Abstract
Acid sphingomyelinase (ASM; E.C. 3.1.4.12) is best known for its involvement in the lysosomal storage disorder Niemann-Pick disease (NPD). Through studies that began by investigating this rare disease, recent findings have uncovered the important role of this enzyme in the initiation of ceramide-mediated signal transduction. This unique function involves translocation of the enzyme from intracellular compartments to the outer leaflet of the cell membrane, where hydrolysis of sphingomyelin into ceramide initiates membrane reorganization and facilitates the formation and coalescence of lipid microdomains. These microdomains are sites of protein-protein interactions that lead to downstream signaling, and perturbation of microdomain formation influences the pathophysiology of many common diseases. The initial observations implicating ASM in this process have come from studies using cells from patients with NPD or from ASM knockout (ASMKO) mice, where the genetic deficiency of this enzymatic activity has been shown to protect these cells and animals from stress-induced and developmental apoptosis. This review will discuss the complex biology of this enzyme in the context of these new findings and its recently reported importance in common human diseases, including cancer, sepsis, cardiovascular, pulmonary, liver, and neurological diseases as well as the potential for using ASM (or ASM inhibitors) as therapeutic agents.—Smith, E. L., Schuchman, E. H. The unexpected role of acid sphingomyelinase in cell death and the pathophysiology of common diseases.
Keywords: ceramide, human pathology, lysosomal enzyme
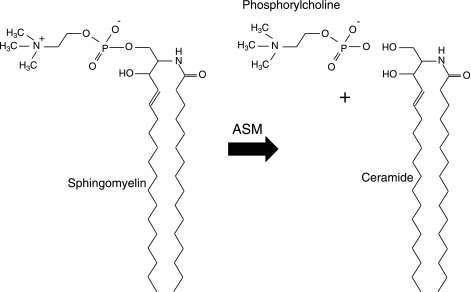
Sphingomyelin is a major lipid component of all mammalian membranes and consists of a phosphorylcholine moiety linked to a ceramide backbone (Fig. 1). Ceramide is a bioactive lipid that has been implicated in the pathogenesis of numerous common diseases, principally through the activation of cell death signaling pathways (for review, see ref. 1). Acid sphingomyelinase (ASM; EC 3.1.4.12) is one member of a family of enzymes that can catalyze the breakdown of sphingomyelin by cleavage of the phosphorylcholine linkage, thereby producing ceramide. The existence of such a “sphingomyelin-cleaving enzyme” was first demonstrated in 1940 by the pioneering work of Thannhauser and Reichel (2), and during the ensuing 25 years several similar enzymatic activities were identified that differed mostly in their tissue distribution and pH optimum. The first clear description of a sphingomyelin cleaving enzyme that worked optimally at acidic pH (i.e., ASM) was made by Gatt and colleagues in 1963 (3). By the late 1960s, researchers reported that the deficiency of this enzyme was responsible for the rare, recessively inherited lysosomal storage disorder, Niemann-Pick disease (NPD; types A and B), which stimulated intensive efforts to purify and characterize it (4).
Figure 1.
Sphingomyelin cleavage by ASM. ASM is a phosphodiesterase that hydrolyzes sphingomyelin to produce phosphorylcholine and ceramide.
In addition to ASM, at least three other sphingomyelinases have been described in mammalian cells that vary in their pH optimum and cofactor dependence (5,6,7). Although these enzymes, and an existing de novo synthetic pathway, are alternative mechanisms for ceramide generation, each with their own implications on cell signaling and disease, this review focuses specifically on the contributions of ASM.
Early investigations identified ASM as a glycoprotein, and the combined facts that the in vitro pH optimum was between 4.5 and 5 and that the majority of storage material in NPD patients was found in lysosomes and late endosomes led to the classification of this enzyme as a lysosomal protein (8). The cDNA and gene encoding ASM (designated SMPD1) were cloned in 1989 and 1992, respectively, and predicted a 629 amino acid polypeptide that included a 46 amino acid signal peptide region, two inframe ATG initiation sites, and 6 potential N-glycosylation sites (9, 10). Subsequent studies revealed that five of the N-glycosylation sites were occupied and that the oligosaccharide side chains contained mannose-6-phosphate residues, typical of lysosomal proteins (11). Mutation analysis in NPD patients also showed that both ATG initiation sites were functional in vivo (12).
SDS-PAGE analysis of ASM purified from various sources revealed an estimated molecular weight of ∼72 kDa; enzymatic deglycosylation reduced the molecular weight to ∼60 kDa (13, 14). Processing studies performed in skin fibroblasts further showed that ASM was synthesized as an ∼75 kDa “prepro” protein that was trafficked to lysosomes. Interestingly, in these same studies a 57 kDa, secreted form of ASM also was identified (15).
In 1999, Chinese hamster ovary (CHO) cells were engineered to overexpress and secrete ASM, facilitating the large-scale purification of the recombinant protein from the culture media (16). This enzyme is now being evaluated in a phase I clinical trial as a treatment for type B NPD. Around this time ASM was found to be a zinc metalloprotein, and a secreted form of the enzyme was identified that was also encoded by the SMPD1 gene. Unlike the lysosomal form, however, the secreted form required exogenous zinc for full activity (17). Interestingly, the SMPD1 gene is within an “imprinted” region of the human genome (chromosomal region 11p15.4) and is preferentially expressed from the maternal chromosome (i.e., paternally imprinted) (18). This form of genetic regulation is typical of genes that play an important role in development.
It is also noteworthy that a zinc-activated, secreted form of ASM was first identified in plasma in the late 1980s, although its biological function remained unknown (19). Tabas and co-workers have suggested that this form may play a role in atherosclerosis, and others have suggested a role in the initiation of cell surface signaling (see below) (20). In addition to zinc, to obtain full ASM activity the terminal cysteine residue (C629) must be removed (21). This is the only cysteine that is not involved in intramolecular disulfide bonds, and it is thought that the retention of this residue in the mature protein may lead to the formation of inactive, high-molecular-weight aggregates.
Until the mid 1990s, interest in ASM was limited primarily to researchers studying NPD. At this time ASM knockout (ASMKO) mice became available (22) and were found to be resistant to radiation (23) and other forms of stress-induced apoptosis (see Table 1 and below). These observations introduced ASM to many new investigators and opened new avenues of research. To date, ASM inhibition (using siRNA or pharmacologic inhibitors, or genetic using NPD cells or knockout mice) has been shown to render cells and animals resistant to the apoptotic effects of diverse stimuli, including Fas/CD95 (24), ischemia (25), radiation (26), chemotherapy (27), tumor necrosis factor-alpha (TNF-α) (28), and others. Based on these observations, investigators have proposed developing ASM inhibitors to treat various common diseases, including emphysema, diabetes, Alzheimer’s, and others. In addition, ASM itself, or methods to enhance ASM activity (e.g., by gene transfer), may be considered as antioncogenic treatments via the production of ceramide in tumors. This review will focus on the complex biology of ASM, with a particular emphasis on its role in cell signaling and the pathogenesis of common diseases.
TABLE 1.
Genetic evidence showing that ASM is necessary for stress-induced cell death
| Evidence | Reference |
|---|---|
| Irradiation in GI microvascular endothelial cells | 26 |
| Irradiation in angiogenic endothelial cells | 23 |
| Irradiation in lymphoblasts | 43 |
| Irradiation in embryonic fibroblasts | |
| Doxorubicin in oocytes | 46 |
| LPS in endothelial cells | 109 |
| TNF-α in lung fibroblasts | 85 |
| TRAIL in splenocytes | 110 |
| CD95 in peripheral blood lymphocytes | 24 |
| CD95 in hepatocytes | 97 |
| Copper in hepatocytes | 47 |
TYPES A AND B NPD
Before understanding the role of ASM in common disease, it is important to appreciate the rare genetic disorder that is caused by its deficiency, types A and B NPD. Both forms of this disorder are inherited as recessive traits, and both are due to mutations in the SMPD1 gene. Type A NPD is the infantile form of ASM deficiency, characterized by a rapidly progressive neurodegenerative course that leads to death by age 2–3. In contrast, type B NPD is the later onset form, in which patients exhibit little or no neurological symptoms but may have severe and progressive visceral organ abnormalities, including hepatosplenomegaly, pulmonary insufficiency, and cardiovascular disease (29). The different clinical presentations of types A and B NPD are likely due to small differences in the amount of residual, functional ASM.
The first patients with NPD (type A) were described in 1914, and by the 1930s the primary lipid accumulating in their tissues was identified as sphingomyelin. It is now known that secondary to sphingomyelin storage, many other lipids (e.g., cholesterol, gangliosides) accumulate in these patients, leading to a plethora of abnormalities in cell function. Many of the clinical findings in NPD are likely related to lipid storage in lysosomes and endosomes, although recent data have also revealed the important role of this enzyme in normal membrane formation and function. Thus, cell membrane abnormalities may contribute to the pathophysiology of NPD as well. To further understand the biology of NPD, ASM “knockout” mice were created in the mid-1990s (see below), and from the analysis of these animals we have learned about the unexpected and important role this enzyme plays in cell signaling and the pathophysiology of many common diseases. Therefore, ASM-deficient NPD provides an excellent example of how analysis of a single, rare genetic disorder can lead to findings that have broad implications in many diverse areas of biomedical science.
ASM AND CELL DEATH
As noted above, in the late 1990s CHO cells were engineered to overexpress and secrete recombinant ASM, facilitating its large-scale purification and characterization. With the availability of recombinant ASM, good antibodies against the enzyme were also soon produced, and several investigators showed by immunocytochemistry that when cells were exposed to various forms of stress, the cellular location of this protein changed from primarily lysosomal/endosomal to the cell surface (e.g., ref. 30). This observation marked a watershed in ASM biology, as it is at the outer leaflet of the cell membrane where ASM initiates cell signaling and thus exerts its impact on the pathogenesis of a diverse group of diseases.
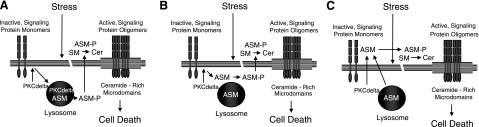
Recently, it was also shown that after ultraviolet irradiation, phosphorylation of a specific serine residue on ASM (S508) by protein kinase C-delta (PKCδ) is required for its translocation to the cell surface (31, 32). These investigators suggested that the phosphorylation occurs within the lysosomes, although it is also possible that a cytosolic pool of ASM serves as the substrate for PKCδ, or that phosphorylation occurs within some other compartment at or near the cell surface (Fig. 2). Regardless of the subcellular location, phosphorylation of ASM may not only facilitate its movement to the cell surface but also its function at physiological pH.
Figure 2.
Proposed involvement of ASM in cell death. In response to stress or developmental signals, ASM may participate in cell death by hydrolysis of sphingomyelin (SM) on the outer leaflet of the plasma membrane, producing ceramide (Cer). This may lead to the formation and coalescence of ceramide-rich microdomains, bringing together inactive, monomeric signaling proteins into active oligomers. In the model of Zeidan and Hannun (31), ASM arrives at the cell surface after phosphorylation by PKCδ within the lysosomes (A). Alternatively, PKCδ may phosphorylate a cytosolic pool of ASM (e.g., Golgi, etc.), facilitating movement to the cell surface (B). Finally, it is possible that upon stress, both ASM and PKCδ move to the cell surface, where the phosphorylation occurs (C). In all cases, phosphorylation of ASM may enhance its activity at the cell surface. Additional experiments are needed to further elucidate the role of ASM in the cell death pathway, and to confirm this hypothetical model.
The role of ASM in cell signaling is tightly linked to its ability to reorganize the plasma membrane. A central thesis of the classic, fluid mosaic model of membranes is that proteins float freely in the lipid bilayer (33). However, protein interactions within the membrane are far more complex than originally proposed, and sphingolipids (particularly sphingomyelin and ceramide) are important membrane components that provide increasing “order” to isolated membrane regions (34). The most prevalent lipid in the outer leaflet of the membrane is sphingomyelin, which ASM can hydrolyze to ceramide. Ceramide molecules in the lipid bilayer are known to interact with each other at the exclusion of other lipids, leading to the formation of isolated lipid “microdomains” (35). Subsequently, either by changing the physical properties of the membrane or by direct ceramide-protein interactions, these microdomains are thought to enhance the density of proteins, including receptors that often require dimerization for their activation, as well as facilitate other protein-protein interactions (36). Current theory predicts that the role of ASM at the cell surface is to reorganize and activate signaling proteins within these microdomains, thus enhancing, or possibly lowering, the threshold for downstream signaling (for review, see ref. 34). Important recent evidence for the involvement of ASM in membrane reorganization was recently provided by Galvan et al. (37), who showed that neurons from ASMKO mice have elevated sphingomyelin in detergent-resistant membrane microdomains, leading to an aberrant distribution of GPI-anchored proteins.
These observations about the function of ASM at the cell surface were initially considered counterintuitive because the enzyme’s housekeeping role resides within lysosomes, and the in vitro pH optimum was clearly acidic. However, an important observation was made in 1998, when it was shown that the secreted form of ASM could degrade sphingomyelin to ceramide within LDL particles at physiological pH, suggesting that the in vitro pH optimum might not predict in vivo function (38). In addition, recent reports have demonstrated acidified microenvironments at the cell surface, and some of these reports have linked such microenvironments to lipid microdomains (39)—the very site of ASM action.
Ceramide is not the only bioactive sphingolipid. It is metabolized by ceramidases to sphingosine, a molecule associated with growth arrest (40). Sphingosine can then be phosphorylated by sphingosine kinases to sphingosine-1-phosphate (S1P), a molecule that promotes cell survival (41). The relative amounts of these three metabolites may ultimately determine the effect of ASM and the fate of a cell (for review, see ref. 42).
As noted above, initial observations relating ASM to cell death were made either using genetic models of ASM deficiency or ASM inhibitors, and showed that such inhibition provided “protection” from stress-induced cell death. The first such paper, published in 1996, found that transformed lymphocytes from patients with NPD were resistant to the irradiation-induced increase in ceramide and subsequent apoptosis that is seen in cells from healthy individuals. Notably, this phenotype was restored after NPD cells were transfected with the ASM cDNA (43). Although these data were initially challenged (44), they have been reproduced by others (45). Over the next decade numerous ASM deficient cell types were shown to be resistant to cell death using a variety of diverse stress agents.
One of the most significant papers published in this regard compared the effect of whole body irradiation of ASMKO mice with p53 null mice. Among a variety of other functions, p53 is required for many cells to enter apoptosis after a lethal (DNA damaging) dose of ionizing irradiation. It was found that in the thymus of p53 null mice, TUNEL staining was drastically reduced when compared to ASMKO mice. However, the reverse was true in the microvascular endothelial cells of the gastrointestinal (GI) tract; i.e., in the small intestine of p53 null mice a significant amount of TUNEL-positive endothelial cells were seen after irradiation that were not seen in the ASMKO mice. The investigators went on to show that ASMKO mice were protected from GI syndrome (fatal bleeding into the GI tract after irradiation), whereas p53 null mice were not (26). Although TUNEL staining is not the most ideal assay to detect apoptosis after irradiation (because radiation itself causes DNA nicking), this paper did show that, at least in the gut, TUNEL staining colocalized with Annexin-V-positive cells. It is obvious from these results that different cell types have independent pathways that are necessary to perform apoptosis after irradiation and, in some (e.g., endothelial cells), ASM is required.
Another convincing example of ASM’s role in stress-induced cell death was demonstrated by Morita et al. (46) in oocytes after ex vivo exposure to doxorubicin. Doxorubicin exposure caused wild-type oocytes to undergo morphological changes typical of apoptosis and to stain positive for TUNEL, whereas oocytes derived from ASMKO mice were resistant to these changes. Additional evidence that might have further supported the role of ASM in oocyte apoptosis would have been to determine whether ASMKO female mice were more resistant to doxorubicin-induced sterility than their wild-type counterparts (as might be expected). Although this paper did not address that possibility, it did look at the protective property of S1P. When administered to wild-type female mice before doxorubicin injection, S1P prevented sterility, further implicating the sphingolipid pathway in cell death (46).
Additional genetic evidence using ASM-deficient mice, cells from these mice, or cells from human NPD patients demonstrated that the lack of functional ASM leads to resistance against an otherwise lethal stress. The list of publications describing these findings is extensive (see Table 1 for a partial list). In addition to this genetic evidence, ASM inhibitors (47) and siRNA (48) have been used to reduce ASM expression in wild-type cells, leading to “protection” from stress-induced cell death. Moreover, many forms of stress have been correlated with ASM activation and or translocation (30, 43), leading to ceramide increases (49). The stress agents that utilize ASM to induce cell death are not only traditional antineoplastic treatments, such as irradiation and chemotherapeutics, but also include a wide variety of other reagents.
ASM AND CANCER
Cancer is a disease of uncontrolled proliferation, and the process of oncogenesis often involves the development of mutations that allow a cell to escape the apoptotic signaling cascade. Thus, induction of the apoptotic pathway is involved in numerous antioncogenic treatments. Notably, the levels of ceramide are significantly decreased in human colon cancers (50), gliomas (51), and ovarian cancers (52). In addition, an inverse relationship has been found between ceramide levels and glioma stratification into high- and low-grade tumors (51). An analysis of the microarray database Oncomine (www.oncomine.org, January 3, 2008) revealed that 12/104 matched cancer vs. normal tissue comparisons underexpressed the ASM mRNA (P<0.0005), with 4/104 comparisons that overexpressed it. In 104 comparisons with a Bonferroni corrected threshold for significance of P < 0.0005, five false positives are expected. This would predict that in at least some cancers ASM may be down-regulated, contributing to their reduced ceramide content and perhaps directing the cells away from apoptosis and toward proliferation. Down-regulation of ASM and ceramide production also may play a role in drug resistance.
In addition to these observations, numerous publications have shown that ASM is important in the response of cancer cells to a variety of antineoplastic treatments. For example, in the Fas-induced apoptotic response of glioma cells, ASM was shown to be activated, and its inhibition caused a Fas-resistant phenotype (53). Paclitaxel-induced apoptosis of human ovarian carcinoma cells also was found to be mediated by ASM-generated ceramide, and cells conditioned to be paclitaxel resistant were characterized by their lack of ceramide generation after treatment (49). Cisplatin treatment of colon cancer cells was similarly shown to induce activation of ASM, changes in membrane fluidity, clustering of CD95, and ultimately apoptosis. Pharmacologic inhibition of ASM decreased membrane changes, CD95 clustering, and apoptosis (54). More recently, this same group has gone on to show that changes in the Na+/H+ transporter activity (NHE1) after cisplatin treatment of these cells is responsible for acidification, contributing to ASM activation at the cell surface and the apoptotic response (55).
In addition, the apoptotic response of neuroblastoma cells to fenretinide was ameliorated by ASM targeted siRNA but not by scrambled siRNA (48). Consistent with the involvement of ASM in the irradiation response (see above), irradiation of a radiosensitive human head and neck squamous carcinoma cell line induced ASM externalization and activation followed by ceramide generation and lipid microdomain formation. However, ASM was not externalized, and subsequent membrane changes did not occur in a matched, radiation-resistant cell line (56). Thus, ASM is involved in the response of many cancers to antioncogenic treatments. Although all of these cancer treatments have other “known” mechanisms of action (e.g., DNA damage, disruption of microtubule assembly, etc.), it is becoming increasingly clear through work on sphingolipids and in other fields that secondary cell membrane changes play an important part in the action of these compounds. Clearly, ASM is an important component of this process.
Ceramide has been suggested to work both through p53 (57), and in a p53-independent manner (58, 59). For example, in neuroblastoma cells an increase in p53 followed by an increase in the Bax/Bcl-2 ratio and ultimately caspase activation is seen after treatment with ceramide. Inhibition of p53 translation by siRNA prevents these changes and subsequent apoptosis (57). However, the relationship between p53 and ASM signaling is not clear. For example, it was shown that irradiation-induced cell death requires ASM, even in E6 oncoprotein-positive glioma cells (58, 59), and as noted above, studies using ASMKO and p53 null mice indicated that these pathways were cell and tissue autonomous (26).
In addition to the role ASM plays in cancer cells themselves, researchers are also becoming aware of its importance in the tumor microenvironment, specifically in angiogenesis. It is well known that solid tumors cannot grow beyond a certain size without the recruitment of a vascular supply, and the development of antiangiogenesis cancer treatments is an extremely active area of research. As mentioned above, the microvasculature of the small intestine relies on ASM for irradiation-induced apoptosis (26). Endothelial cell death after irradiation in the CNS was also found to be dependant on ASM through analysis of ASMKO mice (60, 61). Perhaps the most interesting and substantial finding linking ASM to angiogenesis came from a 2003 study by Garcia-Barros et al. (23). Here, identical tumors were established in either wild-type or ASMKO mice and then irradiated, and it was shown that the tumor response to irradiation was dependant on the ASM status of the recipient mouse. The theory underlying this observation is that even though the tumors were identical, the host endothelium was either responsive (wild-type host) or not (ASMKO host) to the irradiation, and thus the resistant tumors observed in the ASMKO mice were due to a resistant angiogenic vascular supply. Endothelial cells were isolated from these tumors, and it was shown that primary ASMKO angiogenic endothelium were also resistant to irradiation ex vivo (23).
This paper was highly controversial and led to a response by many prominent scientists claiming that they did not believe such an effect could be mediated solely by the antiangiogenic effects of irradiation (62, 63). One explanation that was not considered in the original paper is that the phenotype could have been caused by the absence of secreted ASM in the host mice. As mentioned above, ASM is known to be secreted and present in the blood (20), and is translocated to the outer leaflet of the cell membrane after stress (64). It is possible that the lack of availability of this secreted ASM acting on the tumor itself, in combination with the lack of ASM in the host endothelium, caused the resistant phenotype of the tumors in the ASMKO mice. Work ongoing in our laboratory is aimed at assessing the contribution of ASM to the stress response in both endothelial and tumor cells.
Recently, modulating the ceramide pathway by various approaches has been shown to successfully inhibit cancer growth both in vivo and in vitro. One example is through inhibiting an enzyme that degrades ceramide, acid ceramidase (AC). Systemic administration of AC inhibitors has been shown to inhibit xenograph growth of both head and neck squamous cell carcinomas (65) and hepatomas (66) in mice. Most recently, it was also shown for the first time that transfection of the ASM cDNA into murine glioma cells can sensitize them to the chemotherapy agents doxorubicin and gemcitabine (67). ASM is particularly intriguing as an antioncogenic drug, because preclinical studies performed for the development of NPD enzyme replacement therapy have shown that it can be administered at high doses into normal animals without deleterious effect. In addition, the acidic pH optimum of the enzyme favors its preferential activity within the acidic microenvironment of tumors. GMP-approved recombinant ASM has already been manufactured and is currently being evaluated in clinical trials as a treatment for type B NPD.
Finally, in addition to its role in mediating several antineoplastic treatments, ASM may also be involved in the manifestation of the toxic side effects resulting from these treatments. For example, as noted above irradiation can lead to the GI syndrome, whereas doxorubicin treatment may result in sterility. Both pathways have been shown to involve ASM (23, 46). Therefore, ASM inhibitors targeted at these organs may effectively relieve the side effects of these, and perhaps other, antineoplastic treatments in the future. In addition, ASM targeting through its normal biodistribution pattern (i.e., >95% of the enzyme goes to the liver after i.v. administration) or through antibody conjugation (which is much more feasible in proteins, as opposed to traditional small molecule therapeutics; see ref. 68) can potentially be used to lower the dose of antineoplastic treatment needed to achieve the desired therapeutic response. Thus, cancer therapy based on manipulation of the ceramide pathway, and using ASM in particular, is likely to remain an active area of research in the future.
ASM AND OTHER COMMON DISEASES
The role of ASM in membrane reorganization and cell death led investigators to logically study its involvement in cancer, a disease caused by a dysregulation of normal cell growth. However, ASM has also been implicated in the pathophysiology of a diverse group of other diseases, often as a modulator of cell death, but also through activation of other signaling pathways and physical alterations in the cell membrane. Some of this recent literature is reviewed below.
ASM in cardiovascular disease and diabetes
As noted above, a secreted, zinc-activated form of ASM is found in the plasma, and Tabas and co-workers have suggested that this enzyme plays an important role in the development of atherosclerosis (69). These investigators have also shown that secreted ASM can degrade sphingomyelin in atherogenic LDL particles at physiological pH and that this results in subendothelial aggregation of the particles, increasing their affinity for arterial wall proteoglycans as well as their size (38, 70). Aggregated LDL, in turn, induces macrophage recruitment to the sites of atherosclerotic lesions, which promotes the formation of plaques. It is also known that vascular endothelial cells are a very rich source of secreted ASM and that proinflammatory cytokines, specifically IL-1, enhance ASM secretion in vivo (71). In addition, in patients with chronic heart failure, plasma ASM activity was enhanced by >90% over normal and hypertensive controls and correlated with a number of clinical and other assays, including New York Heart Association (NYHA) class, TNF-α activation, and decreased survival independent of age, NYHA class, and mean blood pressure (72). These observations further suggest that the circulating form of ASM could be biologically active and may contribute to the formation of atherosclerotic plaques and chronic heart failure.
It is a commonly accepted view that diabetes accelerates the development of atherosclerosis. It has also been shown that nonobese patients with type 2 diabetes have elevated levels of plasma ASM, perhaps explaining their increased risk of atherosclerosis (73). Related to this finding, Straczkowski et al. (74) have shown that ceramide levels were elevated in the skeletal muscle of obese men at risk of developing type 2 diabetes, and several groups have demonstrated that small changes in tissue ceramide are enough to antagonize insulin action, providing a link between abnormalities in sphingolipid metabolism and the mechanism of insulin-resistance (for review, see ref. 75). One such recent investigation showed that incubation with ASM or ceramide itself leads to phosphorylation and, thus, negative modulation of the insulin receptor substrate (IRS-1), ultimately leading to an inhibition of insulin signaling (76). In the future it will be extremely important to extend these observations to large numbers of patients and to explore the potential use of ASM inhibitors as a treatment for insulin resistance.
ASM has also been shown to regulate the extent of damage to the heart itself after a period of cardiac ischemia. Rabbit hearts exposed to ischemia exhibited a significant increase in ceramide, peaking after only 5 min. Intravenous administration of the well-documented (although somewhat nonspecific) sphingomyelinase inhibitor, D609, 10 min prior to the induction of ischemia could completely mitigate the increase of tissue ceramide along with decreasing the observed apoptosis and infarct size (77). Similarly, in the perfused rat heart, after the induction of ischemia ceramide levels were increased and sphingomyelin decreased (78). Using this same model, another group went on to show that use of the tricyclic antidepressant desipramine (a cationic amphiphile that triggers ASM proteolysis), before ischemia and reperfusion lowered ceramide from ∼30 nmol/g heart tissue to 6 nmol/g. Baseline levels were generally under 3 nmol/g (79). The measured ischemia-induced decrease in left ventricular pressure, aortic flow, infarct size, the antiapoptotic protein Bcl-2, and the increase in apoptosis were all mitigated by preincubation with desipramine, which correlated with decreased ceramide (79, 80).
Finally, it should be noted that patients with ASM deficiency (types A and B NPD) (81), as well as some carrier (heterozygous) individuals, have an increased incidence of coronary artery disease. For example, of 18 type A or B NPD patients evaluated, several atherosclerotic risk factors were observed that included low HDL (100%), elevated LDL (62%), and high triglycerides (67%), coinciding with the presence of early atherosclerotic plaques (55%) (81). At first glance these observations may be difficult to interpret, given the data of Tabas (20, 38, 69) that suggest that ASM deficiency might protect from atherosclerosis due to a deficiency in secreted ASM. However, in individuals with NPD, the deleterious consequences of the deficient lysosomal form of the enzyme may “trump” any benefits that might occur from a decrease in the secreted form. For example, the deficiency of lysosomal ASM results in enormous sphingomyelin storage followed by subsequent macrophage activation and inflammation (82), as well as perturbed intracellular cholesterol transport, which could contribute to the very low HDL and other lipid abnormalities mentioned above.
ASM in pulmonary diseases
Emphysema is a chronic, obstructive pulmonary disease primarily caused by cigarette smoking. It is characterized by a destructive and permanent enlargement of distal airspaces and alveolar walls, ultimately leading to impaired oxygenation. Historically, the pathogenesis of emphysema has been linked to chronic lung inflammation, and more recently it has been recognized that alveolar cell apoptosis is a crucial step in the pathogenesis of this disease (83). Petrache et al. (84) were the first to show that ceramide is a crucial mediator of alveolar destruction in emphysema. Intratracheal instillation of ceramide into wild-type mice caused emphysema, and inhibition of ceramide, via anticeramide antibodies or inhibition of enzymes controlling de novo ceramide synthesis, prevented it. As a human correlate, increased lung ceramide was found in (cigarette-smoking) patients with emphysema (84).
Notably, treatment of fibroblasts from normal individuals with C8 ceramide led to an important positive feedback mechanism that resulted in an increased amount of secreted ceramides. This did not occur in fibroblasts from individuals with ASM-deficient NPD (84). Thus, de novo synthesis of ceramide, as well as ceramide derived from ASM degradation of sphingomyelin, are important determinants of emphysema. In fact, the importance of ASM in the survival of lung fibroblasts was recently demonstrated by Thon et al. (85), who showed that after exposure to TNF-α in vitro, lung fibroblasts from ASMKO mice were resistant to the ceramide increase and subsequent caspase-independent programmed cell death that wild-type lung fibroblasts exhibited.
It should also be noted that ASMKO mice have chronic lung inflammation but little evidence of emphysema or pulmonary fibrosis. Fibrosis is another common form of interstitial lung disease associated with inflammation and apoptosis. Recent studies from our group (unpublished results) have shown that intratracheal instillation of bleomycin, a chemotherapeutic drug commonly used to cause pulmonary fibrosis in normal mice, leads to an increase in lung ASM activity. Notably, belomycin instillation in ASMKO mice does not cause fibrosis, suggesting that in the future transient inhibition of ASM may play a role in preventing the toxicity of this, and perhaps other, cancer drugs.
ASM also plays a role in the pathology of cystic fibrosis (CF). As discussed in detail below, ASM expression may influence the infection and spread of various bacterial and viral pathogens. Patients with CF often battle persistent lung infections that do not occur in the general population, especially Pseudomonas, leading to increased morbidity and mortality. Several causes, such as increased mucus formation leading to decreased clearance of the bacteria from CF lungs, are well documented. However, ceramide may also be partially involved. A recent paper by Teichgräber et al. (86) found that membrane ceramide levels were elevated in respiratory tissue from two different CF mouse models and patients with CF. Moreover, when CF mice were bred to ASMKO mice to create transgenic CF animals that were heterozygous for the ASM mutant allele, or if ASM was pharmacologically inhibited, the mice were more resistant to Pseudomonas, and their baseline pulmonary inflammation was decreased. Thus, reducing ASM levels inhibited Pseudomonas infection and increased survival in CF mice.
In contrast, however, it also has been shown that Pseudomonas infection is more lethal in homozygous ASMKO mice than in wild-type animals, possibly due to reduced internalization of the bacteria caused by the inability to form ceramide-enriched microdomains (87). In addition, Guilbault et al. (88) reported that patients with CF have significantly lower levels of ceramide than normal control individuals in plasma, lungs, and other organs. They went on to show that by administering fenretinide, a chemotherapeutic known to work by increasing ceramide via ASM (see above and ref. 48), to CFTR knockout mice, ceramide levels went up, and the mice were better able to combat Pseudomonas infections (88). These conflicting results highlight the complexity of ASM in the pathophysiology of CF.
ASM is also involved in acute lung injury, and ceramide derivatives are elevated in patients with acute lung disease (89). Moreover, when wild-type mice are treated with platelet activating factor (PAF), the ensuing pulmonary edema corresponds with increases in serum ASM activity and pulmonary ceramide. ASMKO mice do not have an increase in ceramide and have half as much pulmonary edema as wild-type mice in this model (90).
As mentioned above, decreased pulmonary function is also a major clinical finding in patients with type B NPD. In a study of 53 type B NPD patients, over 70% had decreased pulmonary function tests, and greater the 90% showed lung abnormalities on chest X-ray and CT scan (91). Analysis of the lungs of ASMKO mice showed similar findings (82). The lung pathology seen in this disease may be due, in part, to the lack of ASM surfactant regulation. In fact, in ASMKO mice elevated levels of surfactant lipids (sphingomyelin, as expected, but also three other phospholipids) were found, as well as four surfactant proteins (92, 93). In infantile, acute inflammatory lung disease exogenous surfactant is often administered to improve lung function, although the therapeutic benefit of surfactant is usually short lived. Notably, von Bismarck et al. (94) found that pharmacologically inhibiting ASM in combination with surfactant administration increased the clinical benefit in a piglet model of infantile acute inflammatory lung disease. Here, knowledge that surfactant was elevated in NPD led the way to potentially treating a more common disease.
ASM in neurological diseases
As noted above, the classic form of ASM deficiency (type A NPD) was first identified in 1914 as a severe, neurodegenerative disorder of infancy that leads to death before age 3 (29). This provided the first suggestion that ASM activity is essential for normal brain function. In addition, ASM activity is much higher in the brains of normal mice than other organs (unpublished results), further suggesting its importance in neural function.
In addition to NPD, altered ASM activity has been associated with several common neurological diseases, including depression, Alzheimer’s disease (AD), and ischemia. For example, in a recent, prospective study, ASM activity was found to be higher than normal in a population of patients with a major depressive episode who were free of antidepressant drug therapy for at least 10 days (P<0.05) (95). In addition, many tricyclic antidepressant medications, including imipramine, amitriptyline, and others, reduce ASM activity in cell culture (95). Although these drugs do not act as direct ASM inhibitors (but rather lead to increased proteolysis of the enzyme), several investigators now use tricyclic antidepressants as a method to experimentally inhibit ASM. Although more studies are needed, it is possible that inhibiting ASM is an additional, mechanistic explanation for the antidepressant effects of these medications.
Regarding AD, several reports have found higher levels of ceramide in the brains of AD patients, even in the earliest stages of disease (for review, see ref. 96). Recently, we have found that ASM activity is elevated in the brains of AD patients when compared to healthy, age-matched brains, thus providing a possible mechanism for the elevated ceramide (unpublished results). In addition, using cultured rat neurons we have found that amyloid beta peptide, a main constituent of AD plaques, induced elevated expression of ASM. Based on these observations, ASM-mediated ceramide formation should be examined further as a contributing factor to the neuronal cell death seen in AD patients.
As noted above, ASM also plays a role in cerebral ischemia. In wild-type, but not ASMKO mice, an experimental model of transient focal cerebral ischemia resulted in neuronal increases in ASM, ceramide, and the production of inflammatory cytokines. Wild-type mice also displayed larger infarct size and worse behavioral outcomes than ASMKO mice (25). Thus, as suggested from early observations on type A NPD, ASM plays a complex and varied role in brain development, as well as in the pathogenesis of several common neurological diseases.
ASM in liver diseases
The liver is a major site of pathology in ASM-deficient NPD, suggesting an important role for this enzyme in normal liver function. The hypothesis that ASM is an important component of hepatic toxicity was first tested in 2000, when it was found that unlike normal mice, ASMKO mice, or hepatocytes from these mice were resistant to anti-CD95 exposure (97). The idea that ASM-mediated toxicity is vital in the liver has been confirmed in several disease model systems. For example, a model for hepatic autoimmune disease consists of injecting phytohemagglutinin (PHA) into mice. PHA triggers the up-regulation of CD95 on T-lymphocytes, leading to subsequent hepatic accumulation and apoptosis of hepatocytes via their constitutively expressed CD95 receptor. Knockout mice for either the CD95 receptor (LPR mice) or ASM prevented such T-cell-induced hepatocyte apoptosis (97).
In another example, alcohol-induced liver disease, which at its worst manifests as hepatic cirrhosis, is mediated by the up-regulation of TNF-α, leading to hepatocyte apoptosis. Depletion of mitochondrial glutathione (mGSH) causes normal mouse hepatocytes to become sensitive to TNF-α-induced apoptosis in vitro, whereas ASMKO hepatocytes and mice were more resistant to this form of liver damage (28). As noted above, ASM is essential for TNF-α signaling in lung fibroblasts, as well as hepatocytes.
Cu2+ accumulation, like many heavy metals, can lead to liver cytotoxicity. ATP7B has been identified as the protein responsible for the secretion of Cu2+ from cells. Mutations in ATP7B are responsible for Wilson disease, resulting in severe Cu2+ accumulation followed by a variety of symptoms that affect the nervous, cardiac, hematopoetic, and especially the hepatic systems. This includes hepatic cirrhosis, chronic active hepatitis, and even progressive liver failure. The liver is the primary site of Cu2+ clearance, as a liver transplant is curative. In hepatocytes, ASM activity and ceramide formation was enhanced after Cu2+ exposure at levels that induced apoptosis. Hepatocytes from ASMKO mice, pharmacologic inhibition of ASM, or anti-ASM siRNA treatment in human HepG2 cells all prevented Cu2+-induced apoptosis. Furthermore, patients with Wilson disease have increased plasma ASM activity (47). Finally, pharmacologic inhibition of ASM in a rat model of Wilson disease prevented cirrhosis and increased survival (47). Thus, these common liver diseases represent other important examples where ASM inhibitors may be considered for treatment.
ASM in infection
As mentioned previously, in addition to its role in the initiation of cell death signaling, ASM has an important function in pathogen infection and survival. For example, Gulbins and colleagues have shown that infection with Pseudomonas aeruginosa (87), Neisseria gonorrhoeae (98), and rhinovirus (99) all require a membrane composition change that includes increasing the number and size of ceramide-rich microdomains (for review, see ref. 100). Furthermore, after Pseudomonas infection, ASM was found to relocalize to the outer leaflet of the membrane, similar to its translocation after other stresses, as mentioned above (87), and genetic deficiency of ASM (i.e., in ASMKO mice) prevented the internalization of Pseudomonas by lung epithelial cells and subsequent apoptosis. Without this internalization and apoptosis, IL-1β was drastically increased in the ASMKO infected mice. Thus, although nearly all wild-type mice infected with Pseudomonas could clear the disease, 90% of infected ASMKO mice died within 7 days, most likely due this enhanced inflammatory response (87).
Very similar mortality outcomes were seen when ASMKO mice were challenged with Listeria monocytogenes, but for a different reason. In L. monocytogenes infection, cellular uptake was not a problem. Rather, ASMKO macrophages could not restrict the subsequent growth of this pathogen (101). In both cases enhanced cytokine release was identified in the serum and possibly contributed to sepsis and the increased mortality in the ASM deficient animals (87, 101). Both of these paradigms were seen with other bacteria. For example, in vitro infection of ASMKO macrophages with Salmonella enterica serovar Typhimurium revealed normal internalization but a decrease in early macrophage killing of the bacteria (102). In contrast, N. gonnorrhoea could not be internalized properly by ASMKO phagocytes (98). Clearly, the composition of lipids in the cell membrane, due in part to ASM activity, has an important role in bacterial infection.
Virus-cell interactions are also affected by ASM in diverse ways. For example, infection with rhinovirus induced ASM activation and ceramide-mediated microdomain formation. In addition, genetic deficiency or pharmacological inhibition of ASM has been shown to block human epithelial cell uptake of the virus (99). Steinhart et al. (103), who first showed the involvement of ASM in infection, demonstrated that the yield of infectious herpes virus from ASM-deficient fibroblasts was 97% lower than from wild-type cells, suggesting a role for this enzyme in herpes infection or replication. In contrast to rhino- and herpes viruses, the sindbis virus utilizes sphingomyelin to enter cells. ASM deficiency elevates the sphingomyelin in cell membranes, leading to more rapid replication and spread in the nervous system of ASMKO mice (104).
HIV-1 infection is also influenced by sphingomyelinase-induced microdomain formation in a unique way. HIV-cell fusion is initiated by interaction of the viral gp120 (the receptor binding subunit of the HIV-1 envelope protein) with the CD4 receptor on the surface of cells. Ceramide-enriched microdomains cause the clustering of CD4 receptors and restricts their movement within the membrane. This may spatially isolate the CD4 receptors (105). Intriguingly, incubation of cells with exogenous sphingomyelinase slows the fusion of HIV-1 virus with cells (106).
These observations demonstrate the complex role of ASM in the outcome of infection with both bacterial and viral pathogens. Although the function of ASM in these processes is not entirely understood, presumably the enzyme participates in membrane microdomain reorganization following infection, which can alter cellular internalization and apoptosis. These observations suggest that pharmacologically altering ASM activity might be considered as a treatment for some, but certainly not all, infections, a hypothesis that could be readily investigated in animal models.
One of the early documented effects of ASM was in LPS-induced apoptosis, when it was shown that wild-type mice injected with LPS had serum ASM activity that was increased 2- to 2.5-fold (107). This finding suggested that ASM may play a role in sepsis and that inhibition of serum ASM should be considered as a therapeutic approach for certain infections. Recently, the specific role of ASM in LPS signaling has been further elucidated. The LPS response by macrophages requires activation of the Toll-like receptor 4 (TLR4) complex, which itself requires ceramide-rich lipid microdomains to assemble. Notably, pharmacologic inhibition of ASM prevented TLR4 complex formation after LPS administration, and exogenous ceramide rescued this inhibition (108). These observations, in addition to the above data, suggest a role for ASM in sepsis.
CONCLUSIONS
It is now clear that ASM fulfills a dual role—it has an essential housekeeping function within the lysosomes and late endosomes of virtually all cells, participating in membrane turnover. This includes membranes internalized by endocytosis, as well as those derived from autophagy, including those of mitochondria and other organelles. In addition, a large body of literature supports the original suggestion by Santana et al. (43) that ASM has an important role at the cell surface, which includes reorganization of microdomain structures and the activation of apoptotic signaling. Ceramide-rich microdomain reorganization also affects infection by many pathogens, calcium homeostasis, and many other properties of the cell. These observations suggest a possible role for ASM inhibitors in the treatment of many common diseases, including sepsis, acute lung injury, ischemia, depression, and others.
In addition, the use of ASM as an antioncogenic drug should be investigated further based on the fact that the recombinant enzyme remains one of the most efficient ways to rapidly generate ceramide. It can be safely administered at high doses into normal animals, and it has a preferential activity at acidic pH, consistent with the acidic microenvironment of tumors. It is also currently undergoing FDA-approved clinical trials for the treatment of NPD. Clearly, the study of this enzyme has evolved remarkably and stands as an excellent example of how investigation of a single, ultrarare “orphan” disease (NPD) can stimulate research in far-reaching fields, suggesting new approaches to the treatment of several common disorders.
Acknowledgments
E.H.S. acknowledges the many students, fellows, and technicians who have worked in his laboratory over the past 20 years on ASM. He also thanks the many collaborators who have assisted in these studies, and especially the NPD families who have contributed invaluable research materials. Support for ASM research in his laboratory has been from the National Institutes of Health, March of Dimes Birth Defects Foundation, and Genzyme Corporation. E.L.S. is supported by a MSTP grant (5 T32 GM007280) from the National Institutes of Health.
References
- Gulbins E. Regulation of death receptor signaling and apoptosis by ceramide. Pharmacol Res. 2003;47:393–399. doi: 10.1016/s1043-6618(03)00052-5. [DOI] [PubMed] [Google Scholar]
- Thannhauser S J, Reichel M. Studies on animal lipids. XVI. the occurrence of sphingomyelin as a mixture of sphingomyelin fatty acid ester and free sphingomyelin, demonstrated by enzymatic hydrolysis and mild saponification. J Biol Chem. 1940;135:1–13. [Google Scholar]
- Gatt S. Enzymic hydrolysis and synthesis of ceramides. J Biol Chem. 1963;238:3131–3133. [PubMed] [Google Scholar]
- Brady R O, Kanfer J N, Mock M B, Fredrickson D S. The metabolism of sphingomyelin. II. evidence of an enzymatic deficiency in Niemann-Pick disease. Proc Natl Acad Sci U S A. 1966;55:366–369. doi: 10.1073/pnas.55.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatt S. Magnesium-dependent sphingomyelinase. Biochem Biophys Res Commun. 1976;68:235–241. doi: 10.1016/0006-291x(76)90034-6. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Suzuki K. A novel magnesium-independent neutral sphingomyelinase associated with rat central nervous system meylin. J Biol Chem. 1978;253:4090–4092. [PubMed] [Google Scholar]
- Duan R D, Hertervig E, Nyberg L, Hauge T, Sternby B, Lillienau J, Farooqi A, Nilsson A. Distribution of alkaline sphingomyelinase activity in human beings and animals. tissue and species differences. Dig Dis Sci. 1996;41:1801–1806. doi: 10.1007/BF02088748. [DOI] [PubMed] [Google Scholar]
- Fowler S. Lysosomal localization of sphingomyelinase in rat liver. Biochim Biophys Acta. 1969;191:481–484. doi: 10.1016/0005-2744(69)90271-x. [DOI] [PubMed] [Google Scholar]
- Quintern L E, Schuchman E H, Levran O, Suchi M, Ferlinz K, Reinke H, Sandhoff K, Desnick R J. Isolation of cDNA clones encoding human acid sphingomyelinase: occurrence of alternatively processed transcripts. EMBO J. 1989;8:2469–2473. doi: 10.1002/j.1460-2075.1989.tb08382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchman E H, Levran O, Pereira L V, Desnick R J. Structural organization and complete nucleotide sequence of the gene encoding human acid sphingomyelinase (SMPD1) Genomics. 1992;12:197–205. doi: 10.1016/0888-7543(92)90366-z. [DOI] [PubMed] [Google Scholar]
- Ferlinz K, Hurwitz R, Moczall H, Lansmann S, Schuchman E H, Sandhoff K. Functional characterization of the N-glycosylation sites of human acid sphingomyelinase by site-directed mutagenesis. Eur J Biochem. 1997;243:511–517. doi: 10.1111/j.1432-1033.1997.511_1a.x. [DOI] [PubMed] [Google Scholar]
- Pittis M G, Ricci V, Guerci V I, Marcais C, Ciana G, Dardis A, Gerin F, Stroppiano M, Vanier M T, Filocamo M, Bembi B. Acid sphingomyelinase: Identification of nine novel mutations among Italian Niemann-Pick type B patients and characterization of in vivo functional in-frame start codon. Hum Mutat. 2004;24:186–187. doi: 10.1002/humu.9263. [DOI] [PubMed] [Google Scholar]
- Sakuragawa N. Acid sphingomyelinase of human placenta: purification, properties, and 125 iodine labeling. J Biochem. 1982;92:637–646. doi: 10.1093/oxfordjournals.jbchem.a133974. [DOI] [PubMed] [Google Scholar]
- Lansmann S, Ferlinz K, Hurwitz R, Bartelsen O, Glombitza G, Sandhoff K. Purification of acid sphingomyelinase from human placenta: characterization and N-terminal sequence. FEBS Lett. 1996;399:227–231. doi: 10.1016/s0014-5793(96)01331-2. [DOI] [PubMed] [Google Scholar]
- Ferlinz K, Hurwitz R, Vielhaber G, Suzuki K, Sandhoff K. Occurrence of two molecular forms of human acid sphingomyelinase. Biochem J. 1994;301:855–862. doi: 10.1042/bj3010855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Miranda S R, Xiong X, Dagan A, Gatt S, Schuchman E H. Characterization of human acid sphingomyelinase purified from the media of overexpressing Chinese hamster ovary cells. Biochim Biophys Acta. 1999;1432:251–264. doi: 10.1016/s0167-4838(99)00069-2. [DOI] [PubMed] [Google Scholar]
- Schissel S L, Schuchman E H, Williams K J, Tabas I. Zn2+-stimulated sphingomyelinase is secreted by many cell types and is a product of the acid sphingomyelinase gene. J Biol Chem. 1996;271:18431–18436. doi: 10.1074/jbc.271.31.18431. [DOI] [PubMed] [Google Scholar]
- Simonaro C M, Park J H, Eliyahu E, Shtraizent N, McGovern M M, Schuchman E H. Imprinting at the SMPD1 locus: implications for acid sphingomyelinase-deficient Niemann-Pick disease. Am J Hum Genet. 2006;78:865–870. doi: 10.1086/503750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence M W, Byers D M, Palmer F B, Cook H W. A new Zn2+-stimulated sphingomyelinase in fetal bovine serum. J Biol Chem. 1989;264:5358–5363. [PubMed] [Google Scholar]
- Tabas I. Secretory sphingomyelinase. Chem Phys Lipids. 1999;102:123–130. doi: 10.1016/s0009-3084(99)00080-8. [DOI] [PubMed] [Google Scholar]
- Qiu H, Edmunds T, Baker-Malcolm J, Karey K P, Estes S, Schwarz C, Hughes H, Van Patten S M. Activation of human acid sphingomyelinase through modification or deletion of C-terminal cysteine. J Biol Chem. 2003;278:32744–32752. doi: 10.1074/jbc.M303022200. [DOI] [PubMed] [Google Scholar]
- Horinouchi K, Erlich S, Perl D P, Ferlinz K, Bisgaier C L, Sandhoff K, Desnick R J, Stewart C L, Schuchman E H. Acid sphingomyelinase deficient mice: a model of types A and B Niemann-Pick disease. Nat Genet. 1995;10:288–293. doi: 10.1038/ng0795-288. [DOI] [PubMed] [Google Scholar]
- Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, Fuks Z, Kolesnick R. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- Grassme H, Cremesti A, Kolesnick R, Gulbins E. Ceramide-mediated clustering is required for CD95-DISC formation. Oncogene. 2003;22:5457–5470. doi: 10.1038/sj.onc.1206540. [DOI] [PubMed] [Google Scholar]
- Yu Z F, Nikolova-Karakashian M, Zhou D, Cheng G, Schuchman E H, Mattson M P. Pivotal role for acidic sphingomyelinase in cerebral ischemia-induced ceramide and cytokine production, and neuronal apoptosis. J Mol Neurosci. 2000;15:85–97. doi: 10.1385/JMN:15:2:85. [DOI] [PubMed] [Google Scholar]
- Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, Haimovitz-Friedman A, Cordon-Cardo C, Kolesnick R. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- Dimanche-Boitrel M T, Meurette O, Rebillard A, Lacour S. Role of early plasma membrane events in chemotherapy-induced cell death. Drug Resist Updat. 2005;8:5–14. doi: 10.1016/j.drup.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Garcia-Ruiz C, Colell A, Mari M, Morales A, Calvo M, Enrich C, Fernandez-Checa J C. Defective TNF-alpha-mediated hepatocellular apoptosis and liver damage in acidic sphingomyelinase knockout mice. J Clin Invest. 2003;111:197–208. doi: 10.1172/JCI16010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchman E H. The pathogenesis and treatment of acid sphingomyelinase-deficient Niemann-Pick disease. J Inherit Metab Dis. 2007;30:654–663. doi: 10.1007/s10545-007-0632-9. [DOI] [PubMed] [Google Scholar]
- Grassme H, Jekle A, Riehle A, Schwarz H, Berger J, Sandhoff K, Kolesnick R, Gulbins E. CD95 signaling via ceramide-rich membrane rafts. J Biol Chem. 2001;276:20589–20596. doi: 10.1074/jbc.M101207200. [DOI] [PubMed] [Google Scholar]
- Zeidan Y H, Hannun Y A. Activation of acid sphingomyelinase by protein kinase Cdelta-mediated phosphorylation. J Biol Chem. 2007;282:11549–11561. doi: 10.1074/jbc.M609424200. [DOI] [PubMed] [Google Scholar]
- Zeidan Y H, Wu B X, Jenkins R W, Obeid L M, Hannun Y A. A novel role for protein kinase cdelta-mediated phosphorylation of acid sphingomyelinase in UV light-induced mitochondrial injury. FASEB J. 2008;22:183–193. doi: 10.1096/fj.07-8967com. [DOI] [PubMed] [Google Scholar]
- Singer S J, Nicolson G L. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Grassme H, Riethmuller J, Gulbins E. Biological aspects of ceramide-enriched membrane domains. Prog Lipid Res. 2007;46:161–170. doi: 10.1016/j.plipres.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Chiantia S, Kahya N, Schwille P. Raft domain reorganization driven by short- and long-chain ceramide: a combined AFM and FCS study. Langmuir. 2007;23:7659–7665. doi: 10.1021/la7010919. [DOI] [PubMed] [Google Scholar]
- Hueber A O, Bernard A M, Herincs Z, Couzinet A, He H T. An essential role for membrane rafts in the initiation of Fas/CD95-triggered cell death in mouse thymocytes. EMBO Rep. 2002;3:190–196. doi: 10.1093/embo-reports/kvf022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan C, Camoletto P G, Cristofani F, Van Veldhoven P P, Ledesma M D. Anomalous surface distribution of glycosyl phosphatidyl inositol-anchored proteins in neurons lacking acid sphingomyelinase. Mol Biol Cell. 2008;19:509–522. doi: 10.1091/mbc.E07-05-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schissel S L, Jiang X, Tweedie-Hardman J, Jeong T, Camejo E H, Najib J, Rapp J H, Williams K J, Tabas I. Secretory sphingomyelinase, a product of the acid sphingomyelinase gene, can hydrolyze atherogenic lipoproteins at neutral pH. Implications for atherosclerotic lesion development. J Biol Chem. 1998;273:2738–2746. doi: 10.1074/jbc.273.5.2738. [DOI] [PubMed] [Google Scholar]
- Ro H A, Carson J H. pH microdomains in oligodendrocytes. J Biol Chem. 2004;279:37115–37123. doi: 10.1074/jbc.M403099200. [DOI] [PubMed] [Google Scholar]
- Chao R, Khan W, Hannun Y A. Retinoblastoma protein dephosphorylation induced by D-erythro-sphingosine. J Biol Chem. 1992;267:23459–23462. [PubMed] [Google Scholar]
- Olivera A, Kohama T, Edsall L, Nava V, Cuvillier O, Poulton S, Spiegel S. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J Cell Biol. 1999;147:545–558. doi: 10.1083/jcb.147.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne S, Pyne N J. Sphingosine 1-phosphate signalling in mammalian cells. Biochem J. 2000;349:385–402. doi: 10.1042/0264-6021:3490385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santana P, Pena L A, Haimovitz-Friedman A, Martin S, Green D, McLoughlin M, Cordon-Cardo C, Schuchman E H, Fuks Z, Kolesnick R. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell. 1996;86:189–199. doi: 10.1016/s0092-8674(00)80091-4. [DOI] [PubMed] [Google Scholar]
- Bezombes C, Segui B, Cuvillier O, Bruno A P, Uro-Coste E, Gouaze V, Andrieu-Abadie N, Carpentier S, Laurent G, Salvayre R, Jaffrezou J P, Levade T. Lysosomal sphingomyelinase is not solicited for apoptosis signaling. FASEB J. 2001;15:297–299. doi: 10.1096/fj.00-0466fje. [DOI] [PubMed] [Google Scholar]
- Mikami T, Takahashi T, Ishida A, Minamiya Y, Ida H, Takada G. Signaling pathway for radiation-induced apoptosis in the lymphoblasts from neuronopathic (type A) and non-neuronopathic (type B) forms of niemann-pick disease. J Neurol Sci. 2002;199:39–43. doi: 10.1016/s0022-510x(02)00106-5. [DOI] [PubMed] [Google Scholar]
- Morita Y, Perez G I, Paris F, Miranda S R, Ehleiter D, Haimovitz-Friedman A, Fuks Z, Xie Z, Reed J C, Schuchman E H, Kolesnick R N, Tilly J L. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat Med. 2000;6:1109–1114. doi: 10.1038/80442. [DOI] [PubMed] [Google Scholar]
- Lang P A, Schenck M, Nicolay J P, Becker J U, Kempe D S, Lupescu A, Koka S, Eisele K, Klarl B A, Rubben H, Schmid K W, Mann K, Hildenbrand S, Hefter H, Huber S M, Wieder T, Erhardt A, Haussinger D, Gulbins E, Lang F. Liver cell death and anemia in Wilson disease involve acid sphingomyelinase and ceramide. Nat Med. 2007;13:164–170. doi: 10.1038/nm1539. [DOI] [PubMed] [Google Scholar]
- Lovat P E, Di Sano F, Corazzari M, Fazi B, Donnorso R P, Pearson A D, Hall A G, Redfern C P, Piacentini M. Gangliosides link the acidic sphingomyelinase-mediated induction of ceramide to 12-lipoxygenase-dependent apoptosis of neuroblastoma in response to fenretinide. J Natl Cancer Inst. 2004;96:1288–1299. doi: 10.1093/jnci/djh254. [DOI] [PubMed] [Google Scholar]
- Prinetti A, Millimaggi D, D'Ascenzo S, Clarkson M, Bettiga A, Chigorno V, Sonnino S, Pavan A, Dolo V. Lack of ceramide generation and altered sphingolipid composition are associated with drug resistance in human ovarian carcinoma cells. Biochem J. 2006;395:311–318. doi: 10.1042/BJ20051184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzner M, Bielawska A, Morse M A, Rudiger H A, Sindram D, Hannun Y A, Clavien P A. Induction of apoptotic cell death and prevention of tumor growth by ceramide analogues in metastatic human colon cancer. Cancer Res. 2001;61:1233–1240. [PubMed] [Google Scholar]
- Riboni L, Campanella R, Bassi R, Villani R, Gaini S M, Martinelli-Boneschi F, Viani P, Tettamanti G. Ceramide levels are inversely associated with malignant progression of human glial tumors. Glia. 2002;39:105–113. doi: 10.1002/glia.10087. [DOI] [PubMed] [Google Scholar]
- Rylova S N, Somova O G, Dyatlovitskaya E V. Comparative investigation of sphingoid bases and fatty acids in ceramides and sphingomyelins from human ovarian malignant tumors and normal ovary. Biochemistry (Mosc) 1998;63:1057–1060. [PubMed] [Google Scholar]
- Sawada M, Nakashima S, Kiyono T, Yamada J, Hara S, Nakagawa M, Shinoda J, Sakai N. Acid sphingomyelinase activation requires caspase-8 but not p53 nor reactive oxygen species during fas-induced apoptosis in human glioma cells. Exp Cell Res. 2002;273:157–168. doi: 10.1006/excr.2001.5437. [DOI] [PubMed] [Google Scholar]
- Lacour S, Hammann A, Grazide S, Lagadic-Gossmann D, Athias A, Sergent O, Laurent G, Gambert P, Solary E, Dimanche-Boitrel M T. Cisplatin-induced CD95 redistribution into membrane lipid rafts of HT29 human colon cancer cells. Cancer Res. 2004;64:3593–3598. doi: 10.1158/0008-5472.CAN-03-2787. [DOI] [PubMed] [Google Scholar]
- Rebillard A, Tekpli X, Meurette O, Sergent O, LeMoigne-Muller G, Vernhet L, Gorria M, Chevanne M, Christmann M, Kaina B, Counillon L, Gulbins E, Lagadic-Gossmann D, Dimanche-Boitrel M T. Cisplatin-induced apoptosis involves membrane fluidification via inhibition of NHE1 in human colon cancer cells. Cancer Res. 2007;67:7865–7874. doi: 10.1158/0008-5472.CAN-07-0353. [DOI] [PubMed] [Google Scholar]
- Bionda C, Hadchity E, Alphonse G, Chapet O, Rousson R, Rodriguez-Lafrasse C, Ardail D. Radioresistance of human carcinoma cells is correlated to a defect in raft membrane clustering. Free Radic Biol Med. 2007;43:681–694. doi: 10.1016/j.freeradbiomed.2007.04.031. [DOI] [PubMed] [Google Scholar]
- Kim S S, Chae H S, Bach J H, Lee M W, Kim K Y, Lee W B, Jung Y M, Bonventre J V, Suh Y H. P53 mediates ceramide-induced apoptosis in SKN-SH cells. Oncogene. 2002;21:2020–2028. doi: 10.1038/sj.onc.1205037. [DOI] [PubMed] [Google Scholar]
- Hara S, Nakashima S, Kiyono T, Sawada M, Yoshimura S, Iwama T, Sakai N. Ceramide triggers caspase activation during gamma-radiation-induced apoptosis of human glioma cells lacking functional p53. Oncol Rep. 2004;12:119–123. [PubMed] [Google Scholar]
- Hara S, Nakashima S, Kiyono T, Sawada M, Yoshimura S, Iwama T, Banno Y, Shinoda J, Sakai N. p53-independent ceramide formation in human glioma cells during gamma-radiation-induced apoptosis. Cell Death Differ. 2004;11:853–861. doi: 10.1038/sj.cdd.4401428. [DOI] [PubMed] [Google Scholar]
- Pena L A, Fuks Z, Kolesnick R N. Radiation-induced apoptosis of endothelial cells in the murine central nervous system: Protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res. 2000;60:321–327. [PubMed] [Google Scholar]
- Li Y Q, Chen P, Haimovitz-Friedman A, Reilly R M, Wong C S. Endothelial apoptosis initiates acute blood-brain barrier disruption after ionizing radiation. Cancer Res. 2003;63:5950–5956. [PubMed] [Google Scholar]
- Suit H D, Willers H. Comment on “Tumor response to radiotherapy regulated by endothelial cell apoptosis” (I) Science. 2003;302:1894. doi: 10.1126/science.1089918. [DOI] [PubMed] [Google Scholar]
- Brown M, Bristow R, Glazer P, Hill R, McBride W, McKenna G, Muschel R. Comment on “Tumor response to radiotherapy regulated by endothelial cell apoptosis” (II) Science. 2003;302:1894. doi: 10.1126/science.1089517. [DOI] [PubMed] [Google Scholar]
- Tani M, Ito M, Igarashi Y. Ceramide/sphingosine/sphingosine 1-phosphate metabolism on the cell surface and in the extracellular space. Cell Signal. 2007:229–237. doi: 10.1016/j.cellsig.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Elojeimy S, Liu X, McKillop J C, El-Zawahry A M, Holman D H, Cheng J Y, Meacham W D, Mahdy A E, Saad A F, Turner L S, Cheng J A, Day T, Dong J Y, Bielawska A, Hannun Y A, Norris J S. Role of acid ceramidase in resistance to FasL: therapeutic approaches based on acid ceramidase inhibitors and FasL gene therapy. Mol Ther. 2007;15:1259–1263. doi: 10.1038/sj.mt.6300167. [DOI] [PubMed] [Google Scholar]
- Morales A, Paris R, Villanueva A, Llacuna L, Garcia-Ruiz C, Fernandez-Checa J C. Pharmacological inhibition or small interfering RNA targeting acid ceramidase sensitizes hepatoma cells to chemotherapy and reduces tumor growth in vivo. Oncogene. 2007;26:905–916. doi: 10.1038/sj.onc.1209834. [DOI] [PubMed] [Google Scholar]
- Grammatikos G, Teichgraber V, Carpinteiro A, Trarbach T, Weller M, Hengge U R, Gulbins E. Overexpression of acid sphingomyelinase sensitizes glioma cells to chemotherapy. Antioxid Redox Signal. 2007;9:1449–1156. doi: 10.1089/ars.2007.1673. [DOI] [PubMed] [Google Scholar]
- Garnacho C, Dhami R, Simone E, Dziubla T, Leferovich J, Schuchman E H, Muzykantov V, Muro S. Delivery of acid sphingomyelinase in normal and niemann-pick disease mice using ICAM-1-targeted polymer nanocarriers. J Pharmacol Exp Ther. 2008;325:400–408. doi: 10.1124/jpet.107.133298. [DOI] [PubMed] [Google Scholar]
- Marathe S, Kuriakose G, Williams K J, Tabas I. Sphingomyelinase, an enzyme implicated in atherogenesis, is present in atherosclerotic lesions and binds to specific components of the subendothelial extracellular matrix. Arterioscler Thromb Vasc Biol. 1999;19:2648–2658. doi: 10.1161/01.atv.19.11.2648. [DOI] [PubMed] [Google Scholar]
- Tabas I, Williams K J, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- Wong M L, Xie B, Beatini N, Phu P, Marathe S, Johns A, Gold P W, Hirsch E, Williams K J, Licinio J, Tabas I. Acute systemic inflammation up-regulates secretory sphingomyelinase in vivo: a possible link between inflammatory cytokines and atherogenesis. Proc Natl Acad Sci U S A. 2000;97:8681–8686. doi: 10.1073/pnas.150098097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehner W, Bunck A C, Rauchhaus M, von Haehling S, Brunkhorst F M, Cicoira M, Tschope C, Ponikowski P, Claus R A, Anker S D. Secretory sphingomyelinase is upregulated in chronic heart failure: a second messenger system of immune activation relates to body composition, muscular functional capacity, and peripheral blood flow. Eur Heart J. 2007;28:821–828. doi: 10.1093/eurheartj/ehl541. [DOI] [PubMed] [Google Scholar]
- Gorska M, Baranczuk E, Dobrzyn A. Secretory Zn2+-dependent sphingomyelinase activity in the serum of patients with type 2 diabetes is elevated. Horm Metab Res. 2003;35:506–507. doi: 10.1055/s-2003-41810. [DOI] [PubMed] [Google Scholar]
- Straczkowski M, Kowalska I, Baranowski M, Nikolajuk A, Otziomek E, Zabielski P, Adamska A, Blachnio A, Gorski J, Gorska M. Increased skeletal muscle ceramide level in men at risk of developing type 2 diabetes. Diabetologia. 2007;50:2366–2373. doi: 10.1007/s00125-007-0781-2. [DOI] [PubMed] [Google Scholar]
- Summers S A. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006;45:42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Herschkovitz A, Liu Y F, Ilan E, Ronen D, Boura-Halfon S, Zick Y. Common inhibitory serine sites phosphorylated by IRS-1 kinases, triggered by insulin and inducers of insulin resistance. J Biol Chem. 2007;282:18018–18027. doi: 10.1074/jbc.M610949200. [DOI] [PubMed] [Google Scholar]
- Argaud L, Prigent A F, Chalabreysse L, Loufouat J, Lagarde M, Ovize M. Ceramide in the antiapoptotic effect of ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2004;286:H246–H251. doi: 10.1152/ajpheart.00638.2003. [DOI] [PubMed] [Google Scholar]
- Cordis G A, Yoshida T, Das D K. HPTLC analysis of sphingomylein, ceramide and sphingosine in ischemic/reperfused rat heart. J Pharm Biomed Anal. 1998;16:1189–1193. doi: 10.1016/s0731-7085(97)00260-4. [DOI] [PubMed] [Google Scholar]
- Cui J, Engelman R M, Maulik N, Das D K. Role of ceramide in ischemic preconditioning. J Am Coll Surg. 2004;198:770–777. doi: 10.1016/j.jamcollsurg.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Der P, Cui J, Das D K. Role of lipid rafts in ceramide and nitric oxide signaling in the ischemic and preconditioned hearts. J Mol Cell Cardiol. 2006;40:313–320. doi: 10.1016/j.yjmcc.2005.10.005. [DOI] [PubMed] [Google Scholar]
- McGovern M M, Pohl-Worgall T, Deckelbaum R J, Simpson W, Mendelson D, Desnick R J, Schuchman E H, Wasserstein M P. Lipid abnormalities in children with types A and B Niemann Pick disease. J Pediatr. 2004;145:77–81. doi: 10.1016/j.jpeds.2004.02.048. [DOI] [PubMed] [Google Scholar]
- Dhami R, He X, Gordon R E, Schuchman E H. Analysis of the lung pathology and alveolar macrophage function in the acid sphingomyelinase–deficient mouse model of Niemann-Pick disease. Lab Invest. 2001;81:987–999. doi: 10.1038/labinvest.3780311. [DOI] [PubMed] [Google Scholar]
- Tuder R M, Petrache I, Elias J A, Voelkel N F, Henson P M. Apoptosis and emphysema: The missing link. Am J Respir Cell Mol Biol. 2003;28:551–554. doi: 10.1165/rcmb.F269. [DOI] [PubMed] [Google Scholar]
- Petrache I, Natarajan V, Zhen L, Medler T R, Richter A T, Cho C, Hubbard W C, Berdyshev E V, Tuder R M. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med. 2005;11:491–498. doi: 10.1038/nm1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thon L, Mohlig H, Mathieu S, Lange A, Bulanova E, Winoto-Morbach S, Schutze S, Bulfone-Paus S, Adam D. Ceramide mediates caspase-independent programmed cell death. FASEB J. 2005;19:1945–1956. doi: 10.1096/fj.05-3726com. [DOI] [PubMed] [Google Scholar]
- Teichgräber V, Ulrich M, Endlich N, Riethmuller J, Wilker B, De Oliveira-Munding C C, van Heeckeren A M, Barr M L, von Kurthy G, Schmid K W, Weller M, Tummler B, Lang F, Grassme H, Doring G, Gulbins E. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat Med. 2008;14:382–391. doi: 10.1038/nm1748. [DOI] [PubMed] [Google Scholar]
- Grassme H, Jendrossek V, Riehle A, von Kurthy G, Berger J, Schwarz H, Weller M, Kolesnick R, Gulbins E. Host defense against pseudomonas aeruginosa requires ceramide-rich membrane rafts. Nat Med. 2003;9:322–330. doi: 10.1038/nm823. [DOI] [PubMed] [Google Scholar]
- Guilbault C, De Sanctis J B, Wojewodka G, Saeed Z, Lachance C, Skinner T A, Vilela R M, Kubow S, Lands L C, Hajduch M, Matouk E, Radzioch D. Fenretinide corrects newly found ceramide deficiency in cystic fibrosis. Am J Respir Cell Mol Biol. 2008;38:47–56. doi: 10.1165/rcmb.2007-0036OC. [DOI] [PubMed] [Google Scholar]
- Rauvala H, Hallman M. Glycolipid accumulation in bronchoalveolar space in adult respiratory distress syndrome. J Lipid Res. 1984;25:1257–1262. [PubMed] [Google Scholar]
- Goggel R, Winoto-Morbach S, Vielhaber G, Imai Y, Lindner K, Brade L, Brade H, Ehlers S, Slutsky A S, Schutze S, Gulbins E, Uhlig S. PAF-mediated pulmonary edema: a new role for acid sphingomyelinase and ceramide. Nat Med. 2004;10:155–160. doi: 10.1038/nm977. [DOI] [PubMed] [Google Scholar]
- Mendelson D S, Wasserstein M P, Desnick R J, Glass R, Simpson W, Skloot G, Vanier M, Bembi B, Giugliani R, Mengel E, Cox G F, McGovern M M. Type B niemann-pick disease: findings at chest radiography, thin-section CT, and pulmonary function testing. Radiology. 2006;238:339–345. doi: 10.1148/radiol.2381041696. [DOI] [PubMed] [Google Scholar]
- Buccoliero R, Ginzburg L, Futerman A H. Elevation of lung surfactant phosphatidylcholine in mouse models of sandhoff and of Niemann-Pick A disease. J Inherit Metab Dis. 2004;27:641–648. doi: 10.1023/b:boli.0000042958.22066.6c. [DOI] [PubMed] [Google Scholar]
- Ikegami M, Dhami R, Schuchman E H. Alveolar lipoproteinosis in an acid sphingomyelinase-deficient mouse model of niemann-pick disease. Am J Physiol Lung Cell Mol Physiol. 2003;284:L518–L525. doi: 10.1152/ajplung.00258.2002. [DOI] [PubMed] [Google Scholar]
- Von Bismarck P, Garcia Wistadt C F, Klemm K, Winoto-Morbach S, Uhlig U, Schutze S, Adam D, Lachmann B, Uhlig S, Krause M F. Improved pulmonary function by acid sphingomyelinase inhibition in a newborn piglet lavage model. Am J Respir Crit Care Med. 2008;177:1233–1241. doi: 10.1164/rccm.200705-752OC. [DOI] [PubMed] [Google Scholar]
- Kornhuber J, Medlin A, Bleich S, Jendrossek V, Henkel A W, Wiltfang J, Gulbins E. High activity of acid sphingomyelinase in major depression. J Neural Transm. 2005;112:1583–1590. doi: 10.1007/s00702-005-0374-5. [DOI] [PubMed] [Google Scholar]
- Han X. Lipid alterations in the earliest clinically recognizable stage of Alzheimer’s disease: implication of the role of lipids in the pathogenesis of Alzheimer’s disease. Curr Alzheimer Res. 2005;2:65–77. doi: 10.2174/1567205052772786. [DOI] [PubMed] [Google Scholar]
- Kirschnek S, Paris F, Weller M, Grassme H, Ferlinz K, Riehle A, Fuks Z, Kolesnick R, Gulbins E. CD95-mediated apoptosis in vivo involves acid sphingomyelinase. J Biol Chem. 2000;275:27316–27323. doi: 10.1074/jbc.M002957200. [DOI] [PubMed] [Google Scholar]
- Hauck C R, Grassme H, Bock J, Jendrossek V, Ferlinz K, Meyer T F, Gulbins E. Acid sphingomyelinase is involved in CEACAM receptor-mediated phagocytosis of neisseria gonorrhoeae. FEBS Lett. 2000;478:260–266. doi: 10.1016/s0014-5793(00)01851-2. [DOI] [PubMed] [Google Scholar]
- Grassme H, Riehle A, Wilker B, Gulbins E. Rhinoviruses infect human epithelial cells via ceramide-enriched membrane platforms. J Biol Chem. 2005;280:26256–26262. doi: 10.1074/jbc.M500835200. [DOI] [PubMed] [Google Scholar]
- Gulbins E, Dreschers S, Wilker B, Grassme H. Ceramide, membrane rafts and infections. J Mol Med. 2004;82:357–363. doi: 10.1007/s00109-004-0539-y. [DOI] [PubMed] [Google Scholar]
- Utermohlen O, Karow U, Lohler J, Kronke M. Severe impairment in early host defense against listeria monocytogenes in mice deficient in acid sphingomyelinase. J Immunol. 2003;170:2621–2628. doi: 10.4049/jimmunol.170.5.2621. [DOI] [PubMed] [Google Scholar]
- McCollister B D, Myers J T, Jones-Carson J, Voelker D R, Vazquez-Torres A. Constitutive acid sphingomyelinase enhances early and late macrophage killing of salmonella enterica serovar typhimurium. Infect Immun. 2007;75:5346–5352. doi: 10.1128/IAI.00689-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhart W L, Busch J S, Oettgen J P, Howland J L. Sphingolipid metabolism during infection of human fibroblasts by herpes simplex virus type 1. Intervirology. 1984;21:70–76. doi: 10.1159/000149504. [DOI] [PubMed] [Google Scholar]
- Ng C G, Griffin D E. Acid sphingomyelinase deficiency increases susceptibility to fatal alphavirus encephalomyelitis. J Virol. 2006;80:10989–10999. doi: 10.1128/JVI.01154-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan C M, Rawat S S, Cho E H, Guiffre D L, Lockett S, Merrill A H, Jr, Blumenthal R. Sphingomyelinase restricts the lateral diffusion of CD4 and inhibits human immunodeficiency virus fusion. J Virol. 2007;81:5294–5304. doi: 10.1128/JVI.02553-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan C M, Rawat S S, Puri A, Wang J M, Ruscetti F W, Blumenthal R. Ceramide, a target for antiretroviral therapy. Proc Natl Acad Sci U S A. 2004;101:15452–15457. doi: 10.1073/pnas.0402874101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M L, Xie B, Beatini N, Phu P, Marathe S, Johns A, Gold P W, Hirsch E, Williams K J, Licinio J, Tabas I. Acute systemic inflammation up-regulates secretory sphingomyelinase in vivo: a possible link between inflammatory cytokines and atherogenesis. Proc Natl Acad Sci U S A. 2000;97:8681–8686. doi: 10.1073/pnas.150098097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuschieri J, Bulger E, Billgrin J, Garcia I, Maier R V. Acid sphingomyelinase is required for lipid raft TLR4 complex formation. Surg Infect (Larchmt) 2007;8:91–106. doi: 10.1089/sur.2006.050. [DOI] [PubMed] [Google Scholar]
- Haimovitz-Friedman A, Cordon-Cardo C, Bayoumy S, Garzotto M, McLoughlin M, Gallily R, Edwards C K, 3rd, Schuchman E H, Fuks Z, Kolesnick R. Lipopolysaccharide induces disseminated endothelial apoptosis requiring ceramide generation. J Exp Med. 1997;186:1831–1841. doi: 10.1084/jem.186.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitru C A, Gulbins E. TRAIL activates acid sphingomyelinase via a redox mechanism and releases ceramide to trigger apoptosis. Oncogene. 2006;25:5612–5625. doi: 10.1038/sj.onc.1209568. [DOI] [PubMed] [Google Scholar]