Abstract
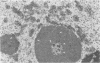
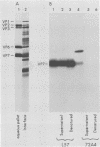
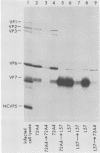
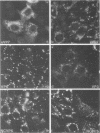
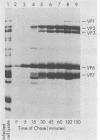
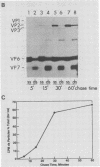
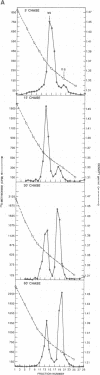
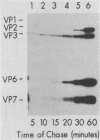
Two pools of the glycoprotein VP7 were detected in the endoplasmic reticulum (ER) of SA11 rotavirus-infected cells. One portion of the newly synthesized protein with VP3 composed the virus outer capsid, while the rest remained associated with the membrane. The two populations could be separated biochemically by fluorocarbon extraction or by immunological methods which used two classes of antibodies. A monoclonal antibody with neutralizing activity recognized VP7 only as displayed on intact virus particles, while a polyclonal antiserum precipitated predominantly the unassembled ER form of the protein and precipitated virus-assembled VP7 poorly. Virus-associated VP7 was localized by immunofluorescence to small punctate structures, presumably corresponding to accumulated virus particles, and to regions of the ER surrounding viroplasmic inclusions, whereas the membrane-associated molecules were distributed in an arborizing reticular pattern throughout the ER. VP3 and the nonstructural glycoprotein NCVP5 displayed a localization similar to that of virus-associated VP7. Intracellular virus particles were isolated from infected cells to determine the kinetics of assembly of VP7 and of the other structural proteins into virions. It was found that incorporation of the inner capsid proteins into single-shelled particles occurred rapidly, while VP7 and VP3 appeared in mature double-shelled particles with a lag time of 10 to 15 min. In addition, the alpha-mannosidase processing kinetics of virus-associated VP7 oligosaccharides showed a 15-min lag compared with that of the membrane-associated form, suggesting that the latter is the precursor to virion VP7. This lag may represent the time required for virus budding and outer capsid assembly.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenburg B. C., Graham D. Y., Estes M. K. Ultrastructural study of rotavirus replication in cultured cells. J Gen Virol. 1980 Jan;46(1):75–85. doi: 10.1099/0022-1317-46-1-75. [DOI] [PubMed] [Google Scholar]
- Andrew M. E., Boyle D. B., Coupar B. E., Whitfeld P. L., Both G. W., Bellamy A. R. Vaccinia virus recombinants expressing the SA11 rotavirus VP7 glycoprotein gene induce serotype-specific neutralizing antibodies. J Virol. 1987 Apr;61(4):1054–1060. doi: 10.1128/jvi.61.4.1054-1060.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chan W. K., Penaranda M. E., Crawford S. E., Estes M. K. Two glycoproteins are produced from the rotavirus neutralization gene. Virology. 1986 Jun;151(2):243–252. doi: 10.1016/0042-6822(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Chasey D. Investigation of immunoperoxidase-labelled rotavirus in tissue culture by light and electron microscopy. J Gen Virol. 1980 Sep;50(1):195–200. doi: 10.1099/0022-1317-50-1-195. [DOI] [PubMed] [Google Scholar]
- Cukor G., Blacklow N. R. Human viral gastroenteritis. Microbiol Rev. 1984 Jun;48(2):157–179. doi: 10.1128/mr.48.2.157-179.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall-Smith M. L., Lazdins I., Tregear G. W., Holmes I. H. Location of the major antigenic sites involved in rotavirus serotype-specific neutralization. Proc Natl Acad Sci U S A. 1986 May;83(10):3465–3468. doi: 10.1073/pnas.83.10.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson B. L., Graham D. Y., Mason B. B., Hanssen H. H., Estes M. K. Two types of glycoprotein precursors are produced by the simian rotavirus SA11. Virology. 1983 Jun;127(2):320–332. doi: 10.1016/0042-6822(83)90147-2. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y., Mason B. B. Proteolytic enhancement of rotavirus infectivity: molecular mechanisms. J Virol. 1981 Sep;39(3):879–888. doi: 10.1128/jvi.39.3.879-888.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass R. I., Keith J., Nakagomi O., Nakagomi T., Askaa J., Kapikian A. Z., Chanock R. M., Flores J. Nucleotide sequence of the structural glycoprotein VP7 gene of Nebraska calf diarrhea virus rotavirus: comparison with homologous genes from four strains of human and animal rotaviruses. Virology. 1985 Mar;141(2):292–298. doi: 10.1016/0042-6822(85)90260-0. [DOI] [PubMed] [Google Scholar]
- Gorziglia M., Larrea C., Liprandi F., Esparza J. Biochemical evidence for the oligomeric (possibly trimeric) structure of the major inner capsid polypeptide (45K) of rotaviruses. J Gen Virol. 1985 Sep;66(Pt 9):1889–1900. doi: 10.1099/0022-1317-66-9-1889. [DOI] [PubMed] [Google Scholar]
- Greenberg H. B., Valdesuso J., van Wyke K., Midthun K., Walsh M., McAuliffe V., Wyatt R. G., Kalica A. R., Flores J., Hoshino Y. Production and preliminary characterization of monoclonal antibodies directed at two surface proteins of rhesus rotavirus. J Virol. 1983 Aug;47(2):267–275. doi: 10.1128/jvi.47.2.267-275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn P. R., Sato F., Powell K. F., Bellamy A. R., Napier J. R., Harding D. R., Hancock W. S., Siegman L. J., Both G. W. Rotavirus neutralizing protein VP7: antigenic determinants investigated by sequence analysis and peptide synthesis. J Virol. 1985 Jun;54(3):791–797. doi: 10.1128/jvi.54.3.791-797.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman S., Theodorakis J. L., Greco J. M., Brown P. H. Processing of MOPC 315 immunoglobulin A oligosaccharides: evidence for endoplasmic reticulum and trans Golgi alpha 1,2-mannosidase activity. J Cell Biol. 1984 Feb;98(2):407–416. doi: 10.1083/jcb.98.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabcenell A. K., Atkinson P. H. Processing of the rough endoplasmic reticulum membrane glycoproteins of rotavirus SA11. J Cell Biol. 1985 Oct;101(4):1270–1280. doi: 10.1083/jcb.101.4.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalica A. R., Flores J., Greenberg H. B. Identification of the rotaviral gene that codes for hemagglutination and protease-enhanced plaque formation. Virology. 1983 Feb;125(1):194–205. doi: 10.1016/0042-6822(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- López S., Arias C. F., Bell J. R., Strauss J. H., Espejo R. T. Primary structure of the cleavage site associated with trypsin enhancement of rotavirus SA11 infectivity. Virology. 1985 Jul 15;144(1):11–19. doi: 10.1016/0042-6822(85)90300-9. [DOI] [PubMed] [Google Scholar]
- Matlin K. S., Simons K. Reduced temperature prevents transfer of a membrane glycoprotein to the cell surface but does not prevent terminal glycosylation. Cell. 1983 Aug;34(1):233–243. doi: 10.1016/0092-8674(83)90154-x. [DOI] [PubMed] [Google Scholar]
- Mellis S. J., Baenziger J. U. Separation of neutral oligosaccharides by high-performance liquid chromatography. Anal Biochem. 1981 Jul 1;114(2):276–280. doi: 10.1016/0003-2697(81)90480-2. [DOI] [PubMed] [Google Scholar]
- Novo E., Esparza J. Composition and topography of structural polypeptides of bovine rotavirus. J Gen Virol. 1981 Oct;56(Pt 2):325–335. doi: 10.1099/0022-1317-56-2-325. [DOI] [PubMed] [Google Scholar]
- Offit P. A., Blavat G., Greenberg H. B., Clark H. F. Molecular basis of rotavirus virulence: role of gene segment 4. J Virol. 1986 Jan;57(1):46–49. doi: 10.1128/jvi.57.1.46-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Shaw R. D., Greenberg H. B. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to surface proteins vp3 and vp7. J Virol. 1986 May;58(2):700–703. doi: 10.1128/jvi.58.2.700-703.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie B. L., Estes M. K., Graham D. Y. Effects of tunicamycin on rotavirus morphogenesis and infectivity. J Virol. 1983 Apr;46(1):270–274. doi: 10.1128/jvi.46.1.270-274.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie B. L., Graham D. Y., Hanssen H., Estes M. K. Localization of rotavirus antigens in infected cells by ultrastructural immunocytochemistry. J Gen Virol. 1982 Dec;63(2):457–467. doi: 10.1099/0022-1317-63-2-457. [DOI] [PubMed] [Google Scholar]
- Petrie B. L., Greenberg H. B., Graham D. Y., Estes M. K. Ultrastructural localization of rotavirus antigens using colloidal gold. Virus Res. 1984;1(2):133–152. doi: 10.1016/0168-1702(84)90069-8. [DOI] [PubMed] [Google Scholar]
- Poruchynsky M. S., Tyndall C., Both G. W., Sato F., Bellamy A. R., Atkinson P. H. Deletions into an NH2-terminal hydrophobic domain result in secretion of rotavirus VP7, a resident endoplasmic reticulum membrane glycoprotein. J Cell Biol. 1985 Dec;101(6):2199–2209. doi: 10.1083/jcb.101.6.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson M. A., Iwamoto A., Ikegami N., Nomoto A., Furuichi Y. Nucleotide sequence of the gene encoding the serotype-specific antigen of human (Wa) rotavirus: comparison with the homologous genes from simian SA11 and UK bovine rotaviruses. J Virol. 1984 Sep;51(3):860–862. doi: 10.1128/jvi.51.3.860-862.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S. C., Mercer L. E., Sonza S., Holmes I. H. Intracellular localization of rotaviral proteins. Arch Virol. 1986;88(3-4):251–264. doi: 10.1007/BF01310879. [DOI] [PubMed] [Google Scholar]
- Sabara M., Babiuk L. A., Gilchrist J., Misra V. Effect of tunicamycin on rotavirus assembly and infectivity. J Virol. 1982 Sep;43(3):1082–1090. doi: 10.1128/jvi.43.3.1082-1090.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabara M., Ready K. F., Frenchick P. J., Babiuk L. A. Biochemical evidence for the oligomeric arrangement of bovine rotavirus nucleocapsid protein and its possible significance in the immunogenicity of this protein. J Gen Virol. 1987 Jan;68(Pt 1):123–133. doi: 10.1099/0022-1317-68-1-123. [DOI] [PubMed] [Google Scholar]
- Shahrabadi M. S., Lee P. W. Bovine rotavirus maturation is a calcium-dependent process. Virology. 1986 Jul 30;152(2):298–307. doi: 10.1016/0042-6822(86)90133-9. [DOI] [PubMed] [Google Scholar]
- Shaw R. D., Vo P. T., Offit P. A., Coulson B. S., Greenberg H. B. Antigenic mapping of the surface proteins of rhesus rotavirus. Virology. 1986 Dec;155(2):434–451. doi: 10.1016/0042-6822(86)90205-9. [DOI] [PubMed] [Google Scholar]
- Soler C., Musalem C., Loroño M., Espejo R. T. Association of viral particles and viral proteins with membranes in SA11-infected cells. J Virol. 1982 Dec;44(3):983–992. doi: 10.1128/jvi.44.3.983-992.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonza S., Breschkin A. M., Holmes I. H. The major surface glycoprotein of simian rotavirus (SA11) contains distinct epitopes. Virology. 1984 Apr 30;134(2):318–327. doi: 10.1016/0042-6822(84)90300-3. [DOI] [PubMed] [Google Scholar]
- Street J. E., Croxson M. C., Chadderton W. F., Bellamy A. R. Sequence diversity of human rotavirus strains investigated by northern blot hybridization analysis. J Virol. 1982 Aug;43(2):369–378. doi: 10.1128/jvi.43.2.369-378.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfeld P. L., Tyndall C., Stirzaker S. C., Bellamy A. R., Both G. W. Location of sequences within rotavirus SA11 glycoprotein VP7 which direct it to the endoplasmic reticulum. Mol Cell Biol. 1987 Jul;7(7):2491–2497. doi: 10.1128/mcb.7.7.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]