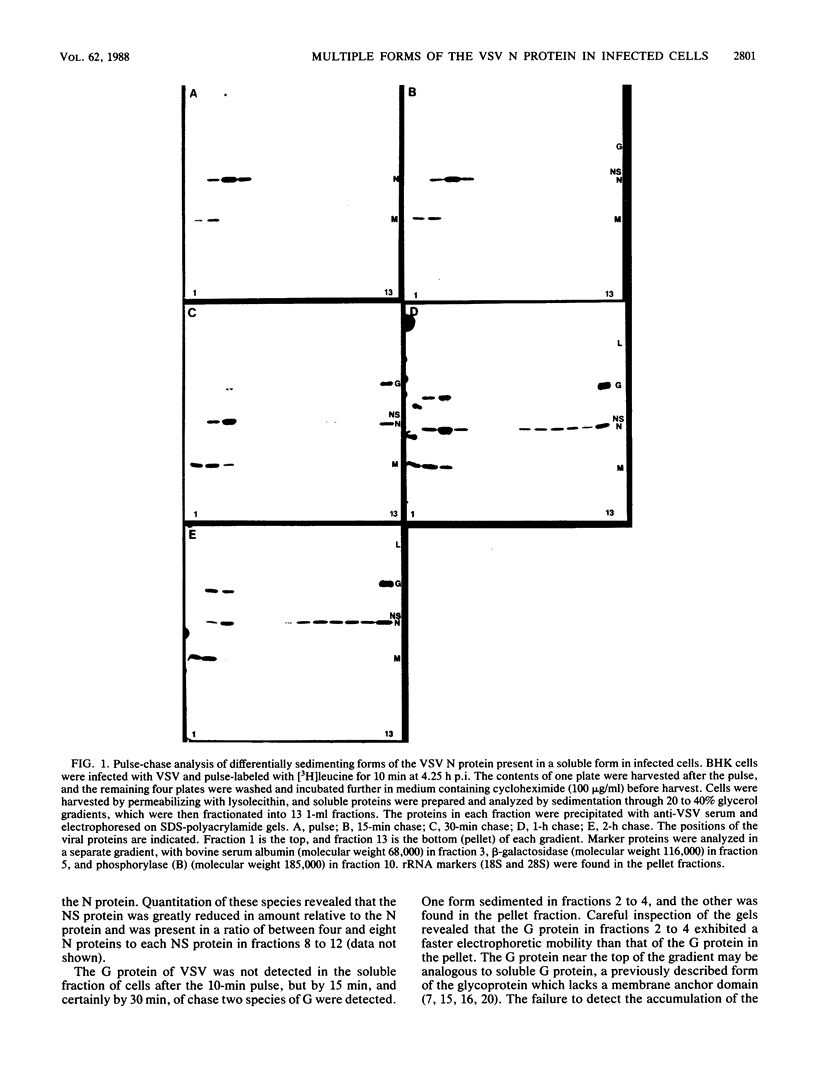
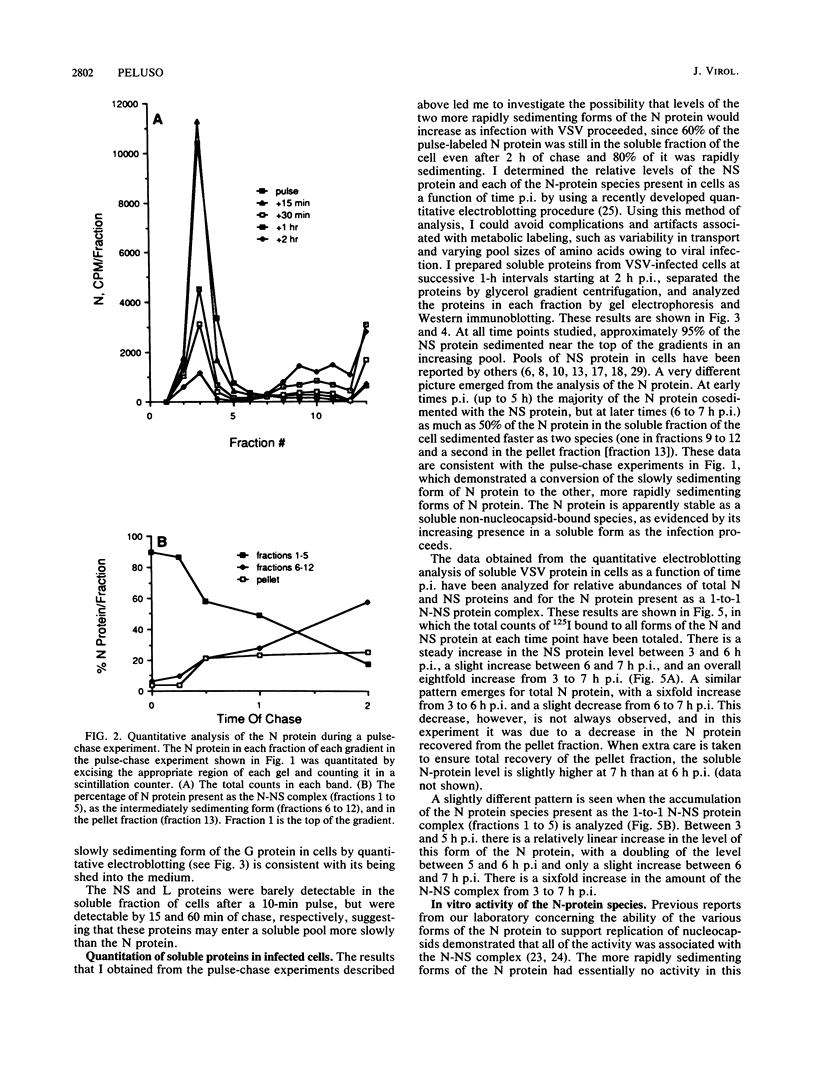
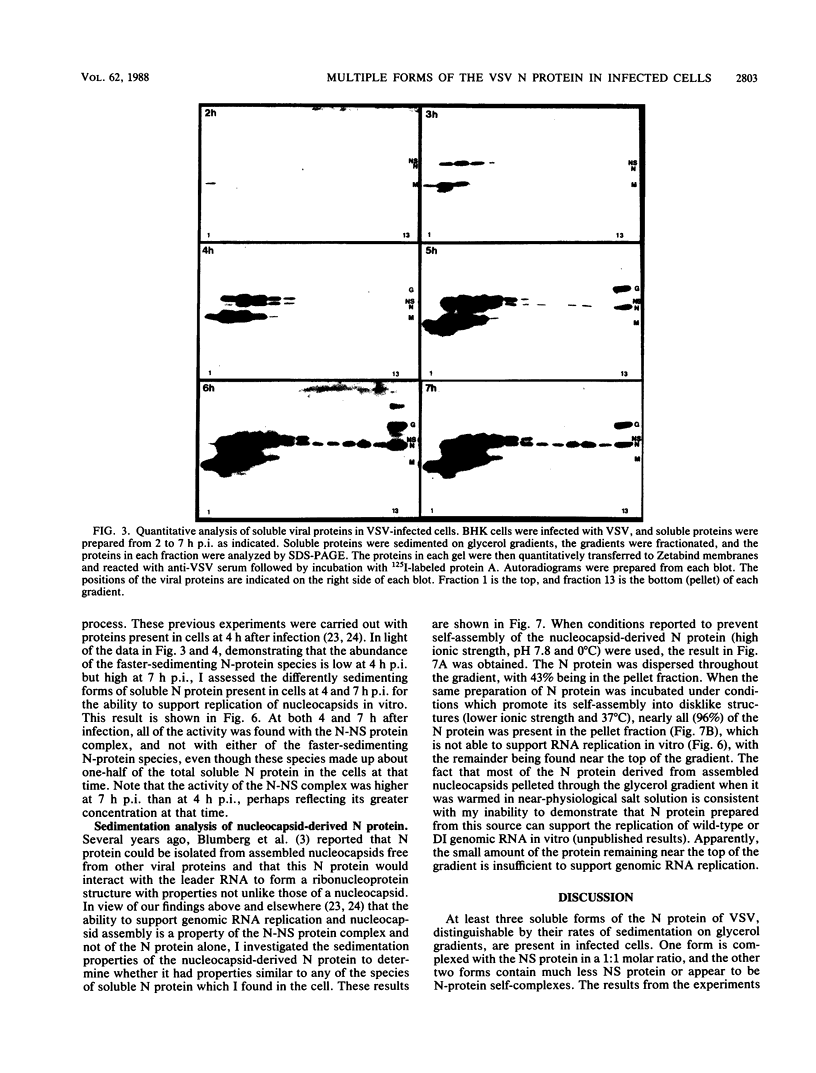
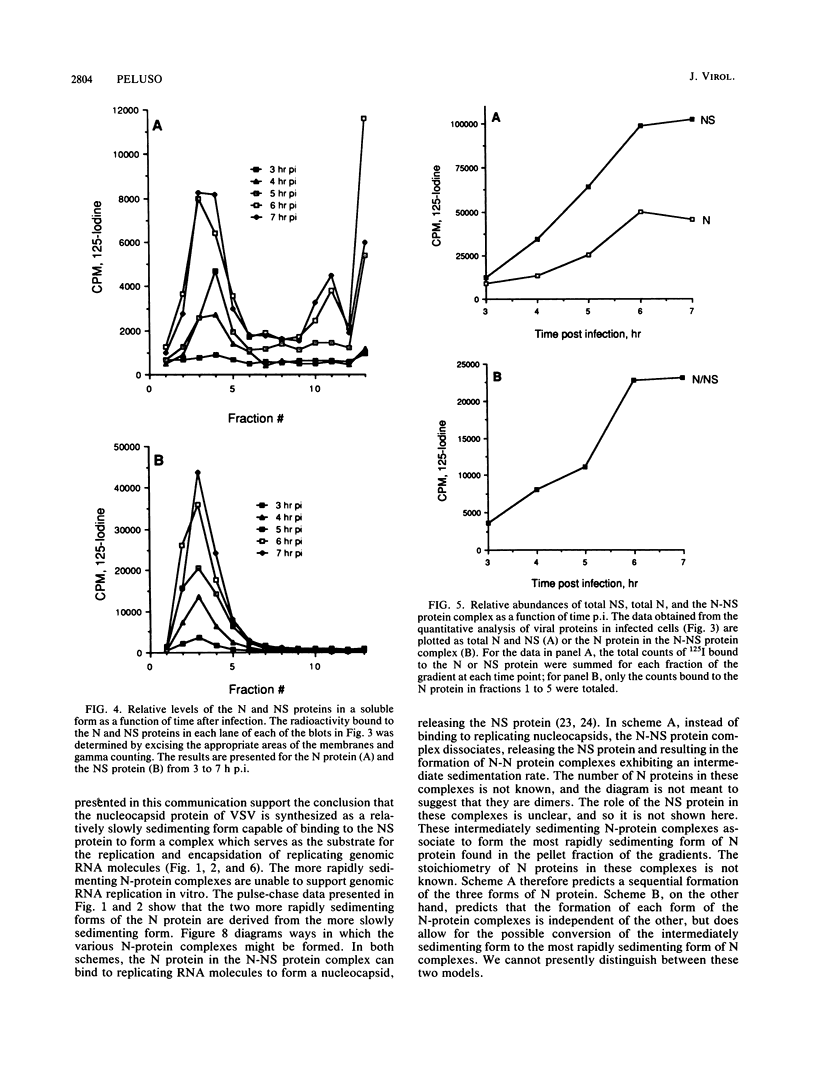
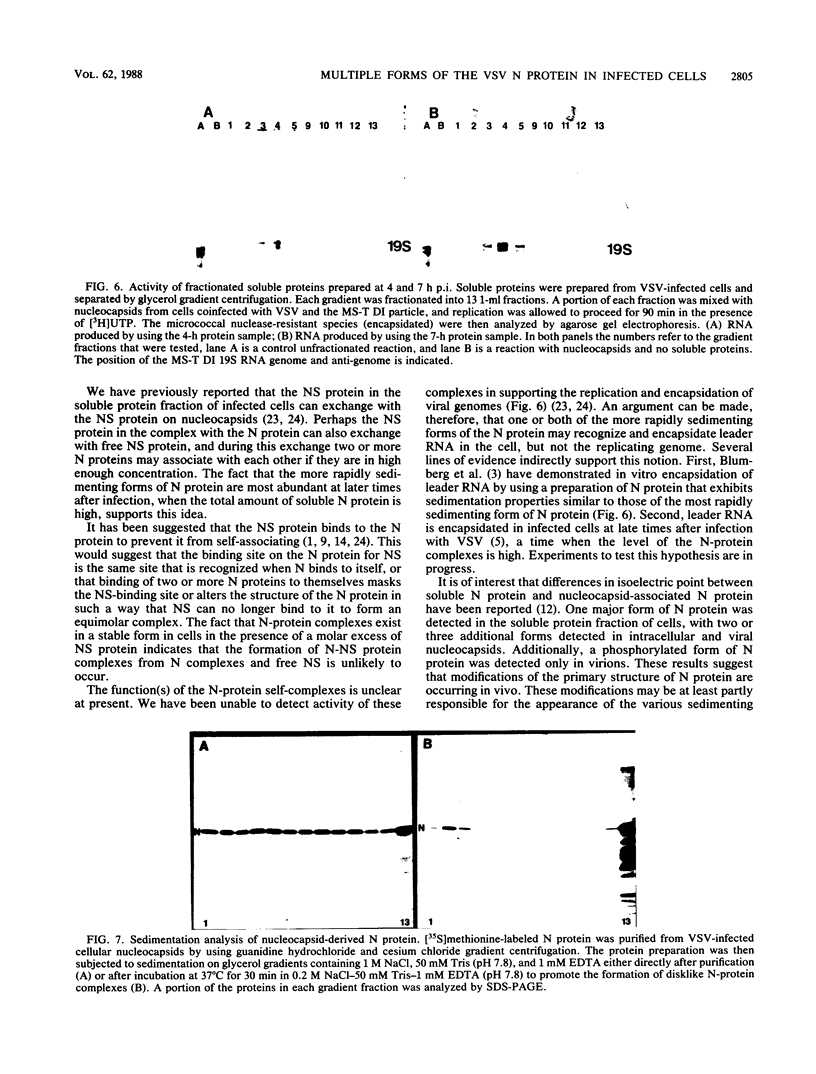
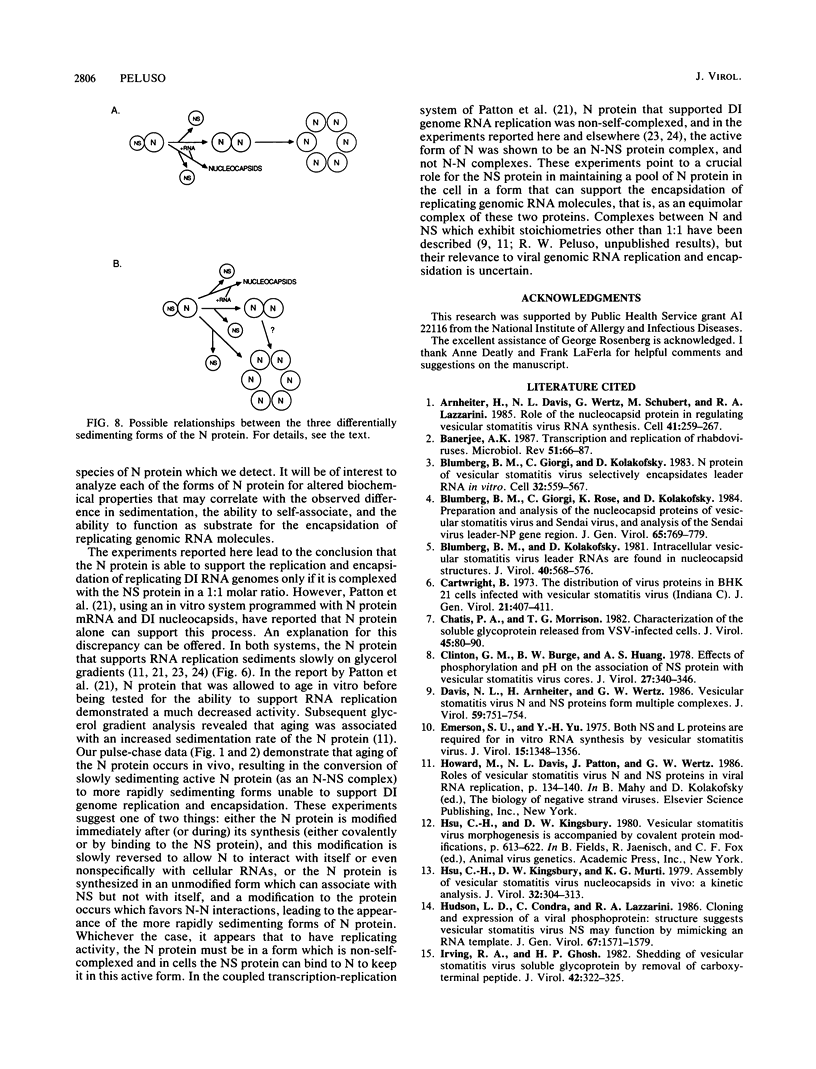
Multiple forms of the vesicular stomatitis virus nucleocapsid protein N have been detected in infected cells. One form is complexed with the viral NS protein in a 1:1 molar ratio, and the other forms are distinguished by their more rapid sedimentation rates on glycerol gradients. I performed a series of experiments designed to analyze the relationships between these forms of the N protein. Pulse-chase experiments demonstrate that the N protein is made first as the form which binds to the NS protein, forming a 1-to-1 molar complex, and that with increasing times of chase it is either assembled into nucleocapsids or converted to the two higher sedimenting forms. Using a newly developed quantitative immunoblotting procedure, I have quantitated the three differentially sedimenting species of the N protein and have shown that at later times postinfection (6 to 7 h), the faster-sedimenting forms of the N protein account for as much as 50% of the soluble N protein in the cell. The activity of these forms has been assessed, with only the 1-to-1 molar N-NS complex demonstrating the ability to support the replication and encapsidation of viral genomic RNA. A model for the conversion of the N protein from the active N-NS complex into the other forms of the protein is presented, and the possible function of the N-protein self-complexes is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnheiter H., Davis N. L., Wertz G., Schubert M., Lazzarini R. A. Role of the nucleocapsid protein in regulating vesicular stomatitis virus RNA synthesis. Cell. 1985 May;41(1):259–267. doi: 10.1016/0092-8674(85)90079-0. [DOI] [PubMed] [Google Scholar]
- Banerjee A. K. Transcription and replication of rhabdoviruses. Microbiol Rev. 1987 Mar;51(1):66–87. doi: 10.1128/mr.51.1.66-87.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B. M., Giorgi C., Kolakofsky D. N protein of vesicular stomatitis virus selectively encapsidates leader RNA in vitro. Cell. 1983 Feb;32(2):559–567. doi: 10.1016/0092-8674(83)90475-0. [DOI] [PubMed] [Google Scholar]
- Blumberg B. M., Giorgi C., Rose K., Kolakofsky D. Preparation and analysis of the nucleocapsid proteins of vesicular stomatitis virus and sendai virus, and analysis of the sendai virus leader-NP gene region. J Gen Virol. 1984 Apr;65(Pt 4):769–779. doi: 10.1099/0022-1317-65-4-769. [DOI] [PubMed] [Google Scholar]
- Blumberg B. M., Kolakofsky D. Intracellular vesicular stomatitis virus leader RNAs are found in nucleocapsid structures. J Virol. 1981 Nov;40(2):568–576. doi: 10.1128/jvi.40.2.568-576.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright B. The distribution of virus proteins in BHK 21 cells infected with vesicular stomatitis virus (Indiana C). J Gen Virol. 1973 Nov;21(2):407–411. doi: 10.1099/0022-1317-21-2-407. [DOI] [PubMed] [Google Scholar]
- Chatis P. A., Morrison T. G. Characterization of the soluble glycoprotein released from vesicular stomatitis virus-infected cells. J Virol. 1983 Jan;45(1):80–90. doi: 10.1128/jvi.45.1.80-90.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton G. M., Burge B. W., Huang A. S. Effects of phosphorylation and pH on the association of NS protein with vesicular stomatitis virus cores. J Virol. 1978 Aug;27(2):340–346. doi: 10.1128/jvi.27.2.340-346.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. L., Arnheiter H., Wertz G. W. Vesicular stomatitis virus N and NS proteins form multiple complexes. J Virol. 1986 Sep;59(3):751–754. doi: 10.1128/jvi.59.3.751-754.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Yu Y. Both NS and L proteins are required for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1975 Jun;15(6):1348–1356. doi: 10.1128/jvi.15.6.1348-1356.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. H., Kingsbury D. W., Murti K. G. Assembly of vesicular stomatitis virus nucleocapsids in vivo: a kinetic analysis. J Virol. 1979 Oct;32(1):304–313. doi: 10.1128/jvi.32.1.304-313.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson L. D., Condra C., Lazzarini R. A. Cloning and expression of a viral phosphoprotein: structure suggests vesicular stomatitis virus NS may function by mimicking an RNA template. J Gen Virol. 1986 Aug;67(Pt 8):1571–1579. doi: 10.1099/0022-1317-67-8-1571. [DOI] [PubMed] [Google Scholar]
- Irving R. A., Ghosh H. P. Shedding of vesicular stomatitis virus soluble glycoprotein by removal of carboxy-terminal peptide. J Virol. 1982 Apr;42(1):322–325. doi: 10.1128/jvi.42.1.322-325.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Prevec L. Proteins of vesicular stomatitis virus. 3. Intracellular synthesis and extracellular appearance of virus-specific proteins. Virology. 1971 Dec;46(3):678–690. doi: 10.1016/0042-6822(71)90070-5. [DOI] [PubMed] [Google Scholar]
- Kang C. Y., Prevec L. Proteins of vesicular stomatitis virus. II. Immunological comparisons of viral antigens. J Virol. 1970 Jul;6(1):20–27. doi: 10.1128/jvi.6.1.20-27.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsford L., Emerson S. U. Transcriptional activities of different phosphorylated species of NS protein purified from vesicular stomatitis virions and cytoplasm of infected cells. J Virol. 1980 Mar;33(3):1097–1105. doi: 10.1128/jvi.33.3.1097-1105.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M., Baltimore D., Lodish H. F. Separate pathways of maturation of the major structural proteins of vesicular stomatitis virus. J Virol. 1977 Mar;21(3):1128–1139. doi: 10.1128/jvi.21.3.1128-1139.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S. P., Huang A. S. Synthesis and distribution of vesicular stomatitis virus-specific polypeptides in the absence of progeny production. Virology. 1977 Aug;81(1):37–47. doi: 10.1016/0042-6822(77)90056-3. [DOI] [PubMed] [Google Scholar]
- Patton J. T., Davis N. L., Wertz G. W. N protein alone satisfies the requirement for protein synthesis during RNA replication of vesicular stomatitis virus. J Virol. 1984 Feb;49(2):303–309. doi: 10.1128/jvi.49.2.303-309.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso R. W., Moyer S. A. Initiation and replication of vesicular stomatitis virus genome RNA in a cell-free system. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3198–3202. doi: 10.1073/pnas.80.11.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso R. W., Moyer S. A. Viral proteins required for the in vitro replication of vesicular stomatitis virus defective interfering particle genome RNA. Virology. 1988 Feb;162(2):369–376. doi: 10.1016/0042-6822(88)90477-1. [DOI] [PubMed] [Google Scholar]
- Peluso R. W., Rosenberg G. H. Quantitative electrotransfer of proteins from sodium dodecyl sulfate-polyacrylamide gels onto positively charged nylon membranes. Anal Biochem. 1987 May 1;162(2):389–398. doi: 10.1016/0003-2697(87)90409-x. [DOI] [PubMed] [Google Scholar]
- Rubio C., Kolakofsky C., Hill V. M., Summers D. F. Replication and assembly of VSV nucleocapsids: protein association with RNPs and the effects of cycloheximide on replication. Virology. 1980 Aug;105(1):123–135. doi: 10.1016/0042-6822(80)90161-0. [DOI] [PubMed] [Google Scholar]
- Soria M., Little S. P., Huang A. S. Characterization of vesicular stomatitis virus nucleocapsids. I. Complementary 40 S RNA molecules in nucleocapsids. Virology. 1974 Sep;61(1):270–280. doi: 10.1016/0042-6822(74)90261-x. [DOI] [PubMed] [Google Scholar]
- Wagner R. R., Kiley M. P., Snyder R. M., Schnaitman C. A. Cytoplasmic compartmentalization of the protein and ribonucleic acid species of vesicular stomatitis virus. J Virol. 1972 Apr;9(4):672–683. doi: 10.1128/jvi.9.4.672-683.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Snyder R. M., Yamazaki S. Proteins of vesicular stomatitis virus: kinetics and cellular sites of synthesis. J Virol. 1970 May;5(5):548–558. doi: 10.1128/jvi.5.5.548-558.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]