Abstract
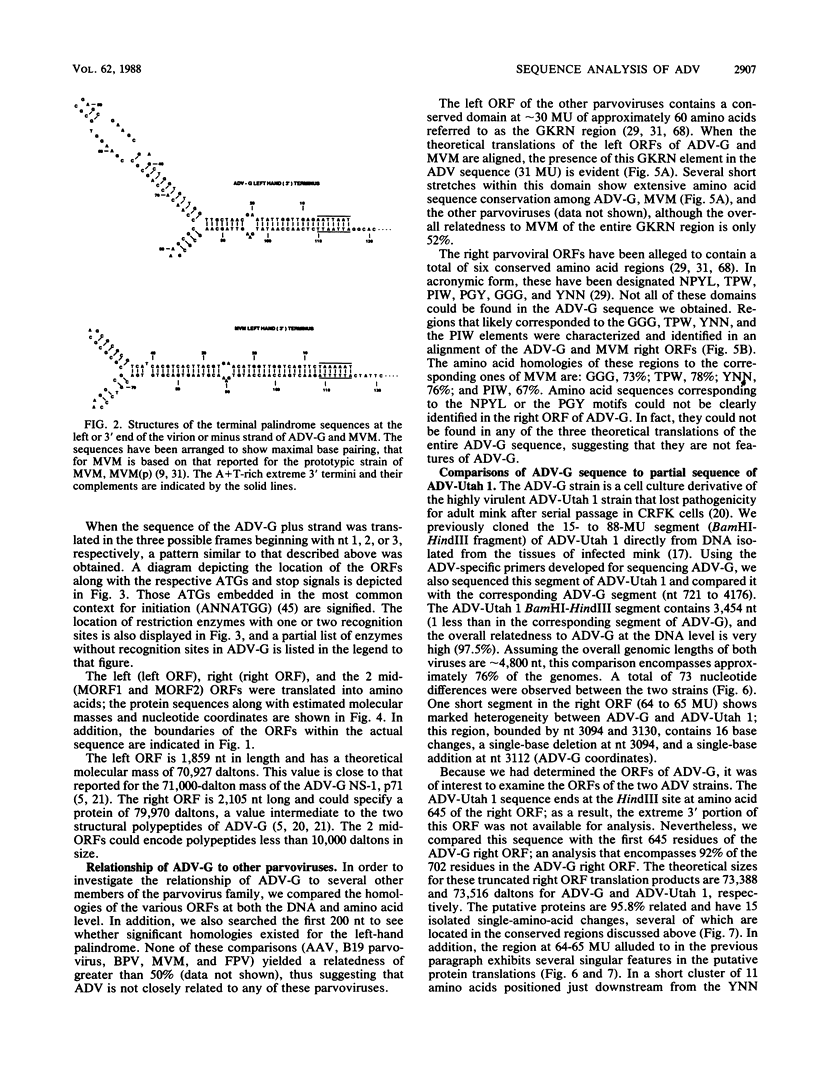
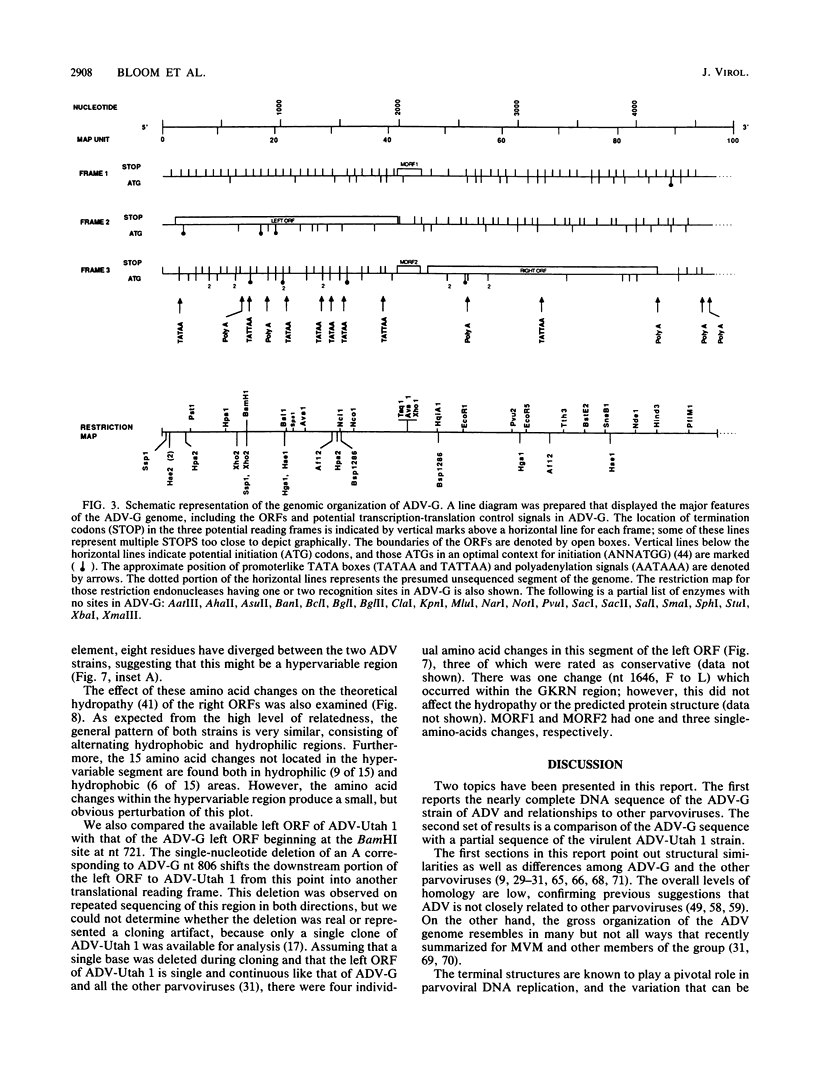
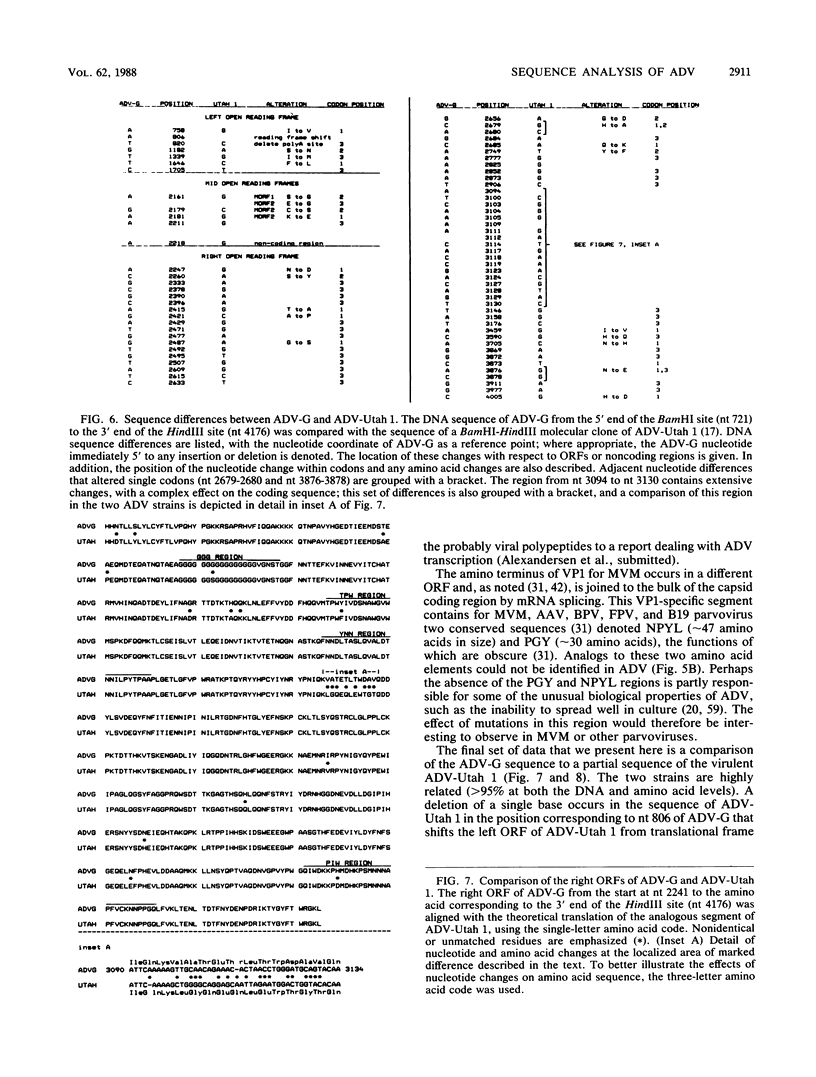
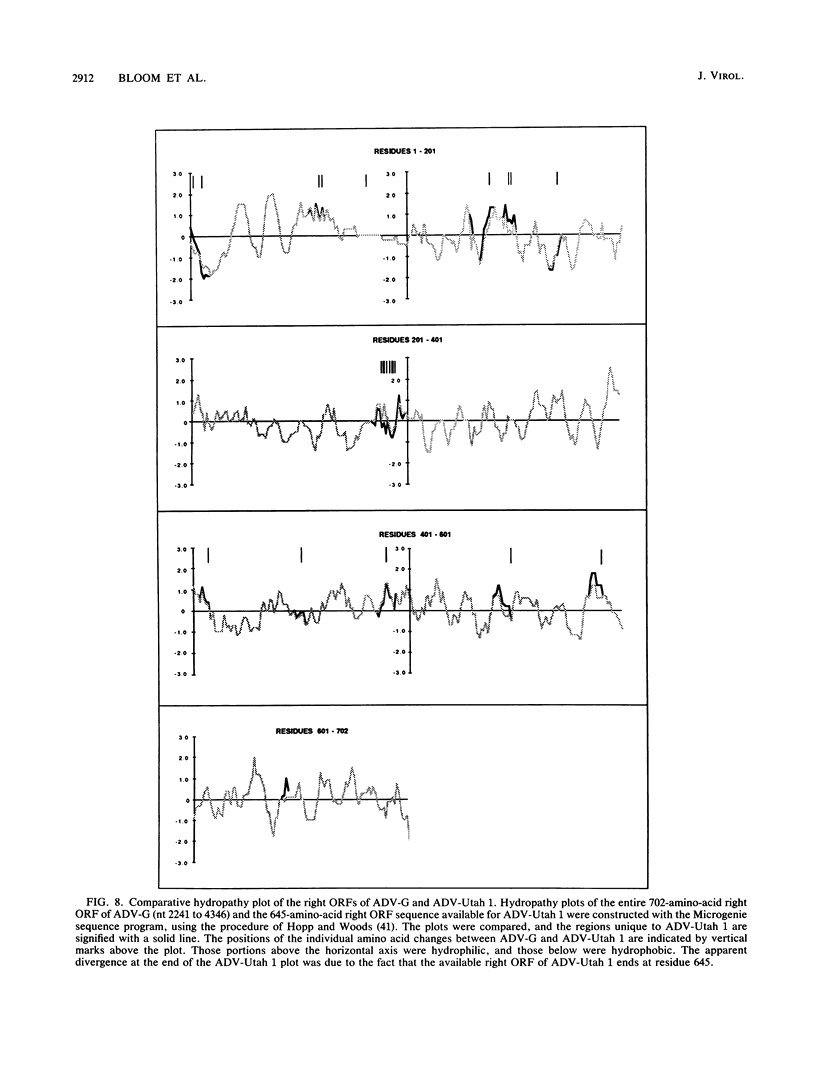
A DNA sequence of 4,592 nucleotides (nt) was derived for the nonpathogenic ADV-G strain of Aleutian mink disease parvovirus (ADV). The 3'(left) end of the virion strand contained a 117-nt palindrome that could assume a Y-shaped configuration similar to, but less stable than, that of other parvoviruses. The sequence obtained for the 5' end was incomplete and did not contain the 5' (right) hairpin structure but ended just after a 25-nt A + T-rich direct repeat. Features of ADV genomic organization are (i) major left (622 amino acids) and right (702 amino acids) open reading frames (ORFs) in different translational frames of the plus-sense strand, (ii) two short mid-ORFs, (iii) eight potential promoter motifs (TATA boxes), including ones at 3 and 36 map units, and (iv) six potential polyadenylation sites, including three clustered near the termination of the right ORF. Although the overall homology to other parvoviruses is less than 50%, there are short conserved amino acid regions in both major ORFs. However, two regions in the right ORF allegedly conserved among the parvoviruses were not present in ADV. At the DNA level, ADV-G is 97.5% related to the pathogenic ADV-Utah 1. A total of 22 amino acid changes were found in the right ORF; changes were found in both hydrophilic and hydrophobic regions and generally did not affect the theoretical hydropathy. However, there is a short heterogeneous region at 64 to 65 map units in which 8 out of 11 residues have diverged; this hypervariable segment may be analogous to short amino acid regions in other parvoviruses that determine host range and pathogenicity. These findings suggested that this region may harbor some of the determinants responsible for the differences in pathogenicity of ADV-G and ADV-Utah 1.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandersen S. Acute interstitial pneumonia in mink kits: experimental reproduction of the disease. Vet Pathol. 1986 Sep;23(5):579–588. doi: 10.1177/030098588602300506. [DOI] [PubMed] [Google Scholar]
- Alexandersen S., Bloom M. E. Studies on the sequential development of acute interstitial pneumonia caused by Aleutian disease virus in mink kits. J Virol. 1987 Jan;61(1):81–86. doi: 10.1128/jvi.61.1.81-86.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersen S., Bloom M. E., Wolfinbarger J. Evidence of restricted viral replication in adult mink infected with Aleutian disease of mink parvovirus. J Virol. 1988 May;62(5):1495–1507. doi: 10.1128/jvi.62.5.1495-1507.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersen S., Bloom M. E., Wolfinbarger J., Race R. E. In situ molecular hybridization for detection of Aleutian mink disease parvovirus DNA by using strand-specific probes: identification of target cells for viral replication in cell cultures and in mink kits with virus-induced interstitial pneumonia. J Virol. 1987 Aug;61(8):2407–2419. doi: 10.1128/jvi.61.8.2407-2419.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersen S., Uttenthal-Jensen A., Aasted B. Demonstration of non-degraded Aleutian disease virus (ADV) proteins in lung tissue from experimentally infected mink kits. Brief report. Arch Virol. 1986;87(1-2):127–133. doi: 10.1007/BF01310549. [DOI] [PubMed] [Google Scholar]
- Anderson M. J. Human parvovirus infections. J Virol Methods. 1987 Aug;17(1-2):175–181. doi: 10.1016/0166-0934(87)90080-2. [DOI] [PubMed] [Google Scholar]
- Anton I. A., Lane D. P. Non-structural protein 1 of parvoviruses: homology to purine nucleotide using proteins and early proteins of papovaviruses. Nucleic Acids Res. 1986 Oct 10;14(19):7813–7813. doi: 10.1093/nar/14.19.7813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astell C. R., Gardiner E. M., Tattersall P. DNA sequence of the lymphotropic variant of minute virus of mice, MVM(i), and comparison with the DNA sequence of the fibrotropic prototype strain. J Virol. 1986 Feb;57(2):656–669. doi: 10.1128/jvi.57.2.656-669.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensimhon M., Gabarro-Arpa J., Ehrlich R., Reiss C. Physical characteristics in eucaryotic promoters. Nucleic Acids Res. 1983 Jul 11;11(13):4521–4540. doi: 10.1093/nar/11.13.4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns K. I., Bohenzky R. A. Adeno-associated viruses: an update. Adv Virus Res. 1987;32:243–306. doi: 10.1016/s0065-3527(08)60479-0. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Labow M. A. Parvovirus gene regulation. J Gen Virol. 1987 Mar;68(Pt 3):601–614. doi: 10.1099/0022-1317-68-3-601. [DOI] [PubMed] [Google Scholar]
- Berzofsky J. A. Intrinsic and extrinsic factors in protein antigenic structure. Science. 1985 Sep 6;229(4717):932–940. doi: 10.1126/science.2410982. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Bloom M. E., Kaaden O. R., Huggans E., Cohn A., Wolfinbarger J. B. Molecular comparisons of in vivo- and in vitro-derived strains of Aleutian disease of mink parvovirus. J Virol. 1988 Jan;62(1):132–138. doi: 10.1128/jvi.62.1.132-138.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Lechner D., Wiedbrauk D. L., Wolfinbarger J. B. Analysis of molecularly cloned DNA reveals minor differences among three virus strains of Aleutian disease of mink parvovirus. Brief report. Arch Virol. 1987;92(1-2):175–181. doi: 10.1007/BF01310071. [DOI] [PubMed] [Google Scholar]
- Bloom M. E., Mayer L. W., Garon C. F. Characterization of the Aleutian disease virus genome and its intracellular forms. J Virol. 1983 Mar;45(3):977–984. doi: 10.1128/jvi.45.3.977-984.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Wolfinbarger J. B. Analysis of Aleutian disease of mink parvovirus infection using strand-specific hybridization probes. Intervirology. 1987;27(2):102–111. doi: 10.1159/000149727. [DOI] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Wolfinbarger J. B. Characterization of Aleutian disease virus as a parvovirus. J Virol. 1980 Sep;35(3):836–843. doi: 10.1128/jvi.35.3.836-843.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Wolfinbarger J. B. Identification of a nonvirion protein of Aleutian disease virus: mink with Aleutian disease have antibody to both virion and nonvirion proteins. J Virol. 1982 Aug;43(2):608–616. doi: 10.1128/jvi.43.2.608-616.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell M. C., Beard C., Astell C. R. In vitro identification of a B19 parvovirus promoter. Virology. 1987 Apr;157(2):534–538. doi: 10.1016/0042-6822(87)90296-0. [DOI] [PubMed] [Google Scholar]
- Boissy R., Astell C. R. An Escherichia coli recBCsbcBrecF host permits the deletion-resistant propagation of plasmid clones containing the 5'-terminal palindrome of minute virus of mice. Gene. 1985;35(1-2):179–185. doi: 10.1016/0378-1119(85)90170-2. [DOI] [PubMed] [Google Scholar]
- Both G. W., Shi C. H., Kilbourne E. D. Hemagglutinin of swine influenza virus: a single amino acid change pleiotropically affects viral antigenicity and replication. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6996–7000. doi: 10.1073/pnas.80.22.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson J. O., Lynde-Maas M. K., Shen Z. D. A nonstructural protein of feline panleukopenia virus: expression in Escherichia coli and detection of multiple forms in infected cells. J Virol. 1987 Feb;61(2):621–624. doi: 10.1128/jvi.61.2.621-624.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Chen K. C., Shull B. C., Moses E. A., Lederman M., Stout E. R., Bates R. C. Complete nucleotide sequence and genome organization of bovine parvovirus. J Virol. 1986 Dec;60(3):1085–1097. doi: 10.1128/jvi.60.3.1085-1097.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. Organization of nonstructural genes of the autonomous parvovirus minute virus of mice. J Virol. 1986 Jun;58(3):724–732. doi: 10.1128/jvi.58.3.724-732.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- DeLange A. M., Reddy M., Scraba D., Upton C., McFadden G. Replication and resolution of cloned poxvirus telomeres in vivo generates linear minichromosomes with intact viral hairpin termini. J Virol. 1986 Aug;59(2):249–259. doi: 10.1128/jvi.59.2.249-259.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond D. C., Jameson B. A., Bonin J., Kohara M., Abe S., Itoh H., Komatsu T., Arita M., Kuge S., Nomoto A. Antigenic variation and resistance to neutralization in poliovirus type 1. Science. 1985 Sep 13;229(4718):1090–1093. doi: 10.1126/science.2412292. [DOI] [PubMed] [Google Scholar]
- Dietzschold B., Wunner W. H., Wiktor T. J., Lopes A. D., Lafon M., Smith C. L., Koprowski H. Characterization of an antigenic determinant of the glycoprotein that correlates with pathogenicity of rabies virus. Proc Natl Acad Sci U S A. 1983 Jan;80(1):70–74. doi: 10.1073/pnas.80.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieser T. M., Tainer J. A., Geysen H. M., Houghten R. A., Lerner R. A. Influence of protein flexibility and peptide conformation on reactivity of monoclonal anti-peptide antibodies with a protein alpha-helix. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8568–8572. doi: 10.1073/pnas.84.23.8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadlow W. J., Race R. E., Kennedy R. C. Comparative pathogenicity of four strains of Aleutian disease virus for pastel and sapphire mink. Infect Immun. 1983 Sep;41(3):1016–1023. doi: 10.1128/iai.41.3.1016-1023.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn E. C., Ramos L., Kenyon A. J. Expression of Aleutian mink disease antigen in cell culture. Infect Immun. 1977 Jan;15(1):204–211. doi: 10.1128/iai.15.1.204-211.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongeneel C. V., Sahli R., McMaster G. K., Hirt B. A precise map of splice junctions in the mRNAs of minute virus of mice, an autonomous parvovirus. J Virol. 1986 Sep;59(3):564–573. doi: 10.1128/jvi.59.3.564-573.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., Gruss P. Enhancer elements. Cell. 1983 Jun;33(2):313–314. doi: 10.1016/0092-8674(83)90410-5. [DOI] [PubMed] [Google Scholar]
- Kimsey P. B., Engers H. D., Hirt B., Jongeneel C. V. Pathogenicity of fibroblast- and lymphocyte-specific variants of minute virus of mice. J Virol. 1986 Jul;59(1):8–13. doi: 10.1128/jvi.59.1.8-13.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Regulation of protein synthesis in virus-infected animal cells. Adv Virus Res. 1986;31:229–292. doi: 10.1016/S0065-3527(08)60265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labieniec-Pintel L., Pintel D. The minute virus of mice P39 transcription unit can encode both capsid proteins. J Virol. 1986 Mar;57(3):1163–1167. doi: 10.1128/jvi.57.3.1163-1167.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre R. B., Riva S., Berns K. I. Conformation takes precedence over sequence in adeno-associated virus DNA replication. Mol Cell Biol. 1984 Jul;4(7):1416–1419. doi: 10.1128/mcb.4.7.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer L. W., Aasted B., Garon C. F., Bloom M. E. Molecular cloning of the Aleutian disease virus genome: expression of Aleutian disease virus antigens by a recombinant plasmid. J Virol. 1983 Dec;48(3):573–579. doi: 10.1128/jvi.48.3.573-579.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt M. A., Imperiale M. J., Ali H., Nevins J. R. Requirement of a downstream sequence for generation of a poly(A) addition site. Cell. 1984 Jul;37(3):993–999. doi: 10.1016/0092-8674(84)90433-1. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchlinsky M. J., Tattersall P. J., Leary J. J., Cotmore S. F., Gardiner E. M., Ward D. C. Construction of an infectious molecular clone of the autonomous parvovirus minute virus of mice. J Virol. 1983 Jul;47(1):227–232. doi: 10.1128/jvi.47.1.227-232.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan W. R., Ward D. C. Three splicing patterns are used to excise the small intron common to all minute virus of mice RNAs. J Virol. 1986 Dec;60(3):1170–1174. doi: 10.1128/jvi.60.3.1170-1174.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K., Ayub J., Hao Y. S., Kurtzman G., Shimada T., Young N. Novel transcription map for the B19 (human) pathogenic parvovirus. J Virol. 1987 Aug;61(8):2395–2406. doi: 10.1128/jvi.61.8.2395-2406.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish C. R., Carmichael L. E. Characterization and recombination mapping of an antigenic and host range mutation of canine parvovirus. Virology. 1986 Jan 15;148(1):121–132. doi: 10.1016/0042-6822(86)90408-3. [DOI] [PubMed] [Google Scholar]
- Pintel D., Dadachanji D., Astell C. R., Ward D. C. The genome of minute virus of mice, an autonomous parvovirus, encodes two overlapping transcription units. Nucleic Acids Res. 1983 Feb 25;11(4):1019–1038. doi: 10.1093/nar/11.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter D. D. Aleutian disease: a persistent parvovirus infection of mink with a maximal but ineffective host humoral immune response. Prog Med Virol. 1986;33:42–60. [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Cox N. A., Porter H. G., Suffin S. C. Isolation of Aleutian disease virus of mink in cell culture. Intervirology. 1977;8(3):129–144. doi: 10.1159/000148888. [DOI] [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Porter H. G. The pathogenesis of Aleutian disease of mink. I. In vivo viral replication and the host antibody response to viral antigen. J Exp Med. 1969 Sep 1;130(3):575–593. doi: 10.1084/jem.130.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race R. E., Chesebro B., Bloom M. E., Aasted B., Wolfinbarger J. Monoclonal antibodies against Aleutian disease virus distinguish virus strains and differentiate sites of virus replication from sites of viral antigen sequestration. J Virol. 1986 Jan;57(1):285–293. doi: 10.1128/jvi.57.1.285-293.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed A. P., Jones E. V., Miller T. J. Nucleotide sequence and genome organization of canine parvovirus. J Virol. 1988 Jan;62(1):266–276. doi: 10.1128/jvi.62.1.266-276.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd, Paradiso P. R. Parvovirus genome: nucleotide sequence of H-1 and mapping of its genes by hybrid-arrested translation. J Virol. 1983 Jan;45(1):173–184. doi: 10.1128/jvi.45.1.173-184.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd trans-Activation of parvovirus P38 promoter by the 76K noncapsid protein. J Virol. 1985 Sep;55(3):886–889. doi: 10.1128/jvi.55.3.886-889.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahli R., McMaster G. K., Hirt B. DNA sequence comparison between two tissue-specific variants of the autonomous parvovirus, minute virus of mice. Nucleic Acids Res. 1985 May 24;13(10):3617–3633. doi: 10.1093/nar/13.10.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shade R. O., Blundell M. C., Cotmore S. F., Tattersall P., Astell C. R. Nucleotide sequence and genome organization of human parvovirus B19 isolated from the serum of a child during aplastic crisis. J Virol. 1986 Jun;58(3):921–936. doi: 10.1128/jvi.58.3.921-936.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl G., Bates R. C., Berns K. I., Carter B. J., Kelly D. C., Kurstak E., Tattersall P. Characteristics and taxonomy of Parvoviridae. Intervirology. 1985;23(2):61–73. doi: 10.1159/000149587. [DOI] [PubMed] [Google Scholar]
- Srivastava A., Lusby E. W., Berns K. I. Nucleotide sequence and organization of the adeno-associated virus 2 genome. J Virol. 1983 Feb;45(2):555–564. doi: 10.1128/jvi.45.2.555-564.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Bratton J. Reciprocal productive and restrictive virus-cell interactions of immunosuppressive and prototype strains of minute virus of mice. J Virol. 1983 Jun;46(3):944–955. doi: 10.1128/jvi.46.3.944-955.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]



