Abstract
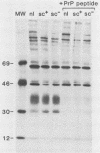
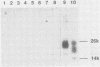
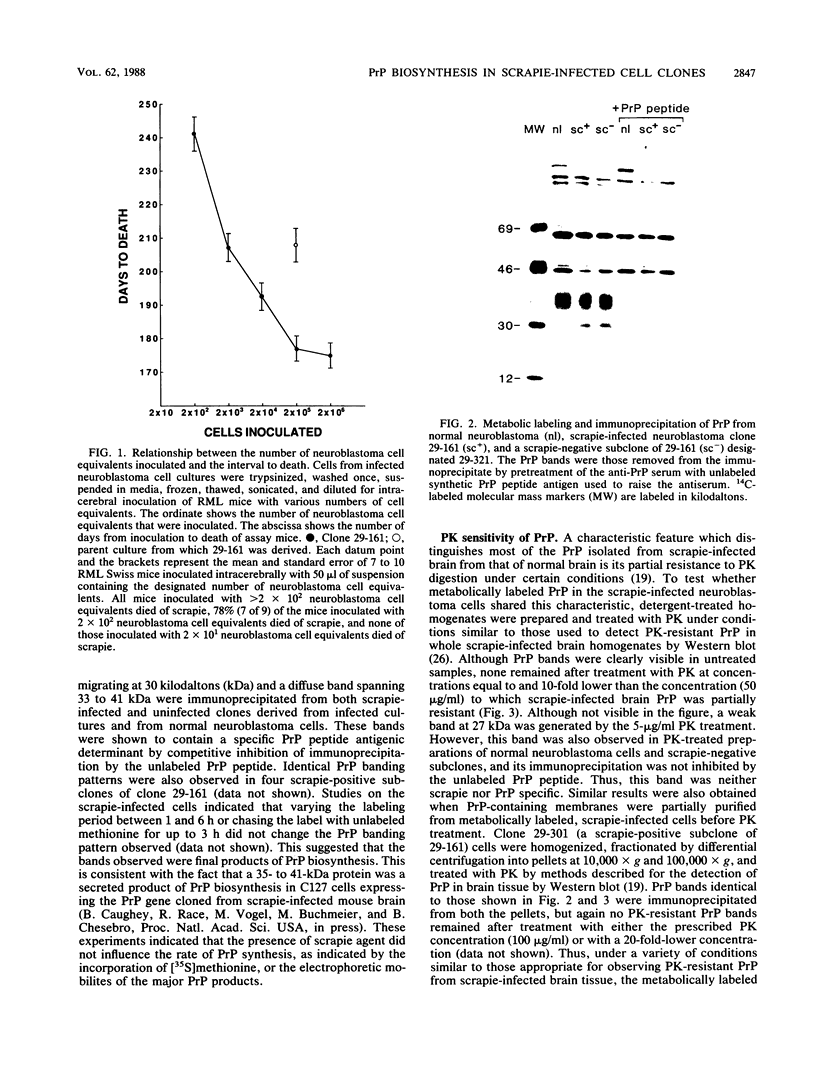
Scrapie, a spongiform encephalopathy of sheep and goats, is caused by a poorly understood transmissible agent in which no nucleic acid has been conclusively identified. Biochemical characterization of agent derived from animal tissues has not been precise because of the tenacious association of the agent with tissue components. As an approach toward obtaining homogeneous preparations of agent generated in vitro, we cloned scrapie-infected neuroblastoma cells. By frequency analysis, nearly every cell in expanded cultures contained scrapie agent. We also analyzed cell-dose infectivity relationships and developed a standard curve which allowed various cultures to be compared. Since a proteinase K (PK)-resistant form of a protein designated prion protein (PrP) has been found in partially purified preparations of scrapie agent from infected animal spleens and brains, we sought to identify this protein in cell cultures. No PK-resistant PrP was found in infected or uninfected cultures, although the PK-sensitive PrP was readily detected. These results suggested that PK-resistant PrP may not be an essential component of the infectious scrapie agent.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Bellinger-Kawahara C., Cleaver J. E., Diener T. O., Prusiner S. B. Purified scrapie prions resist inactivation by UV irradiation. J Virol. 1987 Jan;61(1):159–166. doi: 10.1128/jvi.61.1.159-166.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton D. C., McKinley M. P., Prusiner S. B. Identification of a protein that purifies with the scrapie prion. Science. 1982 Dec 24;218(4579):1309–1311. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- Braig H. R., Diringer H. Scrapie: concept of a virus-induced amyloidosis of the brain. EMBO J. 1985 Sep;4(9):2309–2312. doi: 10.1002/j.1460-2075.1985.tb03931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler D. A., Scott M. R., Bockman J. M., Borchelt D. R., Taraboulos A., Hsiao K. K., Kingsbury D. T., Prusiner S. B. Scrapie-infected murine neuroblastoma cells produce protease-resistant prion proteins. J Virol. 1988 May;62(5):1558–1564. doi: 10.1128/jvi.62.5.1558-1564.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherednichenko YuN, Mikhailova G. R., Rajcáni J., Zhdanov V. M. In vitro studies with the scrapie agent. Acta Virol. 1985 Jul;29(4):285–293. [PubMed] [Google Scholar]
- Clarke M. C., Haig D. A. Evidence for the multiplication of scrapie agent in cell culture. Nature. 1970 Jan 3;225(5227):100–101. doi: 10.1038/225100a0. [DOI] [PubMed] [Google Scholar]
- Dees C., Wade W. F., German T. L., Marsh R. F. Inactivation of the scrapie agent by ultraviolet irradiation in the presence of chlorpromazine. J Gen Virol. 1985 Apr;66(Pt 4):845–849. doi: 10.1099/0022-1317-66-4-845. [DOI] [PubMed] [Google Scholar]
- Diringer H., Gelderblom H., Hilmert H., Ozel M., Edelbluth C., Kimberlin R. H. Scrapie infectivity, fibrils and low molecular weight protein. Nature. 1983 Dec 1;306(5942):476–478. doi: 10.1038/306476a0. [DOI] [PubMed] [Google Scholar]
- Hope J., Morton L. J., Farquhar C. F., Multhaup G., Beyreuther K., Kimberlin R. H. The major polypeptide of scrapie-associated fibrils (SAF) has the same size, charge distribution and N-terminal protein sequence as predicted for the normal brain protein (PrP). EMBO J. 1986 Oct;5(10):2591–2597. doi: 10.1002/j.1460-2075.1986.tb04539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kascsak R. J., Rubenstein R., Merz P. A., Carp R. I., Robakis N. K., Wisniewski H. M., Diringer H. Immunological comparison of scrapie-associated fibrils isolated from animals infected with four different scrapie strains. J Virol. 1986 Sep;59(3):676–683. doi: 10.1128/jvi.59.3.676-683.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markovits P., Dormont D., Delpech B., Court L., Latarjet R. Essais de propagation in vitro de l'agent scrapie dans des cellules nerveuses de souris. C R Seances Acad Sci III. 1981 Nov 2;293(8):413–417. [PubMed] [Google Scholar]
- McKinley M. P., Bolton D. C., Prusiner S. B. A protease-resistant protein is a structural component of the scrapie prion. Cell. 1983 Nov;35(1):57–62. doi: 10.1016/0092-8674(83)90207-6. [DOI] [PubMed] [Google Scholar]
- Merz P. A., Somerville R. A., Wisniewski H. M., Iqbal K. Abnormal fibrils from scrapie-infected brain. Acta Neuropathol. 1981;54(1):63–74. doi: 10.1007/BF00691333. [DOI] [PubMed] [Google Scholar]
- Merz P. A., Somerville R. A., Wisniewski H. M., Manuelidis L., Manuelidis E. E. Scrapie-associated fibrils in Creutzfeldt-Jakob disease. Nature. 1983 Dec 1;306(5942):474–476. doi: 10.1038/306474a0. [DOI] [PubMed] [Google Scholar]
- Meyer R. K., McKinley M. P., Bowman K. A., Braunfeld M. B., Barry R. A., Prusiner S. B. Separation and properties of cellular and scrapie prion proteins. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2310–2314. doi: 10.1073/pnas.83.8.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch B., Groth D. F., Prusiner S. B., Weissmann C. Search for a scrapie-specific nucleic acid: a progress report. Ciba Found Symp. 1988;135:209–223. doi: 10.1002/9780470513613.ch14. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Groth D. F., Cochran S. P., Masiarz F. R., McKinley M. P., Martinez H. M. Molecular properties, partial purification, and assay by incubation period measurements of the hamster scrapie agent. Biochemistry. 1980 Oct 14;19(21):4883–4891. doi: 10.1021/bi00562a028. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., McKinley M. P., Bowman K. A., Bolton D. C., Bendheim P. E., Groth D. F., Glenner G. G. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell. 1983 Dec;35(2 Pt 1):349–358. doi: 10.1016/0092-8674(83)90168-x. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Novel proteinaceous infectious particles cause scrapie. Science. 1982 Apr 9;216(4542):136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- Race R. E., Fadness L. H., Chesebro B. Characterization of scrapie infection in mouse neuroblastoma cells. J Gen Virol. 1987 May;68(Pt 5):1391–1399. doi: 10.1099/0022-1317-68-5-1391. [DOI] [PubMed] [Google Scholar]
- Rohwer R. G. Scrapie infectious agent is virus-like in size and susceptibility to inactivation. Nature. 1984 Apr 12;308(5960):658–662. doi: 10.1038/308658a0. [DOI] [PubMed] [Google Scholar]
- Rubenstein R., Carp R. I., Callahan S. M. In vitro replication of scrapie agent in a neuronal model: infection of PC12 cells. J Gen Virol. 1984 Dec;65(Pt 12):2191–2198. doi: 10.1099/0022-1317-65-12-2191. [DOI] [PubMed] [Google Scholar]
- Rubenstein R., Kascsak R. J., Merz P. A., Papini M. C., Carp R. I., Robakis N. K., Wisniewski H. M. Detection of scrapie-associated fibril (SAF) proteins using anti-SAF antibody in non-purified tissue preparations. J Gen Virol. 1986 Apr;67(Pt 4):671–681. doi: 10.1099/0022-1317-67-4-671. [DOI] [PubMed] [Google Scholar]
- Somerville R. A., Merz P. A., Carp R. I. Partial copurification of scrapie-associated fibrils and scrapie infectivity. Intervirology. 1986;25(1):48–55. doi: 10.1159/000149654. [DOI] [PubMed] [Google Scholar]
- Wiley C. A., Burrola P. G., Buchmeier M. J., Wooddell M. K., Barry R. A., Prusiner S. B., Lampert P. W. Immuno-gold localization of prion filaments in scrapie-infected hamster brains. Lab Invest. 1987 Dec;57(6):646–656. [PubMed] [Google Scholar]
- Yanagihara R. T., Asher D. M., Gibbs C. J., Jr, Gajdusek D. C. Attempts to establish cell cultures infected with the viruses of subacute spongiform encephalopathies. Proc Soc Exp Biol Med. 1980 Nov;165(2):298–305. doi: 10.3181/00379727-165-40974. [DOI] [PubMed] [Google Scholar]