Abstract
The CCAAT/enhancer binding protein α (C/EBPα) and CCAAT/enhancer binding protein β (C/EBPβ) mRNAs are templates for the differential translation of several isoforms. Immunoblotting detects C/EBPαs with molecular masses of 42, 38, 30, and 20 kDa and C/EBPβs of 35, 20, and ∼8.5 kDa. The DNA-binding activities and pool levels of p42C/EBPα and p30C/EBPα in control nuclear extracts decrease significantly whereas the binding activity and protein levels of the 20-kDa isoforms increase dramatically with LPS treatment. Our studies suggest that the LPS response involves alternative translational initiation at specific in-frame AUGs, producing specific C/EBPα and C/EBPβ isoform patterns. We propose that alternative translational initiation occurs by a leaky ribosomal scanning mechanism. We find that nuclear extracts from normal aged mouse livers have decreased p42C/EBPα levels and binding activity, whereas those of p20C/EBPα and p20C/EBPβ are increased. However, translation of 42-kDa C/EBPα is not down-regulated on polysomes, suggesting that aging may affect its nuclear translocation. Furthermore, recovery of the C/EBPα- and C/EBPβ-binding activities and pool levels from an LPS challenge is delayed significantly in aged mouse livers. Thus, aged livers have altered steady-state levels of C/EBPα and C/EBPβ isoforms. This result suggests that normal aging liver exhibits characteristics of chronic stress and a severe inability to recover from an inflammatory challenge.
INTRODUCTION
Recent studies have indicated that the ability to respond to and recover from various stress challenges declines with age. Our studies have shown an age-associated increase in the constitutive expression of the acute phase reactant gene α1-acid glycoprotein (AGP) and an extended lag period in its induction by bacterial LPS (Carter et al., 1991; Post et al., 1991). Other age-associated effects on stress response genes include an increase in the constitutive expression of the T-kininogen (Sierra et al., 1989) gene, failure of hsp70 genes to respond to hyperthermia due to changes in the activity of heat shock gene transcription factors (Liu et al., 1989; 1991; Choi et al., 1990; Heydari et al., 1993), and repression of the androgen receptor gene due to changes in NFκB activity and induction of a repressor protein (Supakar et al., 1995). These studies suggest that the effects of aging on the regulation of families of stress response genes may be due to changes in the structure and/or function of transcription factors that regulate these families of genes. The C/EBPs are a family of trans-acting factors that play a major role in the regulation of families of stress response genes. To determine whether aging affects the structure and/or function of C/EBPα and C/EBPβ isoforms, we studied their DNA-binding activity and protein pool levels in the mouse liver as a function of age and/or LPS treatment.
It has been proposed that the formation of multiple C/EBPα and C/EBPβ isoforms is mediated by the differential use of multiple AUG initiation codons within the same ORF of a single mRNA (Descombes and Schibler, 1991; Lin et al., 1993; Ossipow et al., 1993; Calkhoven et al., 1994; An et al., 1996). Thus, alternative translational initiation produces 42-kDa (p42C/EBPα), 38-kDa, 35-kDa, 30-kDa, and 20-kDa (p20C/EBPα) isoforms; the C/EBPβ isoforms produced by this mechanism are 35 kDa (p35C/EBPβ), 30 kDa (p30C/EBPβ), and 20 kDa (p20C/EBPβ) (An et al., 1996). These isoforms, both C/EBPα and C/EBPβ, differ significantly in their regulatory functions. For example, p42C/EBPα is a powerful trans-activator of several genes coordinately regulated during the differentiation of preadipocytes to adipocytes and has antiproliferative activity associated with adipocyte differentiation (Umek et al., 1991; Lin et al., 1993; Lin and Lane, 1994). The p30C/EBPα, which is initiated from an AUG codon downstream of the first AUG, fails to interfere with adipocyte cell proliferation and to induce complete 3T3-L1 differentiation. The isoform also attenuates transcriptional activation by p42C/EBPα. The C/EBPβ isoforms include p35C/EBPβ, which binds to region D of the albumin promoter and acts as a powerful trans-activator, and p20C/EBPβ whose truncated transcription activation domain antagonizes p35C/EBPβ activity (Descombes et al., 1990; Descombes and Schibler, 1991). Both the 35- and 20-kDa C/EBPs repress transcription of Rous sarcoma virus (Sears and Sealy, 1994); p42C/EBPα has repressor activity for human hepatitis B virus and simian virus 40 (Lopez-Caberra et al., 1990; Pei and Shih, 1990, 1991). We have shown that C/EBPβ is the isoform that binds to the acute phase response element (APRE) of the α1-acid glycoprotein promoter during the LPS-mediated acute phase response (Alam and Papaconstantinou, 1992; Alam et al., 1993) and that when transcription of the AGP gene is activated, it is the 20-kDa C/EBPβ that binds to the APRE, suggesting that p20C/EBPβ may be a trans-activator for this gene (An et al., 1996). These studies have shown that LPS stimulates a dramatic change in the isoforms of the C/EBPα and C/EBPβ family of transcription factors (Baumann et al., 1990, 1992; Chang et al., 1990; Alam and Papaconstantinou, 1992; Alam et al., 1993; An et al., 1996). Using the APRE oligonucleotide we have demonstrated a major shift in the DNA-binding activities from C/EBPα to C/EBPβ during the hepatic response to LPS (Alam et al., 1993; An et al., 1996). Specifically, we have shown that p42C/EBPα is the predominant isoform that binds to the APRE in nuclear extracts from untreated mice and that the p20C/EBPβ becomes the major isoform in the DNA–protein complex formed with nuclear extracts from LPS-treated liver (An et al., 1996). Concomitant with the increase in C/EBPβ-binding activity, the binding activity of C/EBPα is decreased significantly in response to LPS. Furthermore, we demonstrated that during the acute phase response specific C/EBPα and C/EBPβ protein pool levels are up- and down-regulated, respectively; this LPS-mediated regulation of C/EBPα- and C/EBPβ-binding activity and pool levels may play an integral role in gene regulation during the acute phase response. These observations have suggested to us that age-associated changes in the constitutive and LPS-inducible levels of C/EBP isoforms may be important factors in changes in acute phase reactant gene activity seen in aged liver.
In this article, we present our studies on the effects of aging on the DNA-binding activity and protein pool levels of C/EBPα and C/EBPβ isoforms. On the basis of our recent results suggesting that C/EBPα and C/EBPβ isoform synthesis is mediated by LPS treatment, and is due to alternative translation initiation at specific AUG initiation sites in the C/EBPα and C/EBPβ mRNAs, we initiated these studies to determine whether there are age-associated changes in the regulation of alternative translational initiation that alter the differential translation of C/EBPα and C/EBPβ mRNAs.
MATERIALS AND METHODS
Animals
Male C57BL/6J mice from the National Institute on Aging colonies (Bethesda, MD) were obtained through the Charles River Laboratories (Wilmington, MA). All animals were maintained on a light/dark (12-h/12-h) cycle and fed standard chow diet.
Animals were injected intraperitoneally with 10 μg of LPS (Sigma, St. Louis, MO) in pyrogen-free saline and were killed by cervical dislocation at indicated time points. In the course of our previous studies we have noted that 50 μg of LPS produce a maximal acute phase response in young mice. However, this dose of LPS causes a greater than 50% mortality for aged mice (24–28 mo). In view of this high mortality rate, we have used the lower LPS dosage in the present studies.
Preparation of Nuclear Protein Extracts
Preparation of liver nuclear protein extracts from control and LPS-treated mice has been described (Zhang et al., 1990; Alam et al., 1993). Aliquots of the nuclear protein solutions were frozen in liquid nitrogen and stored at −90°C.
Electrophoretic Mobility Shift Assays (EMSAs)
EMSAs were carried out as described by Alam et al. (1993). The following oligonucleotide, i.e., the APRE corresponding to the C/EBP-binding site of the AGP-1 promoter and its complementary strand were used as a probe for EMSA or Southwestern blot analysis after labeling with [γ-32P]ATP by T4 polynucleotide kinase (Fried and Crothers, 1981; Garner and Revzin, 1981): 5′-GAACATTTTGCGCAAGACATTTCCCAAG-3′.
Equal amounts of the two complementary strands were heated at 95°C for 10 min in STE buffer (10 mM Tris-Cl, pH 8.0, 100 mM NaCl, and 1 mM EDTA) and allowed to anneal by slowly cooling to room temperature. For supershift assays, C/EBPα- and C/EBPβ-specific antibodies were preincubated for 20 min with nuclear extracts before adding the probe. The DNA–protein complexes were resolved by electrophoresis in 6% nondenaturing polyacrylamide gels in 0.5× TBE (1× TBE: 25 mM Tris base, 25 mM boric acid, 0.5 mM EDTA).
Southwestern Blot Analysis of Nuclear Proteins Bound to DNA
Southwestern blot analysis procedures have been described by An et al. (1996).
Western Blot Analysis of Nuclear Extracts
Western immunoblot procedures have been described by An et al. (1996).
Antisera
Antisera specific for C/EBPα and C/EBPβ were prepared against specific oligopeptides (Landschulz et al., 1988, 1989; Cao et al., 1991). The oligopeptide used to prepare the anti-C/EBPα was AGPHPDLRTGGGGGGGAGA, which is adjacent to the DNA-binding domain; the C/EBPβ antiserum was prepared using the oligopeptide LRNLFKQLPEPLLASAG, which is at the carboxyl terminus. Antisera specific for the Flag tag sequences inserted into pCMV-C/EBPβ expression vectors were purchased from Eastman Kodak (Rochester, NY).
Polysome Isolation and Analysis of Binding Activity of Nascent Peptide Chains to APRE Oligonucleotide
Polysomes were isolated from control and LPS-treated mouse livers as described previously (Brown and Papaconstantinou, 1977; An et al., 1996). The protein concentration was determined according to the method of Bradford (1976). These polysomal proteins were then used in the EMSA, supershift, and Southwestern blot analyses as described.
Isolation of Proteins Binding to APRE Oligonucleotide
The DNA–protein complexes designated as C1–C5 were isolated as described by An et al. (1996).
Construction of C/EBP Expression Vectors
Amplification vector pMSV-C/EBPβ-SVori was constructed by cloning the PvuII/HindIII inserts of pSVori (Gluzman, 1981; Rabek et al., 1990) into the vector portion of EcoRI-digested pMSV-C/EBPβ (Cao et al., 1991). The identity of the clone was confirmed by restriction enzyme mapping and Southern blot analysis with 32P-labeled SVori probe. The pCMV-C/EBPβ expression vectors were constructed as follows: A 1.45-kb HinfI (blunted)/BamHI restriction fragment, containing the mouse C/EBPβ coding region, 54 bp of 5′ untranslated region and 502 bp of 3′ untranslated region, was cloned into the Xba (blunted)/BamHI sites of pGEM-7zf(+) (Promega, Madison, WI), generating pGEM-7zf-C/EBPβ. To distinguish the protein products of expression vectors from the endogeneously generated C/EBPβ, the Flag tag was attached to the C-terminal of C/EBPβ by annealing oligonucleotides 5′-CCGAGCCGCTCGTGGCCTCGGCGGGCCACTG-3′ with 5′-CAGTGGCCCGCCGAGGCCAGCAGCGGC-3′; 5′-CGACTACAAAGACGATGACGATAAATA-3′ with 5′-CTAGCTATTTATCGTCATCGTCTTTGTAGTCG-3′ (Flag tag codons underlined), respectively, and inserting them in AvaI/NheI sites producing pGEM-7zf-C/EBPβ-Flag. A XbaI/PstI restriction fragment from pGEM-7zf-C/EBPβ was cloned into pGEM-4z. The resultant construct was digested with BamHI/PstI to produce a BamHI/PstI restriction fragment, which along with the PstI/BamHI restriction fragment from pGEM-7zf-C/EBPβ-Flag were cloned into the BamHI site of pCMV-B, yielding the wild-type expression vector pCMV-C/EBPβ-WT. pCMV-B is a derivative from pFlag-CMV 2 (Eastman Kodak) with inactivated BamHI site and substituted SacI/HindIII sequence. Mutation of the 20-kDa AUG start site to produce pCMV-C/EBPβ-MT20 expression vector was prepared by site-specific mutation of the AUG → UUG in the pCMV-C/EBPβ-WT vector. The mutation was achieved by using the oligonucleotide primer 5′-CCGGCCGCCAAGGCGGGCGCGTCG-3′ (mismatch underlined) following the method of Kunkel et al. Construction of the pAPRE-CAT expression vector has been described (Alam et al., 1993). All constructs were confirmed by sequence and restriction analysis.
Cell Culture and Transfection of C/EBPα and C/EBPβ Standards and Expression Vectors
The procedures for the transfection of C/EBP expression vectors into COS-1 cells have been described (An et al., 1996). These nuclear proteins were used as standards in the Southwestern blot and Western blot analyses, as well as for analyses of alternative translational initiation.
RESULTS
Effects of Aging on Binding Activities of C/EBPα and C/EBPβ to the APRE Oligonucleotide of the AGP-1 Promoter
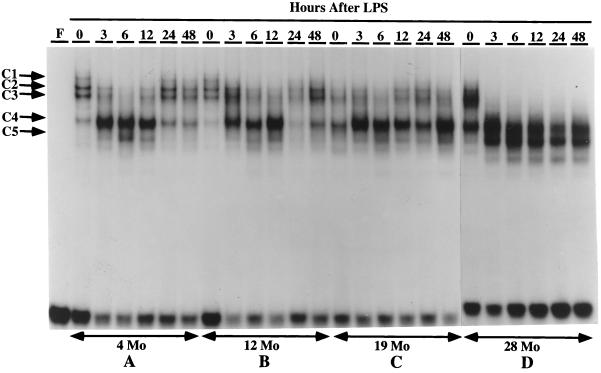
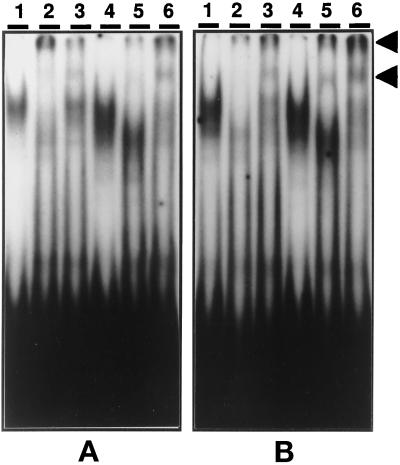
Binding at the C/EBP-binding site of the AGP-1 promoter is altered during the LPS-mediated activation of the acute phase response (Alam and Papaconstantinou, 1991; Alam et al., 1993; An et al., 1996). Using the APRE oligonucleotide, we demonstrated that p42C/EBPα, p35C/EBPβ, and p20C/EBPβ are the isoforms in control nuclear extracts that form the APRE–protein complexes, and that p20C/EBPβ, whose DNA-binding activity is minimal in controls, becomes the predominant isoform in these complexes in LPS-treated mice (Alam and Papaconstantinou, 1992; An et al., 1996). In the present experiments we conducted an EMSA time-course analysis to determine whether the complexes formed between C/EBPα and C/EBPβ isoforms and the APRE are affected by aging. The data in Figure 1 demonstrate significant qualitative and quantitative changes in the APRE–protein complexes formed using liver nuclear extracts from control and LPS-treated 4-, 12-, 19-, and 28-mo-old mice. These experiments demonstrated the formation of four distinct APRE–protein complexes (C1, C2, C3, and C4) with nuclear extracts from untreated 4- and 12-mo-old adults (Figure 1, A and B; An et al., 1996) and that the same DNA–protein-binding pattern is formed with liver nuclear extracts from these mice (An et al., 1996). The DNA-binding pattern for both ages show a loss of the C1 and an increase in the C4 complexes at 3 h, the loss of C2–C3 complexes and further increase in intensity of the C4 complex at 6 and 12 h, and a near recovery to the control pattern at 24–48 h.
Figure 1.
A time course of DNA binding to the C/EBP-binding site (APRE) of the mouse AGP-1 promoter with nuclear proteins from livers of young and aged mice treated with LPS. EMSAs were performed using 3 μg of nuclear proteins isolated from fresh livers of control (0) or LPS-injected C57BL/6 male mice aged 4 mo (A), 12 mo (B), 19 mo (C), and 28 mo (D). LPS (10 μg) was injected at time 0, and mice were killed and nuclear proteins prepared at the times indicated at the top of each lane. The double-stranded oligonucleotide corresponding to the APRE of AGP-1 promoter was labeled with [γ-32P]ATP and T4 polynucleotide kinase. The DNA–protein complexes are indicated as C1, C2, C3, C4, and C5. Lane F contains the free DNA probe.
The EMSA of the nuclear extracts from aged (19- and 28-mo) control and LPS-treated animals are shown in Figure 1, C and 1D. In controls the levels of the C1 and C2 complexes are reduced, whereas the levels of the C3 (in 28 mo) and C4 (in 19 and 28 mo) complexes are increased. These experiments indicate that there is a significant increase in the ratio of C4:C1–C2 in nuclear extracts of the aged, untreated animals and suggest that these animals exhibit properties similar to those observed with LPS-treated young animals. This ratio is significantly larger if the C3–C4 complex is included in this comparison. In addition, the EMSA for the nuclear extracts of LPS-treated 28- mo-old animals show a significant increase in binding activity of the C5 DNA–protein complex (Figure 1D). Although the C5 complex is detectable in the young nuclear extracts, the dramatic increase in binding activity of this isoform appears to be an age-associated characteristic.
The EMSA time-course analyses indicate that the 4- and 12-mo-old (middle-aged) animals recover from the LPS treatment by 24 h (An et al., 1996), whereas the EMSA of the aged animals (19 and 28 mo) indicate that the DNA–protein complexes associated with the inflammatory response persist for up to 48 h (Figure 1, A versus D). Thus, in addition to exhibiting an increased binding activity indicative of an inflammatory response in the absence of a challenge, the aged animal does not recover from this inflammatory challenge as rapidly as the young adult. Studies in progress indicate that recovery occurs within 72–96 h after LPS treatment (our unpublished results).
Identification of the C/EBPs That Bind to the APRE in Control and LPS-treated Nuclear Extracts
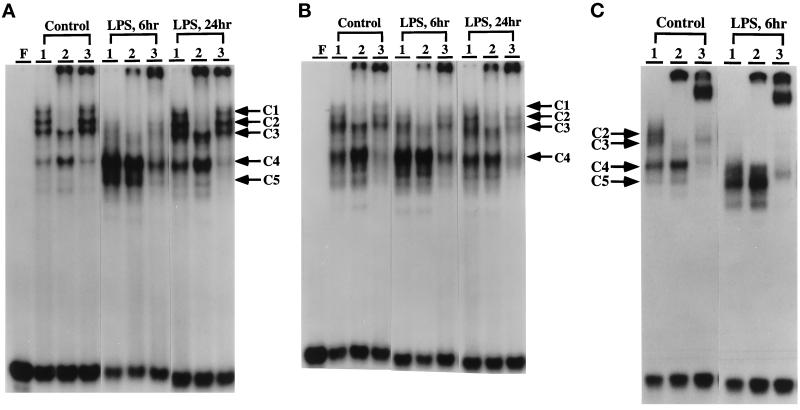
Supershift analyses show that anti-C/EBPα (Figure 2A, control lane 2) displaces the C1, C2, and some of the C3 complexes, whereas anti-C/EBPβ does not exhibit a strong interaction with these complexes (Figure 2A, control lane 3), but does shift the C4 and C5 complexes. Anti-C/EBPδ does not displace any of these complexes (our unpublished results). Analyses of the complexes formed 6 h after LPS treatment indicate that C/EBPα-binding activity decreases, whereas the C/EBPβ-binding activity (C4 and C5) increases during the acute phase response (Figure 2A, 6 h); at 24 h after LPS treatment C/EBPα has increased and C/EBPβ, although still significantly higher than control, has decreased from its peak (An et al., 1996). The supershift analyses indicate that in nuclear extracts of control aged mouse livers (Figure 2, B and C versus A) there is a decrease in C/EBPα (C1–C3) and an increase in C/EBPβ binding activity (C4–C5); after 6 h of LPS treatment the difference in their binding activity increases. These data are in agreement with the EMSA data and indicate that there is an age-associated loss of C/EBPα-binding activity and increase in C/EBPβ-binding activity, resulting in an overall increase in the ratio of C/EBPβ:C/EBPα in control animals. Our data suggest that the C/EBPα–C/EBPβ-binding activities are altered in aged livers, and that this may be due to changes in either their protein pool levels or DNA-binding activity.
Figure 2.
Mobility supershift analyses of the DNA–protein complexes formed with the APRE of the AGP-1 promoter. Supershift assays were done as described in MATERIALS AND METHODS to demonstrate that the nuclear proteins involved in the formation of DNA–protein complexes with the AGP-1 APRE are members of the C/EBP family. Monospecific antibodies against synthetic peptides unique to the C/EBPα and C/EBPβ isoforms were used. Lanes F, free oligonucleotide; lanes 1, no antibody was added to the reaction mixture; lanes 2, mouse anti-C/EBPα; lanes 3, mouse anti-C/EBPβ. Nuclear extracts were prepared 0, 6, and 24 h after LPS injection as indicated. Mice 4, 19, and 28 mo of age were used. The positions of the DNA–protein complexes are noted as C1–C5; the bands formed by supershifting with C/EBPα or C/EBPβ antibody are indicated by the arrows. (A) 4 mo. (B) 19 mo. (C) 28 mo.
Determination of the Molecular Weights of C/EBP Proteins That Bind to the APRE
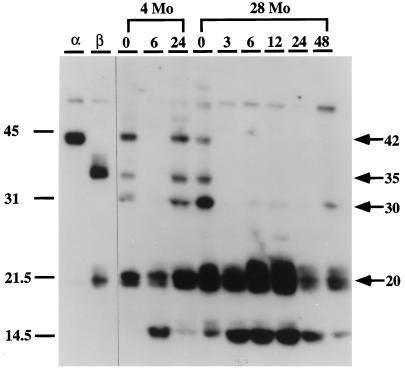
The carboxyl terminal region of C/EBP proteins contains a leucine zipper domain that facilitates formation of homo- and heterodimer (Landschulz et al., 1988). Dimerization is an essential feature of the binding activity of these proteins. In previous studies we used the APRE oligonucleotide for Southwestern blotting analyses to estimate the apparent molecular weights and binding activity of C/EBP homodimers in liver nuclear extracts of control and LPS-treated young mice (An et al., 1996). In the present studies we used this oligonucleotide to determine whether aging affects the binding activity of these C/EBPs (Figure 3). Analysis of nuclear extracts from fresh livers of control and LPS-treated, young (4-mo) and old (28-mo) mice detected bands with apparent molecular masses of 42 kDa, 35 kDa, 30 kDa, and 20 kDa in the nuclear extracts of young untreated livers (Figure 3). The nuclear extracts of aged animals showed the same molecular mass isoforms as well as the low molecular mass isoform of ∼14.5 kDa which is only seen in young mice at 6 h after LPS treatment. Furthermore, the binding activity of the 42-kDa isoform decreased in the aged animal whereas the 30-kDa and 20-kDa isoforms increased. Upon LPS treatment the intensities of the 42-kDa, 35-kDa, and 30-kDa isoforms decreased or disappeared completely 6 h after treatment and reappeared after 24 h in young mice. Similarly, the high molecular mass isoforms decreased, whereas the 20-kDa and ∼14.5-kDa isoforms increased in the aged (28-mo) livers in response to LPS. In general, the pattern of the C/EBP homodimer isoforms detected by Southwestern blot analyses is consistent with the pattern of binding activity demonstrated by EMSA. However, the steady-state and LPS-induced intensities of the 42-kDa, 35-kDa, and 30-kDa isoforms decreased or disappeared completely with the LPS treatment. These experiments also showed the recovery of the DNA-binding activity of the 42-kDa, 35-kDa, and 30-kDa C/EBP proteins in young nuclear extracts after 24 h, whereas this recovery is not detected in aged nuclear extracts even by 48 h. Thus, the kinetics of binding activity determined by EMSA and by Southwestern blot analyses demonstrate the same age-associated differences in C/EBP-binding activity.
Figure 3.
Southwestern blot analysis of liver nuclear proteins that bind to the APRE of the AGP-1 gene after LPS treatment. C57BL/6 mice (4- and 28-mo-old males) were injected with 10 μg of LPS and then killed 3, 6, 12, 24, or 48 h after injection. Nuclear proteins (30 μg) prepared from fresh livers were subjected to SDS-PAGE, blotted onto Westran PVDF membranes, and probed with 106 cpm/ml of 32P-labeled APRE oligonucleotide as described in MATERIALS AND METHODS. Locations of molecular size standards (kDa) are shown on the left. Lanes α and β contain nuclear extracts from COS-1 cells transfected with expression plasmids for C/EBPα and C/EBPβ, respectively, as described in MATERIALS AND METHODS.
Age-associated Effects on the Constitutive and LPS-induced Pool Levels of C/EBPα and C/EBPβ Isoforms
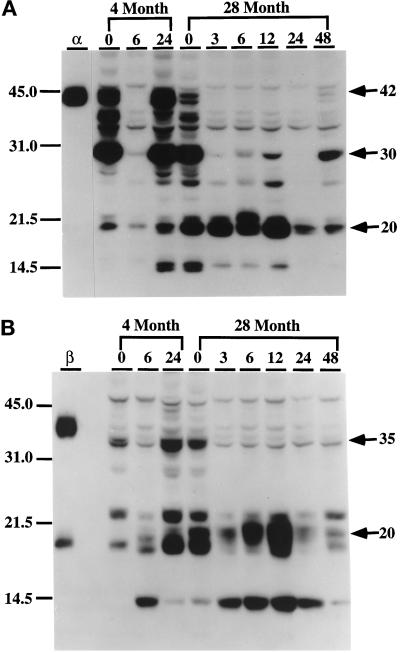
In a recent study we demonstrated by Western immunoblotting that there are multiple C/EBPα and C/EBPβ isoforms in control 4-mo-old liver nuclear extracts and that the pool levels of these isoforms are altered by LPS treatment (An et al., 1996). The Southwestern blot analyses shown in Figure 3 indicate that there are significant qualitative and quantitative changes in the binding activity of C/EBP homodimers in nuclear extracts of both young and aged livers and in their responses to LPS. These changes may be due to either altered protein pool levels or to modification of protein structure that affects binding activity. To determine whether aging affects the C/EBP pool levels of the proteins, we assayed C/EBPα and C/EBPβ protein levels by Western blot analyses using monospecific antibodies. As expected, anti-C/EBPα recognized multiple C/EBP isoforms, with molecular masses of 42 kDa, 38 kDa, 35 kDa, 30 kDa, and 20 kDa, in control young mice (Figure 4A, 4-mo-old, lane 0). Among them the constitutive levels of the 42-kDa, 38-kDa, 35-kDa, and 30-kDa forms were high and that of the 20-kDa isoform level was low. The data also show that the protein pattern detected at 24 h after LPS treatment is similar to that of the control, indicating a recovery from the LPS. The constitutive levels of the 42- kDa, 38-kDa, and 35-kDa isoforms are dramatically reduced in the aged liver nuclear extracts (Figure 4A, 28-mo-old, lane 0), whereas the 30-kDa isoform appears to be at the same level as in the young and the 20-kDa isoform is significantly increased. Although constitutive levels of the 42-kDa, 38-kDa, and 35-kDa isoforms decreased dramatically in the aged animal, a further decrease to undetectable levels occurred after LPS treatment. At the same time, the level of the 20-kDa isoform, whose constitutive level is elevated in the uninduced aged animal, was further induced by treatment with LPS. These data show that the kinetic properties of the DNA-binding activity (Figure 1) and pool levels of p42C/EBPα isoform parallel those associated with the reduction of C/EBPα DNA-binding activity in aged controls and after LPS treatment. Figure 4B shows that anti-C/EBPβ detects 35-kDa and 20-kDa isoforms in nuclear extracts of young control animals and recovery after 24 h. The levels of the 20-kDa and ∼14.5-kDa forms were increased with the LPS treatment, and their inducibility increased with age. Thus, the homodimer-binding activities (Figure 3) and pool levels (Figure 4) follow the same pattern.
Figure 4.
Western blot analysis of the levels of C/EBPα and C/EBPβ isoforms in liver nuclei in response to LPS treatment. Nuclear extracts (30 μg) from control (0) and LPS-injected (3, 6, 12, 24, and 48 h postinjection) C57BL/6 mice (4- and 28-mo-old males) were loaded in each lane and subjected to ECL-Western immunoblot analyses as described in MATERIALS AND METHODS. Immunoblots were incubated with monospecific polyclonal antibodies against C/EBPα (A) or C/EBPβ (B) or preimmune serum as a control (unpublished data). (A) Anti-C/EBPα. (B) Anti-C/EBPβ. Lanes α and β represent nuclear extracts from COS-1 cells transfected with C/EBPα or C/EBPβ expression vectors and were used as standards for each protein. No bands were detected with preimmune serum with liver nuclear extracts or COS-1 nuclear proteins (unpublished data). Positions of molecular mass standards (kDa) are indicated on the left.
Identification of Specific C/EBPα and C/EBPβ Isoforms That Bind to the APRE
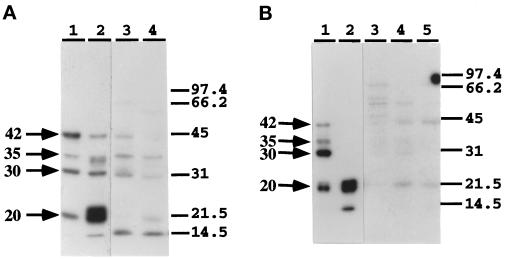
To identify the C/EBP isoforms present in C1–C5, we isolated the complexes from EMSA gels and identified the proteins bound to the APRE by Southwestern blot and Western blot analyses. Previously, we showed by Southwestern blot analyses of proteins from 4-mo-old control liver nuclear extracts that the C1–C3 complexes consist of 42-kDa and 35-kDa isoforms, and that the C3 and C4 complexes formed with LPS-treated nuclear extracts consist mainly of a 20-kDa isoform with detectable amounts of 35-kDa and ∼14.5-kDa proteins (An et al., 1996). Western blot analyses showed that the C1 and C2 complexes in control nuclear extracts consist of p42C/EBPα; the C3 complex consists of the p42C/EBPα and p35C/EBPβ; the C3 and C4 complexes of LPS-treated nuclear extract consist mainly of p20C/EBPβ (An et al., 1996). To identify the specific C/EBP isoforms bound to the C1–C5 complexes of the aged nuclear extracts, these complexes were isolated from EMSA gels and their proteins were analyzed by Southwestern and Western blot analyses. The Southwestern blot data in Figure 5A show the DNA-binding activity of C/EBP isoforms in aged control (lane 1) and LPS-treated (lane 2) nuclear extracts. The C1–C3 complexes showed a slight band at 42 kDa and a more intense band at 35 kDa; the major protein component of these bands is the 20-kDa isoform (Figure 5A, lane 3). The Western blot analyses show that only a very low level of p42C/EBPα can be detected by anti-C/EBPα (just barely detectable), whereas p35C/EBPβ and p20C/EBPβ are detected by anti-C/EBPβ (Figure 5, B and C, lanes 3). These data show that in the aged nuclear extracts, the C1–C3 complexes are predominantly C/EBPβ isoforms. This is consistent with the EMSA and supershift analyses of the aged nuclear extracts which show a virtual loss of the C1–C2 complexes and persistence of the C3 complex.
Figure 5.
Identification of the C/EBP proteins that interact with APRE oligonucleotide to form C1, C2, C3, C4, and C5 complexes in livers of aged mice in response to LPS. The C1–C3, C4, and C5 complexes formed with control or LPS-treated (6 h) nuclear extracts from 28-mo-old C57BL/6 mice were resolved by preparative EMSA. The bands were excised from the gel, eluted, concentrated, and subjected to Southwestern blot and to ECL-Western immunoblot analyses as described in Ref. 3. (A) Southwestern analysis of liver nuclear proteins from 28-mo-old mice that form the C1–C3, C3, C4, and C5 DNA–protein complexes with the APRE oligonucleotide. Lane 1, control NE; lane 2, LPS-treated NE; lane 3, C1–C3 complexes formed with control NE; lane 4, C4 complex formed with control NE. Lanes 5 and 6, C3 and C4 complexes, respectively, formed with mouse liver NE after 6 h of LPS treatment. Lane 7, C5 complex 6 h after LPS treatment. (B and C) Western blot analysis using anti-C/EBPα and anti-C/EBPβ and the same nuclear extracts and complexes as in A.
Southwestern blot analysis of the protein components of the C3, C4, and C5 complexes of aged LPS-treated nuclear extract show that a 20-kDa isoform has the highest level of binding activity (Figure 5A, lanes 4–7); the Western blot analyses of the C3, C4, and C5 complexes identify this protein as p20C/EBPβ with some p14.5C/EBPβ (Figure 5C, lanes 4–7). Furthermore, the data show that only anti-C/EBPβ detects the ∼14.5-kDa isoform.
Identification of C/EBPα and C/EBPβ Isoforms in Polysomes of Control and LPS-treated Livers by EMSA and Supershift Analyses
EMSA and supershift analyses of polysomal proteins from young and aged livers were done to demonstrate that polysomal nascent chains that contain the dimerization domains can be detected by gel-shift analysis, and that the molecular weights of proteins with binding activity are similar to those of the nuclear extracts. We argued that this would demonstrate the presence of nascent chains originating from multiple AUG sites, by alternative translational initiation, within the C/EBPα and C/EBPβ mRNAs. Figure 6 shows EMSA and supershift analyses with polysomal proteins (Figure 6) from livers of young and aged mice with or without LPS. Figure 6A shows that the polysomal proteins from young livers interact with the oligonucleotide to form DNA–protein complex bands. The control bands (Figure 6A, lane 1) show a displacement by anti-C/EBPα (Figure 6A, lane 2) and a low level interaction with anti-C/EBPβ (Figure 6A, lane 3); bands are also formed with proteins from LPS-treated polysomes (Figure 6A, lane 4) which are specifically displaced by anti-C/EBPα (Figure 6A, lane 5) and anti-C/EBPβ (Figure 6A, lane 6). Furthermore, as with the LPS-treated nuclear extracts there is a reduction in the intensity of radioactive complex displaced by anti-C/EBPα (Figure 6A, lanes 2 and 5; Figure 2A).
Figure 6.
EMSA and supershift analysis of mouse liver polysomal proteins that bind to the APRE oligonucleotide. Liver polysomes from young (A, 4-mo-old) and aged (B, 28-mo-old) control and LPS-treated mice were prepared as described in MATERIALS AND METHODS. The [32P]APRE oligonucleotide was prepared as described in MATERIALS AND METHODS. (A) Liver polysomal proteins from 4-mo-old mice; lanes 1–3, control; lane 2, anti-C/EBPα; lane 3, anti-C/EBPβ; lanes 4–6, liver polysomal proteins from 4-mo-old LPS-treated mice; lane 4, no antibody; lane 5, anti-C/EBPα; lane 6, anti-C/EBPβ. (B) Liver polysomal proteins from 28-mo-old mice; lanes 1–3, control; lane 2, anti-C/EBPα; lane 3, anti-C/EBPβ; lanes 4–6, LPS treated; lane 4, no antibody; lane 5, anti-C/EBPα; lane 6, anti-C/EBPβ; lane F, free probe.
The EMSA and supershift analyses of polysomal proteins from aged livers are shown in Figure 6B. DNA–protein complex formation occurs with polysomal proteins from both control and LPS-treated mice (Figure 6B, lanes 1 and 4). Supershift analyses of the polysomal proteins indicates that a C/EBPα–DNA complex is shifted by anti-C/EBPα in control (Figure 6B, lane 2) and LPS-treated polysomes (Figure 6B, lane 5). In addition, supershifting with anti-C/EBPβ detects polysome-associated C/EBPβ in controls (Figure 6B, lane 3) and a significant increase by LPS treatment (Figure 6B, lane 6). Thus, our data suggest that C/EBPα peptides are detected on polysomes from LPS-treated aged livers, even though the level of C/EBPα in the nuclear extract is significantly lower. These results suggest that the nuclear translocation and/or stability of C/EBPα is altered in aged liver. On the other hand, the LPS-mediated increase in polysome-associated C/EBPβ corresponds with the increase in C/EBPβ nuclear extract pool levels seen in LPS-treated aged livers.
Southwestern Blot Analyses of Nuclear and Polysomal Proteins
In a previous article, we proposed that the C/EBPα and C/EBPβ isoforms are products of differential translation of AUG initiation codons within a single mRNA and that the specific pattern of isoform synthesis in response to LPS is mediated via the mechanism of alternative translational initiation. To support our hypothesis, we conducted experiments to analyze the binding activity and molecular weights of nascent chains on C/EBP polysomes (Figure 7A; An et al., 1996). We argued that the presence of C/EBP isoform nascent peptides on the polysomes would support our hypothesis that they are formed by alternative translational initiation. These Southwestern blot analyses showed that proteins isolated from purified polysomes exhibit a similar pattern of DNA-binding activity as that observed with liver nuclear extracts, i.e., the polysomal proteins from control livers exhibited binding activity at 42 kDa, 35 kDa, 30 kDa, 20 kDa, and ∼14.5 kDa (Figure 7A; An et al., 1996). Furthermore, in LPS-treated livers the binding activity of the 42-kDa, 35-kDa, and 30-kDa polysomal proteins is decreased, whereas that of the 20-kDa proteins increased. These data indicated that the nascent chains, i.e., 42 kDa, 35 kDa, 30 kDa, and 20 kDa, whose molecular masses correspond to AUG sites in the mRNAs are the products of differential initiation of translation. To determine whether a similar mechanism occurs in aged livers, we conducted Southwestern blot analysis of the polysomal proteins of aged control and LPS-treated livers. The data in Figure 7B, lanes 1 and 2, show the expected patterns with nuclear extracts from aged control and LPS-treated livers. Similar analysis of the polysomal proteins also detected the expected isoforms, i.e., 42 kDa, 35 kDa, 30 kDa, and 20 kDa (Figure 7B, lanes 3–5). However, in contrast to the data with nuclear extracts, which show that the 42-kDa isoform is not detectable after LPS treatment, we have detected this protein in polysomes of aged livers at 3 and 6 h after LPS treatment. These data suggest that in the aged liver, the translation of the 42-kDa C/EBPα is not down-regulated by LPS. The 20-kDa isoforms, on the other hand, show a pattern of synthesis similar to that seen for polysomal proteins from young livers, i.e., the level of the 20-kDa isoform is low in control and increases in LPS-treated polysomes. These data indicate that although the synthesis of p42C/EBPα persists in aged liver, its decreased pool level and DNA-binding activity in aged nuclear extracts suggest that its translocation to the nucleus may be attenuated or that its stability may be altered.
Figure 7.
Southwestern blot analysis of the mouse liver polysomal proteins from young (A, 4 mo) and aged (B, 28 mo) old livers. (A) Lane 1, control NE; lane 2, LPS-treated NE; lane 3, control polysomal proteins; lane 4, polysomal proteins 3 h after LPS treatment. (B) Lane 1, control NE; lane 2, LPS-treated NE; lane 3, control polysomal proteins; lane 4, polysomal proteins 3 h after LPS treatment; lane 5, polysomal proteins 6 h after LPS treatment.
Analysis of Isoform Production by C/EBPβ Expression Vectors Transfected into COS-1 Cells
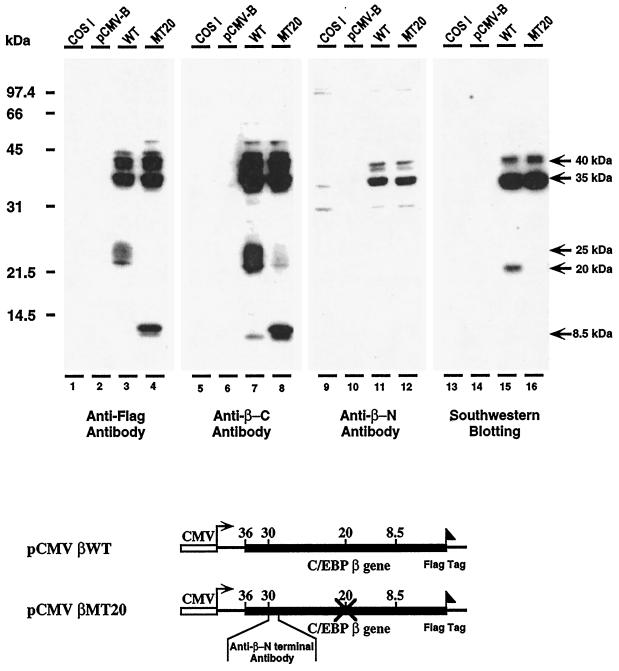
We have hypothesized that the qualitative and quantitative changes in C/EBPα and C/EBPβ isoform pools in response to LPS may occur through the alternative translational initiation of specific AUG start sites. The differential translation of C/EBP mRNAs is supported by reports that the p30C/EBPα, p20C/EBPα, and p20C/EBPβ are products of the alternative translational initiation of the C/EBPα and C/EBPβ mRNAs (Descombes et al., 1990; Descombes and Schibler, 1991; Ossipow, et al., 1993; Lin and Lane, 1994; An et al., 1996). Furthermore, others have presented evidence that the formation of low molecular weight C/EBPα and C/EBPβ isoforms is not due to proteolytic cleavage of the high molecular weight isoforms (Calkhoven et al., 1994; Sears and Sealy, 1994). In these studies we present evidence to support our hypothesis that C/EBPβ isoform synthesis may occur by alternative translational initiation. To demonstrate this, we transfected two C/EBPβ expression vectors into COS-1 cells and analyzed for their ability to produce C/EBPβ isoforms. Each of the expression vectors contained Flag sequences at their C-terminal ends to demonstrate by immunoblot that the origin of their isoforms is from the transfected C/EBPβ expression vectors. The data in Figure 8 show the C/EBPβ isoforms synthesized by the wild-type expression vector pCMV-C/EBPβ (lanes 3, 7, and 11) and an expression vector (pCMV-C/EBPβMT20) in which the 20-kDa AUG start site was mutated to TTG (lanes 4, 8, and 12). Using anti-Flag antibody, we detected the 35-kDa and 20-kDa isoforms synthesized by wild-type pCMV-C/EBPβ (Figure 8, lane 3). The data in lane 7 (Figure 8) show that with the pCMV-C/EBPβMT20 the synthesis of 35-kDa isoform persists whereas the formation of the 20-kDa isoform is completely abolished. Furthermore, the data show that the ∼8.5-kDa isoform appears, suggesting that silencing of the 20-kDa start site may facilitate initiation at a downstream AUG site located within the DNA-binding domain (Figure 8). Similar results were obtained using antibody to the C-terminal sequences of C/EBPβ, i.e., the wild-type mRNA translates 35-kDa and 20-kDa isoforms (Figure 8, lane 7) and the mutation of the 20-kDa start site eliminates formation of this isoform, whereas the 35-kDa isoform is not affected (Figure 8, lane 8).
Figure 8.
The transcription and translation of wild-type and mutant pCMV-C/EBPβ expression vectors in COS-1 cells. Western blot analysis of the C/EBPβ isoforms synthesized in COS-1 cells transfected with wild-type pCMV-C/EBPβ and pCMV-C/EBPβ mutated at the 20-kDa AUG start site. The AUG was mutated to TTG. The expression vectors contain a Flag tag at their C-terminal ends. Western blot analyses were performed on COS-1 nuclear extracts using anti-Flag (lanes 1–4), anti-C-terminal C/EBPβ-antibodies (lanes 5–8); and anti-N-terminal C/EBPβ antibody (lanes 9–13). Southwestern blot analysis was performed on the COS-1 nuclear extracts used for the immunoblot analyses (lanes 13–16). Lanes 1, 5, 9, and 13, control COS-1 nuclear extract; lanes 2, 6, 10, and 14, nuclear extracts of COS-1 cells transfected with expression vector lacking C/EBPβ sequences (pCMV-B); lanes 3, 7, 11, and 15, nuclear extracts of COS-1 cells transfected with wild-type pCMV-C/EBPβ; lanes 4, 8, 12, and 16, nuclear extracts from COS-1 cells transfected with pCMV-C/EBPβ mutated at 20-kDa AUG start site. Maps of the C/EBPβ expression vectors used in these experiments are shown below the autoradiogram.
Although our analyses of polysomal nascent chains (Figure 7) and the studies described above indicate that C/EBP isoforms may be formed by alternative translational initiation, our experiments do not rule out the possible occurrence of a specific proteolytic cleavage of the nascent chains of the high molecular weight isoforms or specific cleavage of the mature polypeptide. Since p20C/EBPβ is a major isoform synthesized in response to LPS (in both young and aged livers and in the COS-1 cells), to address this, we prepared antibody to the N-terminal region, downstream of the p35C/EBPβ AUG start site (Figure 8, lanes 9–12). If proteolytic cleavage occurs, this antibody would detect a 16-kDa N-terminal fragment that has no binding activity. The data in Figure 8 show that only the 35-kDa C/EBPβ isoform is detected in COS-1 cells transfected with both wild-type (lane 11) and mutant (lane 12) pCMV-C/EBPβ expression vectors.
Southwestern blot analyses of the products of these C/EBPβ expression vectors show that the high molecular weight isoforms have DNA-binding activity, whereas the ∼8.5-kDa isoform does not. There is an AUG start site that could account for this isoform that is located within the DNA-binding domain that would explain the lack of binding activity by this protein (Figure 8). Further studies are in progress to demonstrate initiation at this site.
Role of p20C/EBPβ in the Activation of AGP Gene Expression via Its Interaction with the APRE
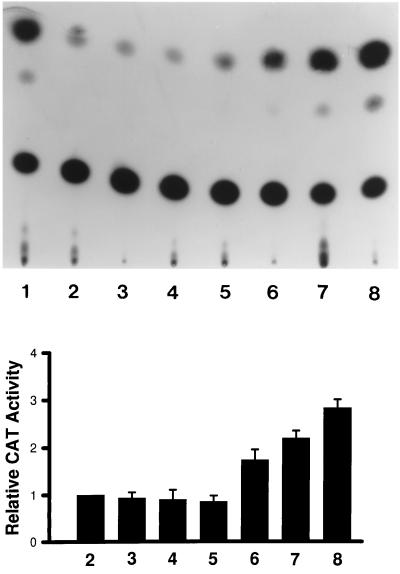
Our studies suggest that the LPS-mediated activation of the AGP gene in aged and young mouse livers may be mediated by the interaction of p20C/EBPβ with the C/EBP binding site (APRE) of the AGP promoter. Other studies have indicated that because of the truncated transcription activation domain, p20C/EBPβ could not serve as an efficient transactivator. It has been shown, for example, that p20C/EBPβ (LIP) has repressor activity with the albumin D promoter binding site (Descombes and Schibler, 1991). To determine whether p20C/EBPβ can function as a transactivator, we cotransfected the pMSV-C/EBPβ20-kDa and pAPRE-CAT expression vectors into COS-1 cells. The data in Figure 9 show that increasing concentrations of transfected pMSV-C/EBPβ20kDa can drive activation of pAPRE-CAT expression, suggesting that the 20-kDa C/EBPβ isoform can function as a transactivator. Since APRE is a composite C/EBP-glucocorticoid receptor binding site, the role of other factors such as glucocorticoid receptor, in maximal activation of the AGP gene via the APRE, remains to be shown.
Figure 9.
The transactivation of pAPRE-CAT expression by p20C/EBPβ. COS-1 cells were cotransfected with pMSV-C/EBPβ20-kDa and pAPRE-CAT expression vectors to determine whether expression of the 20-kDa C/EBPβ isoform could activate expression of pAPRE-CAT. A typical autoradiogram depicting chloramphenicol acetyl transferase activity in response to increasing expression of 20-kDa C/EBPβ is shown in the upper panel. Lane 1, the pSV2-CAT expression vector minus the APRE sequences; lane 2, pAPRE-CAT; lanes 3–5, pAPRE-CAT + 1, 2, and 3 μg of pMSV without the 20-kDa C/EBPβ sequences, respectively; lanes 6–8, pAPRE-CAT + 1, 2, and 3 μg of pMSV-C/EBPβ20-kDa, respectively. A presentation of the data from three separate experiments is shown in the lower panel.
DISCUSSION
In previous studies, we demonstrated the presence of multiple forms of C/EBPα and C/EBPβ proteins in the young mouse liver and dramatic changes in their DNA-binding activities and protein pool levels in response to LPS treatment (An et al., 1996). These studies showed that the level of p42C/EBPα is reduced and the levels of p20C/EBPα and p20C/EBPβ are stimulated by LPS treatment (see maps in Figure 10). We concluded that the formation of C/EBPα and C/EBPβ isoforms occurs by alternative translational initiation at multiple AUG sites within the single C/EBPα and C/EBPβ mRNAs, and that this differential initiation of translation occurs via the leaky ribosomal scanning mechanism (Kozak, 1989, 1992, 1995). In the present studies, we have shown that the DNA-binding activities and pool levels of C/EBPα and C/EBPβ isoforms are significantly altered in the aged liver. This change is particularly significant in control aged livers, where the p42C/EBPα nuclear pool level is decreased and the p20C/EBPβ pool level is increased. This age-associated pattern of binding activity is typical of the inflammatory response in livers of young LPS-treated mice. In particular, the EMSA and super gel-shift pattern of control liver nuclear extract from aged animals show an increase in C/EBPβ-DNA–protein complexes (C3 and C4). Our data further suggest that aging results in an altered regulation of C/EBPα and C/EBPβ isoform synthesis and/or changes in the turnover of these proteins or in their ability to translocate to the nucleus. We conclude that the normal aged liver exhibits characteristics typical of a young liver during an inflammatory response.
Figure 10.
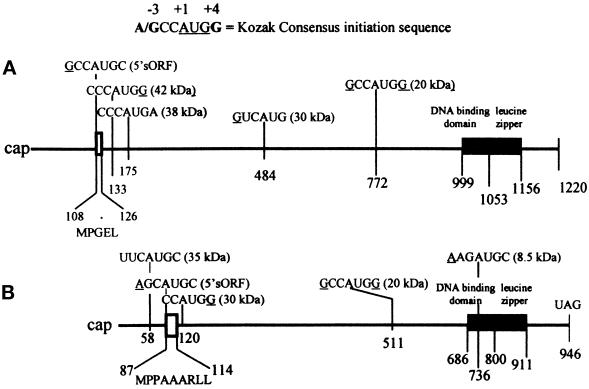
A map of the C/EBPα (A) and C/EBPβ (B) mRNAs. The positions of the AUG initiation sites within each mRNA and the Kozak sequences relative to the A (+1) in each AUG are indicated. The numbers indicate the position of the As in each initiation codon. The molecular masses of isoforms that can be formed by initiation at the designated AUG sites are depicted in parentheses behind each Kozak sequence. The MPGEL is the amino acid sequence of the C/EBPα sORF and MPPAAARLL is the amino acid sequence of the C/EBPβ sORF.
Another interesting age-associated change revealed by the EMSA time course and corresponding protein pool levels is that the recovery from the acute phase response, characterized by the return of the p42C/EBPα–DNA complexes (C1 and C2) to normal levels, is significantly delayed. In the young animal the formation of the p20C/EBPβ–DNA complexes (C3 and C4) persists from 3 to 12 h after LPS whereupon the 42-kDa C/EBPα–DNA complexes (C1 and C2) reappear, indicating recovery from the inflammatory response. The time-course analyses clearly show that in young and 12-mo-old livers, recovery from the acute phase response nears completion between 12 and 24 h after LPS treatment. However, in the aged animals, the EMSA, Southwestern blot, and Western blot analyses show that there is no recovery from this inflammatory response even 48 h after LPS. Our recent preliminary studies indicate that the recovery occurs 72–96 h after LPS (our unpublished results). Thus, the time required for recovery from an inflammatory response is significantly prolonged in the aged animal—a further indication that the physiological stress response processes are altered in normal aged tissue. We speculate that this delayed recovery may be due to a combination of continued stimulation of p20C/EBPβ synthesis and a failure to reinitiate p42C/EBPα synthesis. However, the analysis of polysomal peptides indicates that the p42C/EBPα is synthesized, which suggests that there may be a failure of nuclear translocation or a destabilization of C/EBPα. We propose, therefore, that the control, unchallenged, aged animal exhibits characteristics of chronic inflammatory stress as indicated by the qualitative and quantitative pool levels of C/EBP isoforms and their DNA-binding activities, which mimic the acute phase response, and that upon challenges such as an induced acute phase response, the recovery is significantly delayed.
Immunological and DNA-binding activity analyses have demonstrated the in vivo and in vitro formation of multiple C/EBPα and C/EBPβ DNA-binding proteins with varying molecular weights which also have various transcriptional activities (Descombes and Schibler, 1991; Lin et al., 1993; Ossipow et al., 1993; Calkhoven et al., 1994; Lin and Lane, 1994; Sears and Sealy, 1994; Clarkson et al., 1995; An et al., 1996). It has been proposed that synthesis of these proteins occurs by alternative translational initiation of multiple AUG sites within the single C/EBPα and C/EBPβ mRNAs. In our studies we showed that LPS specifically mediates the reduction of p42C/EBPα synthesis and induction of synthesis of p20C/EBPα and p20C/EBPβ isoforms, and we propose that this may occur by differential initiation of translation at specific AUG sites. Our hypothesis is supported by the observation that the DNA-binding activity of polysomal nascent peptide chains corresponds closely with the patterns observed with nuclear extract (An et al., 1996). Since C/EBP DNA-binding activity is dependent on their ability to dimerize, analyses of the polysomal nascent chains only detected those that contain the leucine zipper sequences at the carboxyl terminus. The ability to detect the C/EBP homodimers in polysomal protein extracts by binding activity supports our hypothesis that these isoforms are produced by an alternative translational initiation mechanism. Further support for the alternative translational initiation of the C/EBPβ mRNA is provided by our observations that C/EBPβ isoforms (35 kDa and 20 kDa) are synthesized in COS-1 cells transfected with wild-type pCMV-C/EBPβ expression vector. Mutation of the 20-kDa AUG start site to TTG did not affect the synthesis of the 35-kDa isoform while formation of the 20-kDa isoform was abolished. Our data suggest that production of the 20-kDa C/EBPβ isoform occurs via alternative translational initiation rather than via the proteolytic cleavage of the high molecular mass 35-kDa isoform.
Our immunological and DNA-binding activity data have demonstrated significant changes in the levels of high and low molecular weight C/EBPα and C/EBPβ isoforms. Analysis of the nascent chains from polysomes of aged livers indicated that the p42C/EBPα, which is down-regulated on young polysomes, is not affected, or may even be up-regulated by LPS in aged polysomes. Since the nuclear pool levels of p42C/EBPα are reduced in control aged liver and further reduced by LPS treatment, our data suggest that two major regulatory changes may occur in the aged liver. The first involves an age-associated failure to down-regulate C/EBPα nascent chains on polysomes, which we observed to occur in young livers (An et al., 1996). This suggests an age-associated attenuation of the alternative translational initiation mechanism that conditions the scanning ribosome to bypass the 42-kDa AUG initiation site in response to LPS. At the same time, since the 20-kDa C/EBP isoform is stimulated, this indicates that the alternative translational initiation at this AUG site does occur. However, our results do not rule out the possibility of a specific processing or proteolytic cleavage of the high molecular weight nascent chains, which does not occur in young polysomes. The report that m-calpain specifically cleaves several transcription factors, leaving the binding and dimerization domains intact, suggests that some low molecular weight isoform may be a product of such cleavage (Watt and Molloy, 1993). Whether m-calpain can cleave polysomal nascent chains is presently being investigated. Sequence analysis of the isoforms, which is also in progress, would demonstrate whether the N-terminal amino acid residue is methionine.
The second age-associated regulation involves a possible attenuation of the nuclear translocation of p42C/EBPα. This is suggested by the observation that p42C/EBPα synthesis persists in polysomes from aged livers, whereas the nuclear pool level decreases, and by the fact that the p20C/EBPβ isoforms in these polysomes and their nuclear pool levels increase. A consequence of the decreased efficiency of nuclear translocation of p42C/EBPα and continued translocation of C/EBPβ could be a factor in the failure of the liver to recover from the inflammatory response. The studies of Yin et al. (1996) have shown that tumor necrosis factor-α, a mediator of the inflammatory response, initiates a rapid posttranscriptional activation and nuclear translocation of C/EBPβ and C/EBPδ in hepatocytes. They also confirmed our observation that the C/EBPα is decreased in this inflammatory response. These observations support our proposal that nuclear translocation of C/EBPα may be affected by aging. This reduced level of p42C/EBPα in the nucleus may favor C/EBPβ binding and may in part cause the prolonged stress response indicated by our EMSA data. We propose that the inability of p42C/EBPα to translocate to the nucleus favors the prolonged presence of the p20C/EBPβ–APRE complex, which may be a factor in the prolonged inflammatory response in aged liver.
Alternative translational initiation at the p20C/EBPα- and p20C/EBPβ-specific AUGs occurs in both young and aged livers in response to LPS. However, as we argued above, our experiments do not completely rule out the occurrence of a very specific proteolytic cleavage of the nascent chain of a high molecular weight isoform. To address this possibility, we prepared an antibody to the NH-terminal region downstream of the p35C/EBPβ start site which would detect the product of proteolytic cleavage at either the 30-kDa or 20-kDa start sites. If proteolysis occurs, this antibody would detect a 6-kDa and/or a 16-kDa NH-terminal fragment that would not have DNA-binding activity. Our results detect only the 35-kDa C/EBPβ isoform in the COS-1 cells transfected with the expression vector mutated at the 20-kDa start site. These results suggest that p30C/EBPβ and p20C/EBPβ are not products of proteolytic cleavage at the second and third AUG start sites. Similar analyses with antibodies to the NH-terminal region of C/EBPα are in progress.
Our experiments indicate that the LPS-induced increase in the newly synthesized polysome-associated p20C/EBPβ in aged (these studies) as well as young (An et al., 1996) livers is far less than the increase in nuclear pool level of this protein. These data suggest that in addition to the translational increase there is a stabilization of p20C/EBPβ which results in its increased nuclear pool level. We speculate that posttranslational modification, such as phosphorylation or protein–protein interactions associated with nuclear translocation and the formation of DNA–protein complexes in the nucleus, may be important factors in the stabilization of this protein and its elevated pool level in the nucleus. Similarly, the decreased pool level of p42C/EBPα in aged nuclear extract may be due to age-associated changes in posttranslational modifications, which may be a factor in destabilization or the basis for the isoform’s decreased translocation to the nucleus. Studies from other laboratories have shown that phosphorylation of C/EBPα and C/EBPβ occurs at specific sites and that some of these modifications affect the binding activity of these proteins (Metz and Ziff, 1991; Mahoney et al., 1992; Wegner et al., 1992; Trautwein et al., 1993). We propose that aging may affect the interactions of signaling pathways which may modify C/EBPα and alter its stabilization and translocation. It is interesting that a model of the structure of the C/EBPβ molecule and its mechanism of activation is based on its ability to adopt a tightly folded conformation that masks the DNA-binding and activation domains, and that phosphorylation causes the protein to unfold and unmask these domains (Williams et al., 1995). Such a structure may also serve to stabilize this protein for nuclear translocation and may be the basis for the stability of the p20C/EBPβ in both young and aged tissues. A similar model has not, however, been proposed for C/EBPα.
The differential translation of the C/EBPα and C/EBPβ mRNAs is supported by reports that the p30C/EBPα and p20C/EBPβ are products of alternative translational initiation (Descombes and Schibler, 1991; Lin et al., 1993; Ossipow et al., 1993; Lin and Lane, 1994; An, et al., 1996). Further supporting evidence is provided by Calkhoven et al. (1994), who showed that increased synthesis of p42C/EBPα caused a decrease in the synthesis of the 30-kDa isoform. These results suggest that there is no precursor–product relationship in the production of the p30C/EBPα isoform. Similarly, the studies of Sears and Sealy (1994) and our studies with C/EBPβ-transfected COS-1 cells showed that there is no precursor–product relationship between the high molecular weight and low molecular weight C/EBPβ isoforms (20 kDa). These results indicate that p20C/EBPβ is not a product of proteolytic cleavage of the high molecular weight forms. Based on these experiments, we propose that the changes in C/EBPα and C/EBPβ isoform production in livers of young and aged mice and in response to LPS treatment result from the differential initiation of specific AUG start sites in their respective mRNAs. Furthermore, the regulation of translation at this level appears to be altered in aged tissues to produce a pattern that mimics the LPS response in young mice.
The observed qualitative and quantitative changes in C/EBPα and C/EBPβ isoform pool levels suggest that LPS or an LPS-stimulated factor may regulate the selection of AUG start sites. Calkhoven et al. (1994) showed that a small 5′ ORF located just upstream of the first C/EBPα AUG codon is the start site for the synthesis of a pentapeptide (MPGEL) that is essential for generating translation start site multiplicity within the mRNA, and that this is the basis for the formation of C/EBPα isoforms (Figure 10A). We have recently shown that mutation of the AUG of the C/EBPβ small ORF reduces initiation at the 35-kDa and 20-kDa AUG sites (Xiong et al., unpublished results). These studies suggest the existence of regulatory proteins that may act as translational trans-acting factors as well as processes that involve leaky ribosomal scanning; we proposed that aging may affect the function of these proteins.
It is well established that C/EBPα and C/EBPβ isoforms play a key role in the regulation of complex biological processes such as cell proliferation, differentiation, energy metabolism, and inflammation. These regulatory processes include transcriptional activation and repression as well as posttranslational regulation (Timchenko et al., 1996, 1997). Interestingly, the regulatory functions of the C/EBPs are localized within the transcription activation domains. Thus, the production of multiple isoforms, with truncated activation domains, by the alternative translational initiation of a single mRNA is a regulatory mechanism of major significance because of the global regulatory functions of these proteins. For example, this mechanism provides a genetic diversity to a single gene by utilizing differential translational initiation in response to growth factors and stress signals and warrants further investigation into understanding how these proteins are regulated as well as how they regulate their targeted genes. Our studies suggest that transcription of the AGP gene may be activated in aged liver, as in young liver, by the interaction of the p20C/EBPβ with the APRE (Alam et al., 1993). The ability of p20C/EBPβ to function as a transactivator of the AGP gene via its interaction with the APRE is suggested by the activation of pAPRE-CAT by overexpression of the 20-kDa isoform in COS-1 cells. This is the first direct indication that this isoform can function as a trans-activator. Since the p20C/EBPβ pool level is elevated in control aged liver, we propose that this increase may be the basis for the age-associated increased constitutive level of AGP expression (Carter et al., 1991; Post et al., 1991). Until now, the 20-kDa C/EBPβ isoform has been shown to function as a repressor when it interacts with the region D binding site of the albumin promoter. We propose that the activator or repressor activity of this isoform may depend on its protein–protein interactions with adjacent DNA-binding or coactivator proteins.
Our studies pose an important question concerning the recovery from the acute phase response. Since this process would require the reappearance of p42C/EBPα, the mechanism of how the translation of this isoform is reinitiated in young liver and why regulation of this isoform does not occur in aged liver becomes relevant to understanding how aging affects the mechanisms of stress response. Addressing these questions is an important goal of our future research.
ACKNOWLEDGMENTS
Publication No. 66 was supported by United States Public Health Service grant PO1 AG10514 awarded by the National Institute on Aging. We thank Dr. David Konkel for the review of this manuscript and Ms. Marg Ridgeway for her assistance in the preparation of this manuscript.
REFERENCES
- Alam T, An MR, Mifflin RC, Hsieh C-C, Ge X, Papaconstantinou J. Trans-activation of the α1-acid-glycoprotein gene acute phase responsive element by multiple isoforms of C/EBP and glucocorticoid receptor. J Biol Chem. 1993;268:15681–15688. [PubMed] [Google Scholar]
- Alam T, Papaconstantinou J. Interaction of acute-phase-inducible and liver enriched nuclear factors with the promoter region of the mouse α1-acid glycoprotein gene-1. Biochemistry. 1992;31:1928–1936. doi: 10.1021/bi00122a005. [DOI] [PubMed] [Google Scholar]
- An MR, Hsieh C-C, Reisner PD, Rabek JR, Scott SG, Kuninger DT, Papaconstantinou J. Evidence for posttranscriptional regulation of C/EBPα and C/EBPβ isoform expression during the lipopolysaccharide-mediated acute-phase response. Mol Cell Biol. 1996;16:2295–2306. doi: 10.1128/mcb.16.5.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H, Jahreis GP, Morella KK. Interaction of cytokine- and glucocorticoid-response elements of acute-phase plasma protein genes. J Biol Chem. 1990;265:22275–22281. [PubMed] [Google Scholar]
- Baumann H, Morella KK, Campos SP, Cao Z, Jahreis GP. Role of CAAT-enhancer binding protein isoforms in the cytokine regulation of acute-phase plasma protein genes. J Biol Chem. 1992;267:19744–19751. [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown PC, Papaconstantinou J. Identification of albumin synthesizing polysomes from mouse liver and a mouse hepatoma cell line. Biochem Biophys Res Commun. 1977;76:121–128. doi: 10.1016/0006-291x(77)91676-x. [DOI] [PubMed] [Google Scholar]
- Calkhoven CF, Bouwman PRJ, Snippe L, Geert AB. Translation start site multiplicity of the CCAAT/enhancer binding protein α mRNA is dictated by a small 5′ open reading frame. Nucleic Acids Res. 1994;22:5540–5547. doi: 10.1093/nar/22.25.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Umek RM, McKnight SL. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3–L1 cells. Genes Dev. 1991;5:1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- Carter KC, Post DJ, Papaconstantinou J. Differential expression of the mouse α1-acid glycoprotein genes (AGP-1 and AGP-2) during inflammation and aging. Biochim Biophys Acta. 1991;1089:197–205. doi: 10.1016/0167-4781(91)90008-a. [DOI] [PubMed] [Google Scholar]
- Chang C-J, Chen T-T, Lei H-Y, Chen D-S, Lee S-C. Molecular cloning of a transcription factor, AGP/EBP, that belongs to members of the C/EBP family. Mol Cell Biol. 1990;10:6642–6653. doi: 10.1128/mcb.10.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H-S, Lin Z, Li B, Liu AY-C. Age-dependent decrease in the heat-inducible DNA sequence-specific binding activity in human diploid fibroblasts. J Biol Chem. 1990;265:18005–18011. [PubMed] [Google Scholar]
- Clarkson R-WE, Chen CM, Harrison S, Wells C, Muscat AEO, Waters MJ. Early responses of trans-activating factors to growth hormone in preadipocytes; differential regulation of CCAAT enhancer-binding protein-P (C/EBPβ) and C/EBPδ. Mol Endocrinol. 1995;9:108–120. doi: 10.1210/mend.9.1.7760844. [DOI] [PubMed] [Google Scholar]
- Descombes P, Chojkier M, Lichtsteiner S, Falvey E, Schibler U. LAP, a novel member of the C/EBP gene family, encodes a liver-enriched transcriptional activator protein. Genes Dev. 1990;4:1541–1551. doi: 10.1101/gad.4.9.1541. [DOI] [PubMed] [Google Scholar]
- Descombes P, Schibler U. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Genes Dev. 1991;67:569–579. doi: 10.1016/0092-8674(91)90531-3. [DOI] [PubMed] [Google Scholar]
- Fried MG, Crothers DM. Equilibria and kinetics of LAC repressor operator interaction by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981;9:6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner MM, Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions. Nucleic Acids Res. 1981;9:3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981;23:175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Heydari AR, Wu B, Takahashi R, Strong R, Richardson A. Expression of heat shock protein 70 is altered by age and diet at the level of transcription. Mol Cell Biol. 1993;13:2909–2918. doi: 10.1128/mcb.13.5.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989;108:229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. A consideration of alternative models for the initiation of translation in eukaryotes. Crit Rev Biochem Mol Biol. 1992;27:385–402. doi: 10.3109/10409239209082567. [DOI] [PubMed] [Google Scholar]
- Kozak M. Adherence to the first AUG rule when a second AUG codon follows closely upon the first. Proc Natl Acad Sci USA. 1995;92:2662–2666. doi: 10.1073/pnas.92.7.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel TA, Roberts JD, Zakour RA. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Landschulz WH, Johnson PF, McKnight SL. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988;240:1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Landschulz WH, Johnson PF, McKnight SL. The DNA binding domain of the rat liver nuclear protein C/EBP is bipartite. Science. 1989;243:1681–1688. doi: 10.1126/science.2494700. [DOI] [PubMed] [Google Scholar]
- Lin F-T, Lane MD. CCAAT/enhancer binding protein alpha is sufficient to initiate the 3T3–L1 adipocyte differentiation program. Proc Natl Acad Sci USA. 1994;91:8757–8761. doi: 10.1073/pnas.91.19.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F-T, MacDougald OA, Diehl AM, Lane MD. A 30 kDa alternative translation product of the CCAAT/enhancer binding protein alpha message: transcriptional activator lacking antimitotic activity. Proc Natl Acad Sci USA. 1993;90:9606–9610. doi: 10.1073/pnas.90.20.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AY-C, Choi HS, Lu Y-K, Chen KY. Molecular events involved in transcriptional activation of heat shock genes become progressively refractory to heat stimulation during aging of human diploid fibroblasts. J Cell Physiol. 1991;149:560–566. doi: 10.1002/jcp.1041490327. [DOI] [PubMed] [Google Scholar]
- Liu AY-C, Lin Z, Choi HS, Sorhage F, Li B. Attenuated induction of heat shock gene expression in aging diploid fibroblasts. J Biol Chem. 1989;264:12037–12045. [PubMed] [Google Scholar]
- Lopez-Cabrera M, Letovsky J, Hu K, Siddaqui I. Multiple liver-specific factors bind to the hepatitis B virus core/pregenomic promoter: trans-activation and repression by CCAAT/enhancer binding protein. Proc Natl Acad Sci USA. 1990;87:5069–5073. doi: 10.1073/pnas.87.13.5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney CW, Shuman J, McKnight SL, Chen H-C, Huang K-P. Phosphorylation of CCAAT-enhancer binding protein by protein kinase C attenuates site-selective DNA binding. J Biol Chem. 1992;267:19396–19403. [PubMed] [Google Scholar]
- Metz R, Ziff EB. cAMP stimulates the C/EBP-related transcription factor rNFIL-6 to translocate to the nucleus and induce c-fos transcription. Genes Dev. 1991;5:1754–1776. doi: 10.1101/gad.5.10.1754. [DOI] [PubMed] [Google Scholar]
- Ossipow V, Descombes P, Schibler U. CCAAT/enhancer binding protein mRNA is translated into multiple proteins with different transcription activation potentials. Proc Natl Acad Sci USA. 1993;90:8219–8223. doi: 10.1073/pnas.90.17.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei D, Shih C. Transcriptional activation and repression by a cellular DNA binding protein C/EBP. J Virol. 1990;64:1517–1522. doi: 10.1128/jvi.64.4.1517-1522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei D, Shih C. An “attenuator domain” is sandwiched by two distinct transactivation domains in transcription factor C/EBP. Mol Cell Biol. 1991;11:1480–1487. doi: 10.1128/mcb.11.3.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post DJ, Carter KC, Papaconstantinou J. The effect of aging on constitutive mRNA levels and LPS inducibility of acute phase genes. Ann NY Acad Sci. 1991;621:66–77. [PubMed] [Google Scholar]
- Rabek JP, Hoyt PR, Zhang D-E, Izban MG, Papaconstantinou J. Derepression of a mouse α-fetoprotein expression factor in COS-1 cells by amplification of specific cis-acting sequences of the AFP promoter. Nucleic Acids Res. 1990;18:6677–6682. doi: 10.1093/nar/18.22.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears RC, Sealy L. Multiple forms of C/EBPβ bind the EFII enhancer sequence in the Rous Sarcoma Virus long terminal repeat. Mol Cell Biol. 1994;14:4855–4871. doi: 10.1128/mcb.14.7.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra F, Fey GH, Guigoz YU. T-kininogen gene expression is induced during aging. Mol Cell Biol. 1989;9:5610–5616. doi: 10.1128/mcb.9.12.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supakar PC, Jung MH, Song CS, Chatterjee B, Roy AK. Nuclear factor κB functions as a negative regulator for the rat androgen receptor gene and NFκB activity increases during the age-dependent desensitization of the liver. J Biol Chem. 1995;270:837–842. doi: 10.1074/jbc.270.2.837. [DOI] [PubMed] [Google Scholar]
- Timchenko NA, Harris TE, Wilde M, Bilyeu TA, Burgess-Beusse L, Finegold MJ, Darlington GJ. CCAAT/enhancer binding protein α regulates p21 protein and hepatocyte proliferation in newborn mice. Mol Cell Biol. 1997;17:7353–7361. doi: 10.1128/mcb.17.12.7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timchenko NA, Wilde M, Nakanisi M, Smith JR, Darlington GJ. CCAAT/enhancer-binding protein alpha (C/EBP alpha) inhibits cell proliferation through the p21 (WAF-1/CIP-1/SDI-1) protein. Genes Dev. 1996;10:804–815. doi: 10.1101/gad.10.7.804. [DOI] [PubMed] [Google Scholar]
- Trautwein C, Caelles C, van der Geer P, Hunter T, Karin M, Chojkier M. Transactivation by NF-IL6/LAP is enhanced by phosphorylation of its activation domain. Nature. 1993;364:544–547. doi: 10.1038/364544a0. [DOI] [PubMed] [Google Scholar]
- Umek RM, Friedman AD, McKnight SL. CCAAT-enhancer binding protein: a component of a differentiation switch. Science. 1991;251:288–292. doi: 10.1126/science.1987644. [DOI] [PubMed] [Google Scholar]
- Watt F, Malloy L. Specific cleavage of transcription factors by the thiol protease, m-calpain. Nucleic Acids Res. 1993;21:5092–5100. doi: 10.1093/nar/21.22.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner M, Cao Z, Rosenfeld MG. Calcium-regulated phosphorylation with in the leucine zipper of C/EBP beta. Science. 1992;256:370–373. doi: 10.1126/science.256.5055.370. [DOI] [PubMed] [Google Scholar]
- Williams SC, Baer M, Dillner AJ, Johnson PF. CRP (C/EBPβ) contains a bipartite regulatory domain that controls transcriptional activation, DNA binding and cell specificity. EMBO J. 1995;14:3170–3183. doi: 10.1002/j.1460-2075.1995.tb07319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin M, Yang SQ, Lin HZ, Lane MD, Chatterjee S, Diehl AM. Tumor necrosis factor α promotes nuclear localization of cytokine-inducible CCAAT/enhancer binding protein isoforms in hepatocytes. J Biol Chem. 1996;271:17974–17978. doi: 10.1074/jbc.271.30.17974. [DOI] [PubMed] [Google Scholar]
- Zhang D-E, Hoyt PR, Papaconstantinou J. Localization of DNA protein-binding sites in the proximal and distal promoter regions of the mouse α-fetoprotein gene. J Biol Chem. 1990;265:3382–3391. [PubMed] [Google Scholar]