Abstract
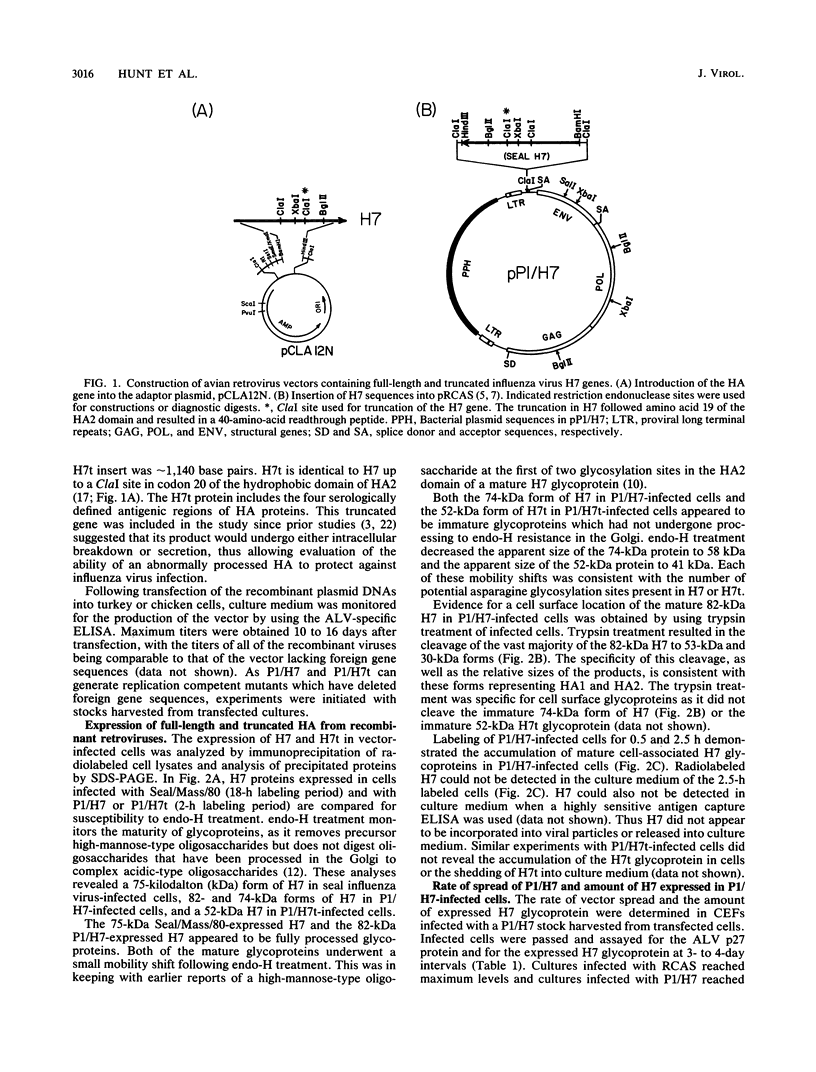
An influenza virus hemagglutinin gene, H7, has been expressed in a replication-competent Schmidt-Ruppin Rous sarcoma virus-derived vector. This virus, P1/H7, expressed a glycosylated precursor of the H7 protein which was processed to a mature form and transported to the cell surface. The expressed H7 glycoprotein could not be detected in P1/H7 virus particles. A P1/H7 stock which expressed 5 to 10% of the level of H7 observed in influenza virus-infected chicken embryo fibroblasts was used to immunize 1-month-old chickens. This immunization resulted in low or undetectable levels of hemagglutination-inhibiting and neutralizing antibody. Despite the low serum response, challenge with a highly pathogenic H7N7 virus revealed complete protection against lethal infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Copeland C. S., Doms R. W., Bolzau E. M., Webster R. G., Helenius A. Assembly of influenza hemagglutinin trimers and its role in intracellular transport. J Cell Biol. 1986 Oct;103(4):1179–1191. doi: 10.1083/jcb.103.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle C., Sambrook J., Gething M. J. Analysis of progressive deletions of the transmembrane and cytoplasmic domains of influenza hemagglutinin. J Cell Biol. 1986 Oct;103(4):1193–1204. doi: 10.1083/jcb.103.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., McCammon K., Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986 Sep 12;46(6):939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Hughes S. H., Greenhouse J. J., Petropoulos C. J., Sutrave P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J Virol. 1987 Oct;61(10):3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S., Kosik E. Mutagenesis of the region between env and src of the SR-A strain of Rous sarcoma virus for the purpose of constructing helper-independent vectors. Virology. 1984 Jul 15;136(1):89–99. doi: 10.1016/0042-6822(84)90250-2. [DOI] [PubMed] [Google Scholar]
- Hughes S., Mellstrom K., Kosik E., Tamanoi F., Brugge J. Mutation of a termination codon affects src initiation. Mol Cell Biol. 1984 Sep;4(9):1738–1746. doi: 10.1128/mcb.4.9.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt L. A., Davidson S. K., Golemboski D. B. Unusual heterogeneity in the glycosylation of the G protein of the hazelhurst strain of vesicular stomatitis virus. Arch Biochem Biophys. 1983 Oct 1;226(1):347–356. doi: 10.1016/0003-9861(83)90301-6. [DOI] [PubMed] [Google Scholar]
- Kawai S., Nishizawa M. New procedure for DNA transfection with polycation and dimethyl sulfoxide. Mol Cell Biol. 1984 Jun;4(6):1172–1174. doi: 10.1128/mcb.4.6.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil W., Niemann H., Schwarz R. T., Klenk H. D. Carbohydrates of influenza virus. V. Oligosaccharides attached to individual glycosylation sites of the hemagglutinin of fowl plague virus. Virology. 1984 Feb;133(1):77–91. doi: 10.1016/0042-6822(84)90427-6. [DOI] [PubMed] [Google Scholar]
- Kida H., Brown L. E., Webster R. G. Biological activity of monoclonal antibodies to operationally defined antigenic regions on the hemagglutinin molecule of A/Seal/Massachusetts/1/80 (H7N7) influenza virus. Virology. 1982 Oct 15;122(1):38–47. doi: 10.1016/0042-6822(82)90375-0. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mackett M., Smith G. L. Vaccinia virus expression vectors. J Gen Virol. 1986 Oct;67(Pt 10):2067–2082. doi: 10.1099/0022-1317-67-10-2067. [DOI] [PubMed] [Google Scholar]
- Morin J. E., Lubeck M. D., Barton J. E., Conley A. J., Davis A. R., Hung P. P. Recombinant adenovirus induces antibody response to hepatitis B virus surface antigen in hamsters. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4626–4630. doi: 10.1073/pnas.84.13.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Flexner C. Vaccinia virus expression vectors. Annu Rev Immunol. 1987;5:305–324. doi: 10.1146/annurev.iy.05.040187.001513. [DOI] [PubMed] [Google Scholar]
- Naeve C. W., Webster R. G. Sequence of the hemagglutinin gene from influenza virus A/Seal/Mass/1/80. Virology. 1983 Sep;129(2):298–308. doi: 10.1016/0042-6822(83)90169-1. [DOI] [PubMed] [Google Scholar]
- Nestorowicz A., Kawaoka Y., Bean W. J., Webster R. G. Molecular analysis of the hemagglutinin genes of Australian H7N7 influenza viruses: role of passerine birds in maintenance or transmission? Virology. 1987 Oct;160(2):411–418. doi: 10.1016/0042-6822(87)90012-2. [DOI] [PubMed] [Google Scholar]
- Palese P., Tobita K., Ueda M., Compans R. W. Characterization of temperature sensitive influenza virus mutants defective in neuraminidase. Virology. 1974 Oct;61(2):397–410. doi: 10.1016/0042-6822(74)90276-1. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Rodgers L., White J., Gething M. J. Lines of BPV-transformed murine cells that constitutively express influenza virus hemagglutinin. EMBO J. 1985 Jan;4(1):91–103. doi: 10.1002/j.1460-2075.1985.tb02322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sveda M. M., Markoff L. J., Lai C. J. Cell surface expression of the influenza virus hemagglutinin requires the hydrophobic carboxy-terminal sequences. Cell. 1982 Sep;30(2):649–656. doi: 10.1016/0092-8674(82)90261-6. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Hinshaw V. S., Bean W. J., Van Wyke K. L., Geraci J. R., St Aubin D. J., Petursson G. Characterization of an influenza A virus from seals. Virology. 1981 Sep;113(2):712–724. doi: 10.1016/0042-6822(81)90200-2. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Rott R. Influenza virus A pathogenicity: the pivotal role of hemagglutinin. Cell. 1987 Aug 28;50(5):665–666. doi: 10.1016/0092-8674(87)90321-7. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Skehel J. J. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- Wood J. M., Kawaoka Y., Newberry L. A., Bordwell E., Webster R. G. Standardization of inactivated H5N2 influenza vaccine and efficacy against lethal A/Chicken/Pennsylvania/1370/83 infection. Avian Dis. 1985 Jul-Sep;29(3):867–872. [PubMed] [Google Scholar]




