Abstract
Mesangioproliferative glomerulonephritis is associated with overactive PDGF receptor signal transduction. We show that the phytoalexin resveratrol dose dependently inhibits PDGF-induced DNA synthesis in mesangial cells with an IC50 of 10 μM without inducing apoptosis. Remarkably, the increased SIRT1 deacetylase activity induced by resveratrol was not necessary for this inhibitory effect. Resveratrol significantly blocked PDGF-stimulated c-Src and Akt kinase activation, resulting in reduced cyclin D1 expression and attenuated pRb phosphorylation and cyclin-dependent kinase-2 (CDK2) activity. Furthermore, resveratrol inhibited PDGFR phosphorylation at the PI 3 kinase and Grb-2 binding sites tyrosine-751 and tyrosine-716, respectively. This deficiency in PDGFR phosphorylation resulted in significant inhibition of PI 3 kinase and Erk1/2 MAPK activity. Interestingly, resveratrol increased the activity of protein tyrosine phosphatase PTP1B, which dephosphorylates PDGF-stimulated phosphorylation at tyrosine-751 and tyrosine-716 on PDGFR with concomitant reduction in Akt and Erk1/2 kinase activity. PTP1B significantly inhibited PDGF-induced DNA synthesis without inducing apoptosis. These results for the first time provide evidence that the stilbene resveratrol targets PTP1B to inhibit PDGFR mitogenic signaling.—Venkatesan, B., Ghosh-Choudhury, N., Das, F., Mahimainathan, L., Kamat, A., Kasinath, B. S., Abboud, H. E., Choudhury, G. G. Resveratrol inhibits PDGF receptor mitogenic signaling in mesangial cells: role of PTP1B.
Keywords: SIRT1, Akt kinase, CDK2
Four polypeptide chains are assembled by disulfide bonds to form platelet-derived growth factor isoforms AA, AB, BB, CC, and DD (1). These isoforms bind to either one or both tyrosine kinase receptors to exert their biological effects. PDGF A-, B-, and C-chains bind PDGF receptor-α, while PDGF receptor-β (PDGFR) interacts with B- and D-chains (2). These receptors play an important role during development and in adult life, including cell fate determination, proliferation, survival, and migration (3). Both are composed of a large extracellular domain flanked by an intracellular juxtamembrane domain and a C-terminal tail (4). Ligand binding to the PDGF receptor induces a conformational change that relieves autoinhibitory constraints of the juxtamembrane and C-terminal domains on the intrinsic tyrosine kinase activity of the receptor (5,6,7). Subsequently, the PDGF receptors undergo transphosphorylation at a number of tyrosine residues in the intracellular domain. These act as docking sites for cytoplasmic signaling proteins such as PLCγ, c-Src, Grb-2, and phosphatidylinositol (PI) 3 kinase (4, 8, 9). Recently, we and others have shown essential roles of c-Src and PI 3 kinase in PDGF-induced cell proliferation (8,9,10).
Proliferative glomerular injury, including IgA nephropathy, membranoproliferative glomerulonephritis, and lupus nephritis, is characterized by increased mesangial cell proliferation (11, 12). Although a variety of growth factors stimulate growth of mesangial cells, PDGF has been shown to be the most potent mitogen for these cells in vitro and in vivo (7, 11, 13,14,15,16,17). In fact, most growth factors operate via autocrine induction of PDGF to elicit their mitogenic effect in mesangial cells (18). Furthermore, inactivation of PDGF BB and PDGFR blocks mesangioproliferative glomerulonephritis in rats (14, 16, 19). Mice deficient for PDGFR or PDGF BB show abnormal glomeruli due to lack of mesangial cell development (7, 20,21,22). Thus, PDGF BB-PDGFR signal transduction is essential for glomerular development and pathogenesis of proliferative glomerulonephritis.
Resveratrol (3,5,4′-trihydroxy-trans-stilbene), a phytoalexin present in Leguminosae family of plants, exhibits beneficial effects in the control of atherosclerosis, heart disease, arthritis and autoimmune disorders (23, 24). Free radical scavenging and antioxidant properties of this stilbene have been suggested to explain its beneficial effects. Resveratrol also interacts with many proteins, including protein kinase C, MEK1, NF-κB, TNF-α, p53, mitochondrial complex III, ATP synthase and fatty acid synthase; these interactions may be responsible for its biological effects (25). More recently, resveratrol has been shown to increase the deacetylase activity of a Sirtuin family member, which acts to increase life span of various organisms (26, 27). Also, activation of AMP-activated protein kinase by resveratrol protected against liver damage in diabetic mice and increased survival of mice fed a high-fat diet (28, 29). Apart from these activities, resveratrol has gained considerable attention because of its potent antiproliferative activity in vitro and in vivo (24, 30,31,32,33,34,35). Although inhibition of signaling pathways, down-regulation of proinflammatory mediators, alteration of eicosanoid synthesis, or inhibition of activated immune cells have been postulated for the beneficial effects of resveratrol, the mechanism varies significantly in a cell and context-dependent manner.
In the present study, we show that resveratrol dose-dependently inhibited PDGF-induced DNA synthesis in mesangial cells without inducing apoptosis. We found resveratrol blocked tyrosine phosphorylation of PDGFR, including tyrosine 751 and 716, the binding sites for PI 3 kinase and Grb2, resulting in inhibition of Akt kinase and Erk1/2 MAPK. The stilbene inhibited cyclin D1 expression, which led to attenuated PDGF-induced phosphorylation of the retinoblastoma protein and CDK2 activity. In addition, we provide the first evidence that resveratrol increases the activity of the tyrosine phosphatase PTP1B, which dephosphorylates PDGFR to inhibit PDGF-induced signal transduction, resulting in attenuation of DNA synthesis. These results represent a novel mechanism of resveratrol-mediated inhibition of PDGF-induced mesangial cell proliferation.
MATERIALS AND METHODS
Materials
Tissue culture materials were purchased from Gibco BRL (Carlsbad, CA, USA). PDGF was obtained from R&D Systems (Minneapolis, MN, USA). Phospho-Src, Src, phospho-Akt (Ser-473), phospho-pRb (Ser-809/811), phopsho-Erk1/2 (Thr-202/Tyr 204), and Erk1/2 antibodies were obtained from Cell Signaling (Beverly, MA, USA). Anti-phospho-tyrosine (4G10), phospho-PDGFR (tyrosine-751), phospho-PDGFR (tyrosine-716), PDGFR, and Akt antibodies were obtained from Upstate Technology (Lake Placid, NY, USA). CDK2 and cyclin D1 antibodies were purchased from Santa Cruz (Santa Cruz, CA, USA). Anti-PTP1B was from Abcam (Cambridge, MA, USA). Histone H1, myelin basic protein (MBP), PI, resveratrol, antitubulin, and anti-FLAG antibodies were purchased from Sigma (St. Louis, MO, USA). SIRT1 assay and apoptosis detection kits were obtained from Biomol (Plymouth Meeting, PA, USA) and Calbiochem (San Diego, CA, USA), respectively. Fugene HD transfection reagent was purchased from Roche (Indianapolis, IN, USA). Plasmid expressing a mutant SIRT1H363Y, which acts as a dominant negative enzyme, was purchased from Addgene (Cambridge, MA, USA) (36). Adenovirus vector expressing wild-type PTP1B was kindly provided by Dr. Michael Bryer-Ash (University of California, Los Angeles, CA, USA).
Cell culture and adenovirus infection and transfection
Rat and human mesangial cells were grown in RPMI 1640 and Dulbecco Modified Eagle Medium (DMEM) with 17% fetal bovine serum, respectively, as described previously (8, 37). Cells were made quiescent by serum starvation for 48 h in the same media. Cells were treated with resveratrol 1 h prior to the addition of PDGF. In experiments involving adenovirus infection, the cells were infected with adenovirus vector, essentially as described previously (38,39,40). Briefly, mesangial cells were infected with 50 moi (multiplicity of infection) Ad PTP1B at room temperature for 1 h. Adenovirus vector expressing green fluorescence protein (Ad GFP) was used as control. The media were changed with fresh serum-free medium for 24 h before stimulation with PDGF. Mesangial cells were transfected with plasmid DNA using Fugene, according to the vendor’s protocol (41).
DNA synthesis and cell proliferation assay
DNA synthesis was determined as incorporation of 3H-thymidine into trichloroacetic acid-insoluble material as described (8, 10, 37, 38, 42, 43). Mesangial cells were serum starved for 24 h prior to incubation with resveratrol for 1 h. PDGF was added for indicated periods of time. The cells were trypsinized and counted in a hemocytometer.
Detection of apoptosis
Apoptosis of mesangial cells was determined using a commercial kit, utilizing Annexin V-FITC and propidium iodide. The cells were analyzed by flow cytometry in the University of Texas Health Science Center Core Facility, as described previously (44, 45).
SIRT1 activity
SIRT1 activity was measured using a kit, which utilizes a synthetic substrate (Fluor de Lys-SIRT1 substrate), consisting of four amino acids with one acetylated lysine group (Arg-His-Lys-Lys-Ac) in the presence of a fluorochrome (7-amino-4-methylcoumarin). After deacetylation, the fluorochrome is released from the deacetylated substrate when Developer II is added. Essentially, the cell-based SIRT1 assay was performed with slight modifications of the method described by de Boer et al. (46). Briefly, mesangial cells were washed with PBS and incubated with phenol red-free medium supplemented with 25 μM Fluor de Lys SIRT1 substrate in the presence and absence of 2.5 mM nicotinamide (NAM). After 2 h following addition of the substrate, cells were lysed in SIRT1 lysis buffer containing 0.5× developer solution (provided in the kit). The cells were scraped and centrifuged at 10,000 g for 10 min. The supernatant was analyzed using a spectrofluorometer with excitation at 355 nm and emission at 460 nm. The relative fluorescence was calculated by subtracting the values in the presence of NAM from that in the absence.
Immunoblotting
Cells were lysed in RIPA buffer at 4°C as described previously (39, 40). After centrifugation of the cell extracts, protein concentration was determined in the supernatant. Equal amounts of protein were separated by SDS-PAGE. The proteins were transferred to polyvinylidene difluoride (PVDF) membrane, and immunoblotting was performed using specific antibodies, as described previously (39, 40, 42,43,44).
CDK2 assay
Equal amounts of cell lysates were immunoprecipitated with CDK2 antibody, as described previously (37). The immunobeads were suspended in CDK2 kinase assay buffer (Tris-HCl, pH 7.5; 4 mM MgCl2; and 25 μM ATP). One microgram of Histone H1 was used as substrate. The reaction was started by adding 10 μCi of γ32P-ATP and incubated at 30°C for 30 min as described (37, 44). The radiolabeled histone H1 was separated by SDS-PAGE. The gel was dried on a filter paper followed by autoradiography.
PDGFR tyrosine kinase assay
The mesangial cell lysates were immunoprecipitated with PDGFR antibody. The tyrosine kinase activity of the receptor was measured directly on the immunobeads, essentially as described previously (47, 48).
PI 3 kinase assay
PDGFR immunoprecipitates were suspended in PI 3 kinase assay buffer (20 mM Tris-HCl, pH 7.5; 0.1 M NaCl; and 0.5 mM EGTA) in the presence of PI and incubated at 25°C for 10 min. Then 1 μl of 1 M MgCl2 and 10 μCi of γ32P-ATP were added, and the incubation was continued for 10 more minutes. The reaction was stopped essentially as described previously (8, 38). The reaction product was separated by thin-layer chromatography (38, 48). The PI 3-P spot was visualized by autoradiography.
PTP1B assay
Myelin basic protein (MBP) was labeled on tyrosine residues using γ32P-ATP and recombinant c-Src tyrosine kinase as described (49). PTP1B immunoprecipitates were incubated with radiolabeled MBP at 30°C. The released 32Pi was determined in a scintillation counter as described (50).
Statistics
The significance of the data was determined by ANOVA followed by Student-Newman-Keuls analysis (39, 42,43,44,45).
RESULTS
Resveratrol inhibits PDGF-induced DNA synthesis
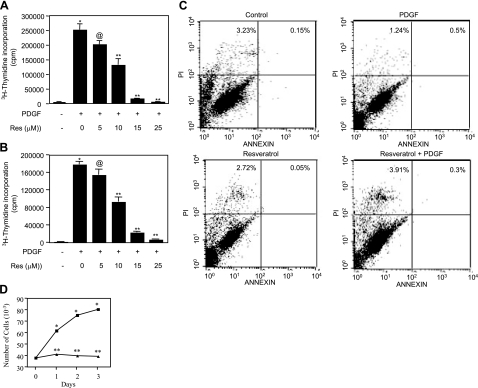
PDGF is a potent mitogen for mesangial cells and plays important roles in pathogenesis of mesangioproliferative glomerulonephritis (8, 10, 11, 37, 38, 51). The polyphenol resveratrol has been shown to inhibit growth of many cells (24, 52). We examined the effect of resveratrol on mesangial cell DNA synthesis. Resveratrol treatment of both rat and human mesangial cells resulted in inhibition of PDGF-induced DNA synthesis in a dose-dependent manner with a half-maximal inhibitory concentration of 10 μM (Fig. 1A, B). Since reduction in 3H-thymidine incorporation may result from cell death and since resveratrol is a potent proapoptotic agent (24, 31, 53), we examined the apoptotic effect of resveratrol on rat mesangial cells, which were used for subsequent studies. Detection of phosphatidylserine by the binding of anticoagulant Annexin V demonstrates apoptosis of cells (54). In addition, propidium iodide distinguishes between viable and nonviable cells. The cells were analyzed by flow cytometry for FITC and propidium iodide fluorescence. Three to four percent of cells bound propidium iodide, indicating the presence of nonviable cells. More than 95% of cells did not bind Annexin V (Fig. 1C). These results indicate that resveratrol did not induce apoptosis of mesangial cells. To confirm the effect of resveratrol on mesangial cell proliferation, we performed cell count assay. PDGF increased the proliferation of mesangial cells in a time-dependent manner (Fig. 1D). Incubation of cells with resveratrol significantly inhibited mesangial cell proliferation at each time point (Fig. 1D). These data demonstrate that resveratrol blocks mesangial cell DNA synthesis, resulting in attenuation of proliferation.
Figure 1.
Effect of resveratrol on PDGF-induced DNA synthesis and proliferation. A, B) Rat (A) and human (B) mesangial cells were incubated with indicated concentrations of resveratrol for 1 h prior to incubation with 20 ng/ml PDGF. DNA synthesis was determined as described in Materials and Methods. A representative experiment of 4 independent experiments performed in triplicate is shown. *P < 0.001 vs. control; @P < 0.05 vs. PDGF; **P < 0.001 vs. PDGF. C) Rat mesangial cells were treated as described in A. Apoptosis was measured using an apoptosis detection kit as described in text. Numbers in the quadrants indicate percentage of apoptotic and necrotic cells. D) PDGF-stimulated rat mesangial cell proliferation in the presence (solid triangles) and absence (solid squares) of resveratrol was determined at indicated time periods as described in text. Data represent means ± se of quadruplicate measurements. *P < 0.001 vs. control; **P < 0.001 vs. PDGF.
Resveratrol-stimulated SIRT1 activity is not necessary for its inhibition of DNA synthesis
Recently, resveratrol has been reported to allosterically increase the activity of SIRT1, an NAD-dependent class III histone deacetylase (26, 55). Many biological activities of resveratrol including increased life span and insulin sensitivity with high-fat diet have been ascribed to its effect on SIRT1 activity (25, 28, 56). We measured SIRT1 activity in resveratrol-treated mesangial cells. Resveratrol significantly increased SIRT1 activity, whereas PDGF alone or in conjunction with resveratrol did not have any further stimulatory effect (Fig. 2A). These data suggest that increased SIRT1 activity by resveratrol may play a role in inhibition of DNA synthesis, which we observed in Fig. 1A, B. We examined this hypothesis using NAM, a SIRT1 inhibitor (57, 58). Incubation of mesangial cells with nicotinamide did not block PDGF-induced DNA synthesis (Fig. 2B). Furthermore, NAM did not prevent resveratrol-mediated inhibition of PDGF-induced DNA synthesis (Fig. 2B). To confirm these results, we used a plasmid vector expressing dominant negative SIRT1H363Y mutant (36). Transfection of mesangial cells with this vector resulted in time-dependent expression of dominant negative SIRT1 (Fig. 2C). Expression of dominant negative SIRT1 did not have any effect on PDGF-induced DNA synthesis (Fig. 2D). These results indicate that endogenous, as well as resveratrol-mediated increase in SIRT1 activity, is not required for its inhibitory effect on DNA synthesis in response to PDGF.
Figure 2.
Role of SIRT1 in resveratrol regulation of PDGF-induced DNA synthesis. A) Mesangial cells were incubated with resveratrol (25 μM) for 1 h prior to treatment with PDGF. SIRT1 activity was determined in the cell lysate as described in Materials and Methods. Data represent means ± se of 6 replicates. *P < 0.001 vs. control. B) Mesangial cells were incubated with 2.5 mM NAM for 1 h prior to incubation with resveratrol for 1 h. Cells were then incubated with PDGF, and DNA synthesis was determined as described in text. A representative experiment of 4 independent experiments is shown; means ± se of triplicate measurements. *P < 0.001 vs. control; **P < 0.001 vs. PDGF. C) Mesangial cells were transfected with dominant negative SIRT1H363Y plasmid. The cells were harvested at indicated periods of time. The cell lysates were immunoblotted with anti-FLAG (to detect dominant negative SIRT1) and tubulin antibodies, respectively. D) Mesangial cells were transfected with vector or dominant negative SIRT1 plasmids as indicated for 24 h prior to incubation with PDGF. DNA synthesis was determined as described in text. Data are means ± se of 6 measurements. *P < 0.001 vs. control.
Resveratrol inhibits c-Src, Akt, and cell cycle regulatory proteins
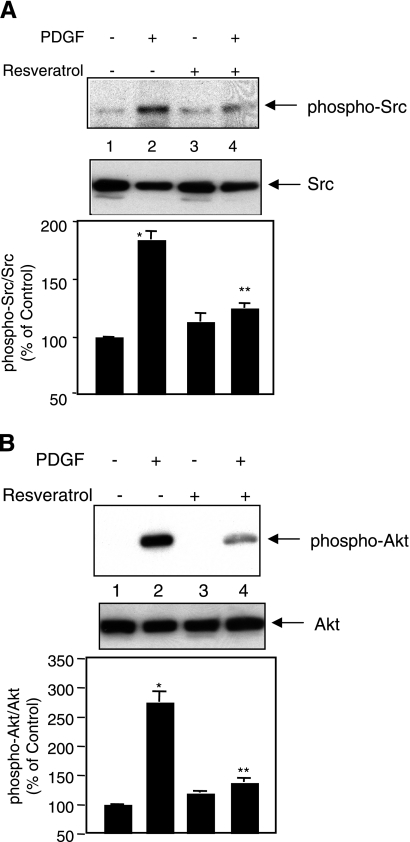
We have recently shown that PDGF-induced DNA synthesis is regulated by c-Src kinase (42). Therefore, we examined whether resveratrol affects c-Src activity. PDGF increased phosphorylation of c-Src, indicating its activation (Fig. 3A). Preincubation of cells with resveratrol significantly inhibited PDGF-induced phosphorylation of c-Src (Fig. 3A, compare lanes 4 and 2). Previously, we showed that PDGF-stimulated Akt kinase activity is necessary for DNA synthesis in mesangial cells (37). Also, we demonstrated that Akt kinase acts downstream of c-Src in mesangial cells (42). We examined the effect of resveratrol on Akt activation by measuring its phosphorylation at serine-473 residue. PDGF increased phosphorylation of Akt (Fig. 3B). Resveratrol significantly blocked PDGF-induced phosphorylation of Akt (Fig. 3B, compare lanes 4 and 2).
Figure 3.
Effect of resveratrol on PDGF-stimulated Src and Akt activation. Mesangial cells were treated with resveratrol prior to incubation with PDGF. The cell lysates were immunoblotted with phospho-Src, Src (A), phospho-Akt and Akt (B) antibodies. Representative blots from 6–12 experiments are shown. Histograms at the bottom show means ± se of quantitation of the protein bands. *P < 0.001 vs. control; **P < 0.001 vs. PDGF.
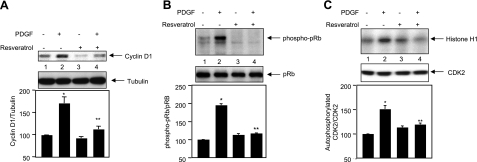
Mitogens increase the abundance of cyclin D1 in the G1 phase of the cell cycle to allow entry of cells into the S phase. We tested whether resveratrol affects PDGF-induced expression of cyclin D1. Preincubation of cells with resveratrol significantly inhibited expression of cyclin D1 in response to PDGF (Fig. 4A). Cyclin D1 increases CDK4 activity in the late G1 phase, resulting in phosphorylation of the retinoblastoma protein (pRb) on serine 809/811 residues. Phosphorylation at these residues is necessary for cell cycle progression (59). PDGF increased phosphorylation of pRb in serine 809/811, which was significantly inhibited by resveratrol (Fig. 4B, compare lanes 4 and 2). Along with CDK4, the activity of cyclin E-dependent kinase, CDK2, is also necessary for progression of cells from G1 to S-phase (60). We have recently shown the requirement of CDK2 in PDGF-induced DNA synthesis (37, 42). Therefore, the effect of resveratrol on CDK2 activity was tested. PDGF significantly increased CDK2 activity in mesangial cells (Fig. 4C). Resveratrol abolished PDGF-induced CDK2 activity (Fig. 4C, compare lanes 4 and 2). Together, these results demonstrate that resveratrol intercepts c-Src kinase, which regulates DNA synthesis by modulating Akt kinase and cyclin-dependent kinases (42).
Figure 4.
Effect of resveratrol on cell cycle proteins. A, B) Mesangial cells were treated with resveratrol for 1 h prior to incubation with PDGF for 20 h. The cell lysates were immunoblotted with cyclin D1 and tubulin (A) and phospho-pRb and pRb (B) antibodies. C) The cell lysates were immunoprecipitated with CDK2 antibody. The immunoprecipitates were used to assay CDK2 activity using histone H1 as substrate, as described in the text. Middle panel shows immunoblot analysis of the same samples with CDK2 antibody. Bottom panels show quantitation of the protein bands. Data are means ± se of 6–12 experiments. *P < 0.001 vs. control; **P < 0.001 vs. PDGF.
Resveratrol inhibits tyrosine phosphorylation of PDGFR
The signaling cascade activated by the PDGFR is initiated by tyrosine phosphorylation of the receptor itself and then several downstream proteins (5, 6). Tyrosine phosphorylated PDGFR serves as the docking site for signaling proteins such as c-Src, described above, activation of which is necessary for DNA synthesis (42). Therefore, we tested the hypothesis that resveratrol inhibits tyrosine phosphorylation of PDGFR. PDGF increased tyrosine phosphorylation of several proteins, including the PDGFR in mesangial cells (Fig. 5A). Resveratrol significantly inhibited PDGF-induced tyrosine phosphorylation (Fig. 5A, compare lanes 4 and 2). Binding of PDGF to PDGFR increases the intrinsic tyrosine kinase activity of the receptor, which is necessary for its biological function (4, 5). Immunocomplex kinase assays of PDGFR immunoprecipitates were performed from lysates of mesangial cells incubated with PDGF. PDGF increased tyrosine kinase activity of the PDGFR, as judged by autophosphorylation of the receptor (Fig. 5B). Preincubation of cells with resveratrol prior to treatment with PDGF inhibited PDGF-induced tyrosine kinase activity of the receptor (Fig. 5B, compare lanes 4 and 2). These results suggest a possibility that resveratrol may act as an inhibitor of PDGFR tyrosine kinase activity. PDGFR was immunoprecipitated from PDGF-treated mesangial cells and incubated with resveratrol for 1 h in vitro, before performing tyrosine kinase assay. Resveratrol did not inhibit PDGFR tyrosine kinase activity (Fig. 5C). These data conclusively demonstrate that resveratrol is not a PDGFR tyrosine kinase inhibitor. Furthermore, these results indicate that resveratrol does not directly act on PDGFR to modify its tyrosine kinase activity.
Figure 5.
Effect of resveratrol on PDGFR tyrosine phosphorylation. A, B) Mesangial cells were treated with resveratrol and PDGF as described in Fig. 3. Equal amounts of cell lysates were immunoblotted with antiphosphotyrosine and tubulin antibodies; arrow indicates tyrosine phosphorylated PDGFR (A). PDGFR immunoprecipitates were used in an in vitro immunocomplex tyrosine kinase assay in the presence of γ32P-ATP as described in the text; arrow indicates autophosphorylated PDGFR (B). C) PDGFR immunoprecipitates from PDGF-stimulated mesangial cells were incubated with 25 μM resveratrol for 1 h in the immunocomplex kinase assay buffer. Then tyrosine kinase assay was performed as described in the text. The arrow indicates the autophosphorylated PDGFR. Bottom panels show quantitation of PDGFR. Data are means ± se of 3–12 independent experiments. *P < 0.001 vs. control; **P < 0.01 vs. PDGF-stimulated.
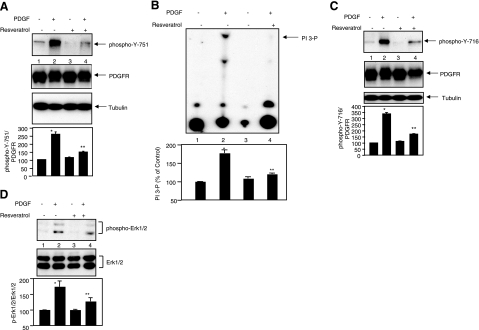
We have shown that PI 3 kinase activity is necessary for PDGF-induced mesangial cell proliferation (8). We have previously shown association of PI 3 kinase with PDGFR in mesangial cells (38). PDGF-induced activation of PI 3 kinase depends on its association with tyrosine phosphorylated PDGFR at tyrosine 751 (9). Resveratrol significantly inhibited PDGF-induced phosphorylation of PDGFR at tyrosine 751 (Fig. 6A, compare lanes 4 and 2). Because tyrosine 751 is involved in association and activation of PI 3 kinase, we examined the lipid kinase activity of the latter. Resveratrol significantly reduced PI 3 kinase activity in response to PDGF (Fig. 6B, compare lanes 4 and 2). PI 3 kinase regulates PDGF-stimulated Akt activity; therefore, these results explain the observation that Akt activation is inhibited by resveratrol (Fig. 3B).
Figure 6.
Effect of resveratrol on PDGF-induced PI 3 kinase and Erk1/2 activation. Mesangial cells were treated with resveratrol and PDGF as described in Fig. 3. Equal amounts of lysates were immunoblotted with indicated antibodies (A, C, D). PDGFR immunoprecipitates were assayed for PI 3 kinase activity as described in Materials and Methods; arrow indicates PI 3-P (B). Bottom panels show quantitation of the protein bands and the intensity of the PI 3-P signals, respectively. Data are means ± se of 6–12 experiments. *P < 0.001 vs. control; **P < 0.001 vs. PDGF (A, B, C); **P < 0.05 vs. PDGF (D).
Stimulation of the PDGFR results in activation of Erk1/2 MAPK. We have previously shown that MAPK partially regulates PDGF-induced DNA synthesis (37). Activation of MAPK is initiated by a cascade of events initiated by Grb-2-SOS complex in the plasma membrane. SOS exchanges GTP for GDP in Ras, which, in turn, initiates the kinase cascade to stimulate MAPK phosphorylation and activity. Previously, Grb-2 was shown to bind phosphorylated tyrosine-716 of the PDGFR and recruit the Grb-2-SOS complex to the plasma membrane (4). Therefore, we examined the phosphorylation of tyrosine-716 in the PDGFR. PDGF increased phosphorylation of tyrosine-716 (Fig. 6C), which was inhibited by resveratrol (Fig. 6C, compare lanes 4 and 2). Next, we tested activation of MAPK. Resveratrol partially inhibited PDGF-induced activating phosphorylation of Erk1/2 MAPK (Fig. 6D, compare lanes 4 and 2). These results for the first time demonstrate that resveratrol specifically inhibits phosphorylation of PDGFR at specific sites, resulting in attenuation of two downstream targets PI 3 kinase and MAPK, both of which are necessary for PDGF-induced DNA synthesis (37).
Resveratrol stimulates protein tyrosine phosphatase PTP1B activity in mesangial cells
We have shown that resveratrol inhibits tyrosine phosphorylation of PDGFR at select sites without having any effect on its intrinsic tyrosine kinase activity (Fig. 5). To investigate the mechanism, we considered dephosphorylation of PDGFR. Receptor tyrosine phosphorylation is known to be regulated by protein tyrosine phosphatases (61, 62). We have previously shown abundant expression of the cytoplasmic tyrosine phosphatase PTP1B in mesangial cells (50). PTP1B has been shown to dephosphorylate tyrosine phosphorylated receptor tyrosine kinases, resulting in attenuation of downstream signal transduction (61, 62). We examined whether PTP1B regulated the effect of resveratrol. Incubation of mesangial cells with resveratrol significantly increased PTP1B enzymatic activity (Fig. 7A). PDGF alone or in combination with resveratrol did not increase PTP1B activity (Fig. 7A). To test whether resveratrol directly activates PTP1B activity, we immunoprecipitated PTP1B from lysates of mesangial cells. Immunocomplexes were incubated with resveratrol for 1 h, and phosphatase assays were performed. Resveratrol did not have any effect on PTP1B activity in vitro (Fig. 7B). These results suggest that resveratrol does not directly modulate the PTP1B activity. The intracellular signaling induced by resveratrol may regulate PTP1B activity.
Figure 7.
Effect of resveratrol on PTP1B activity. A) Mesangial cells were treated with resveratrol followed by incubation with PDGF as described in Fig. 3. PTP1B immunoprecipitates from the cell lysates were used for enzymatic activity as described in Materials and Methods. Data are means ± se of 6 measurements. *P < 0.001 vs. control. B) PTB1B immunoprecipitates from the lysates of mesangial cells were incubated with resveratrol for 1 h prior to assay for PTP1B activity in vitro. Data are means ± se of 7 measurements. *P < 0.01 vs. control. C) Nonimmune serum (IgG) or PTP1B immunoprecipitates were incubated with radiolabeled autophosphorylated PDGFR. The product was separated by SDS gel electrophoresis followed by autoradiography, as described in text. A representative experiment of 6 independent experiments is shown. Right histogram represents quantitation of the PDGFR band. Data are means ± se of 6 experiments. *P < 0.001 vs. control.
To determine whether PDGFR is a substrate for PTP1B, we immunoprecipitated PDGFR from PDGF-stimulated mesangial cells and labeled it with γ32P-ATP in an autophosphorylation assay. This autophosphorylated PDGFR was incubated with PTP1B immunoprecipitates. PTP1B significantly dephosphorylated the PDGFR (Fig. 7C). These data show that autophosphorylated PDGFR is a substrate for PTP1B. To confirm this observation in vivo with more specificity, we used an adenovirus vector expressing PTP1B (Ad PTP1B) (Fig. 8A). Mesangial cells were infected with Ad PTP1B prior to incubation with PDGF. The cell lysates were immunoblotted with antibody that recognizes phospho-Tyr-751 PDGFR. Expression of PTP1B significantly inhibited tyrosine-751 phosphorylation (Fig. 8B, compare lanes 4 and 2). Similarly, we examined the effect of PTP1B on tyrosine-716 phosphorylation. PTP1B blocked PDGF-induced tyrosine-716 phosphorylation of the PDGFR (Fig. 8C, compare lanes 4 and 2). Since tyrosine-751 in the receptor regulates PI 3 kinase activity, we investigated this signaling pathway by measuring activating phosphorylation of Akt, a downstream target of the lipid kinase. Expression of PTP1B significantly reduced PDGF-stimulated phosphorylation of Akt (Fig. 9A, compare lanes 4 and 2). PDGFR tyrosine-716 phosphorylation regulates the activation of MAPK. Expression of PTP1B inhibited PDGF-stimulated MAPK phosphorylation (Fig. 9B, compare lanes 4 and 2). Together, these data indicate that resveratrol-induced increased PTP1B activity may dephosphorylate activated PDGFR at tyrosine-716 and -751 to inhibit downstream mitogenic signaling.
Figure 8.
Effect of PTP1B on PDGFR tyrosine phosphorylation. A) Mesangial cells were infected with 50 moi of Ad PTP1B for the indicated periods of time. Cell lysates were immunoblotted with PTP1B and tubulin antibodies. A longer exposure of the blot revealed the endogenous level of PTP1B in lane 1 (not shown). B, C) Mesangial cells were infected with 50 moi of Ad PTP1B for 24 h followed by incubation with PDGF for 5 min. The cell lysates were immunoblotted with PDGFR phospho-tyrosine-751 (B) and PDGFR phospho-tyrosine-716 (C) and the indicated antibodies. A representative experiment of 6 independent experiments is shown. Bottom panels in B and C show quantitation as the means ± se. *P < 0.001 vs. control; **P < 0.001 vs. PDGF.
Figure 9.
Effect of PTP1B on PDGF-induced phosphorylation of Akt and Erk1/2. Mesangial cells were infected with Ad PTP1B followed by PDGF, as described in Fig. 8. The cell lysates were immunoblotted with indicated antibodies. Bottom panels show quantitation ± se of 6 independent experiments. *P < 0.001 vs. control; **P < 0.001 vs. PDGF (A); **P < 0.05 vs. PDGF (B).
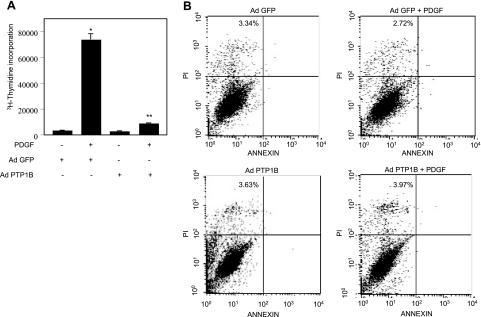
We examined this possibility using the Ad PTP1B. Mesangial cells were infected with Ad PTP1B prior to incubation with PDGF and DNA synthesis was determined by 3H-thymidine incorporation. PTP1B significantly inhibited PDGF-stimulated DNA synthesis (Fig. 10A). A decrease in thymidine incorporation may arise from apoptosis of mesangial cells after PTP1B expression; therefore, apoptotic cells were assessed by annexin V staining. More than 96% cells did not bind annexin V (Fig. 10B). These data indicate that PTP1B expression did not induce apoptosis of mesangial cells.
Figure 10.
Effect of PTP1B on PDGF-induced DNA synthesis. A) Mesangial cells were infected with 50 moi of Ad PTP1B for 24 h. The cells were incubated with PDGF as described in Fig. 1. DNA synthesis was determined as described in Materials and Methods. Data are means ± se of 12 measurements. *P < 0.001 vs. control; **P < 0.001 vs. PDGF. B) Mesangial cells were treated as described in A. Apoptosis was measured using a detection kit as described in text. Numbers in each quadrant indicate the percentage of apoptotic and necrotic cells.
DISCUSSION
In the present study, we provide evidence that resveratrol inhibits PDGF-induced DNA synthesis in the absence of induction of apoptosis. We show that resveratrol-stimulated SIRT1 activity is not necessary for this inhibitory activity. Furthermore, we demonstrate that resveratrol induces PDGFR dephosphorylation at specific residues, which regulate PI 3 kinase activation that is necessary for PDGF-induced DNA synthesis. As a mechanism, we provide the first evidence that resveratrol increases the tyrosine phosphatase PTP1B activity, which targets autophosphorylated PDGFR to inhibit the mitogenic signaling.
Mesangial cell proliferation is a principle feature of a number of renal disorders. The identification of naturally occurring compounds, such as resveratrol, may open new avenues to treat these diseases. Resveratrol has been extensively studied as a potent inhibitor of tumor cell growth and has strong proapoptotic effects (24, 31, 33, 34). The mechanism of action includes activation of p53; increased nitric oxide, cAMP and ceramide production; and down-regulation of various proliferative and antiapoptotic gene products. Resveratrol has been shown to inhibit growth of tumor cells without having any effect on normal fibroblast and prostate epithelial cell growth (63, 64). In contrast to these results in the present study, we observed significant inhibition of DNA synthesis in normal mesangial cells (Fig. 1). Also, in contrast to previous reports, we could not implicate apoptosis in resveratrol-induced growth inhibition (24, 31, 33, 34). Furthermore, resveratrol has been shown to exert a mild proliferative effect at low concentration (5–10 μM) (53, 65). However, in contrast to these results, we observed significant inhibition of PDGF-induced DNA synthesis with 10 μΜ resveratrol concentration (Fig. 1).
Many biological effects of resveratrol have been attributed to increased deacetylase activity of SIRT1. SIRT1 was originally described as a factor regulating longevity, apoptosis, and DNA repair (66, 67). SIRT1 deacetylates many transcription factors and effector proteins, resulting in both positive and negative regulatory functions (36, 68,69,70,71,72,73). We explored the possibility that resveratrol-mediated inhibition of PDGF-induced DNA synthesis may result from increased SIRT1 activity. In fact, we detected increased SIRT1 activity in the presence of resveratrol in mesangial cells (Fig. 2A). However, SIRT1 inhibitor nicotinamide, as well as expression of dominant negative SIRT1, did not have any effect on PDGF-stimulated DNA synthesis (Figs. 2B, D). These results conclusively demonstrate a SIRT1-independent effect of resveratrol on PDGF mitogenic signaling (25, 74).
We have previously established that c-Src and Akt-regulate PDGF-induced DNA synthesis in mesangial cells (37, 42). Thus, our results showing inhibition of phosphorylation of c-Src and Akt by resveratrol further support our previous observation. Activation of cyclin D1, cyclin E, and their dependent kinases CDK4 and CDK2 is necessary for cells to pass from early through late phase of G1 and finally to S-phase (60). In fact, increased phosphorylation of pRb by CDK4 at serine 809/811 facilitates cell cycle progression. Our results show that resveratrol inhibits increased expression of cyclin D1 and the associated increased phosphorylation of pRb in response to PDGF (Fig. 4A, B). Recent experiments, where cyclin D1 deficiency was functionally rescued by knocking in cyclin E, suggested that CDK2 activity is essential for cell cycle progression (75). We demonstrate that PDGF-induced CDK2 activity is significantly attenuated by resveratrol (Fig. 4C). These results indicate that resveratrol targets cell cycle machinery to inhibit PDGF-induced DNA synthesis.
How resveratrol mediates its effect on the cell cycle machinery is not clearly understood. We have shown that PI 3 kinase/Akt and Erk1/2 MAPK play a role in PDGF-induced cell cycle regulation (37, 42, 51). Both of these kinase cascades have been reported to become targets of resveratrol for its inhibitory activity. For example, to inhibit growth of breast tumor or osteosarcoma cells, resveratrol increased the MAPK activity (76, 77). On the other hand, resveratrol inhibited MAPK in epidermal carcinoma, cardiac fibroblast, and bovine endothelial cells to elicit its growth inhibitory effect (78,79,80). In support of the latter observation, our results in mesangial cells demonstrate a modest inhibition of PDGF-induced MAPK activity by resveratrol (Fig. 6D). Because activation of this kinase depends on tyrosine phosphorylation of PDGFR, this inhibitory effect may result from dephosphorylation of PDGFR tyrosine kinase. In fact, we detected significant dephosphorylation of tyrosine-716, which recruits Grb-2-SOS complex to initiate the MAPK activation (4). However, it was reported that mutation of this residue did not have any effect on MAPK activation (81). These data indicate that resveratrol-mediated modest inhibition of MAPK in mesangial cell may result from inhibition of PDGFR-mediated signaling other than tyrosine-716 of the receptor, e.g., tyrosine-751, which regulates PDGF-induced PI 3 kinase activity. In fact, we have demonstrated previously that PI 3 kinase partially regulates PDGF-induced MAPK activity in mesangial cells (8).
Both positive and negative regulatory actions of resveratrol on PI 3 kinase activation have been reported. In T47D breast tumor cells, PI 3 kinase inhibition abolished the inhibitory action of resveratrol (76). Also, in MCF7 breast cancer cells, resveratrol (10 μM) increased PI 3 kinase activity associated with estrogen receptor α (ERα) (82). At concentrations higher than 50 μM, resveratrol inhibited the ERα-associated PI 3 kinase activity, not by a direct action on PI 3 kinase but through proteasome-mediated degradation of ERα (82). In contrast, resveratrol has been shown to inhibit PI 3 kinase activity in EGF and insulin signal transduction pathways (24, 83). However, contradictory results have been reported about direct inhibition of PI 3 kinase by resveratrol (30, 83). In mesangial cells, we observed significant inhibition of PDGFR tyrosine 751 phosphorylation and associated PI 3 kinase activity (Fig. 6A, B). These results show an indirect effect of resveratrol on the PDGFR signal transduction.
Inhibition of PI 3 kinase can be achieved via phosphatases. Largely, on the basis of overexpression studies, multiple protein tyrosine phosphatases have been implicated in dephosphorylation of receptor tyrosine kinases (61, 62). PTP1B, a cytosolic, ubiquitously expressed protein tyrosine phosphatase, has been shown to dephosphorylate the tyrosine kinases JAK2 and TYK2 to inhibit cytokine signal transduction (62, 84). However, PTP1B null mouse displayed tissue-specific insulin sensitivity and resistance to diet-induced diabetes and obesity (85, 86). Similar observation was reported in resveratrol-fed mice. At a high dose, resveratrol inhibited obesity-induced insulin resistance possibly by increased SIRT1 activity (87). Also, administration of a low dose of resveratrol to high-fat fed mice has been shown to increase insulin sensitivity (28). This effect of resveratrol was thought to be independent of its effect on SIRT1. Our results show that very low dose of resveratrol was sufficient to inhibit PDGF-induced DNA synthesis in mesangial cells, although this dose was capable of activating SIRT1 (Fig. 2).
Resveratrol-fed mice and PTP1B null mice show similar phenotypes with improved insulin sensitivity. A recent significant report demonstrated that a low dose of resveratrol reduced expression of PTP1B via increased SIRT1 activity, thus providing a mechanism of insulin sensitivity by insulin receptor tyrosine kinase (61, 62, 88, 89). In contrast to these results, our data show a significant increase in PTP1B activity in the presence of resveratrol (Fig. 7). PTP1B-deficient mice did not show any defect in PDGFR signaling (85, 86); however, substrate trap mutant of PTP1B physically interacted with overexpressed PDGFR (90). Although fibroblasts isolated from PTP1B null mice did not show increased basal phosphorylation of PDGFR, PDGF stimulated a sustained increase in PDGFR receptor phosphorylation (91). However, no increase in Akt activation was observed in PTP1B null fibroblasts. In addition, overexpression of PTP1B was reported to inhibit PDGFR phosphorylation in smooth muscle cells (92). On the other hand, in 3T3 L1, myocytes, and hepatoma cells, expression of PTP1B did not have any effect on PDGFR tyrosine phosphorylation (93, 94). These results suggest both positive and negative regulatory role of PTP1B in PDGFR signaling. Our results, for the first time, demonstrate an increase in PTP1B activity in mesangial cells in response to resveratrol (Fig. 7A). Furthermore, immunopurified PTP1B from mesangial cells significantly dephosphorylated tyrosine phosphorylated PDGFR (Fig. 7C). These data indicate that PTP1B may cell-specifically regulate PDGF signal transduction by directly dephosphorylating PDGFR. This notion was also supported by our results, showing specific dephosphorylation of tyrosine-751 and tyrosine-716 of the PDGF-stimulated PDGFR (Fig. 8). Furthermore, expression of PTP1B inhibited PDGF-induced phosphorylation of Akt and Erk1/2 (Fig. 9). We should emphasize that dephosphorylation of Erk1/2 was partial, similar to the observation we obtained with resveratrol (Fig. 6D). The inhibition of Akt activation resulted in attenuation of PDGF-induced DNA synthesis in the absence of any detectable apoptosis (Fig. 10). These data support our previous results, demonstrating an essential requirement of Akt kinase in PDGF-induced DNA synthesis (37).
The precise effect of resveratrol on various mesangial cell functions remains to be explored. Our results provide the first evidence that resveratrol increases the cytosolic PTP1B activity, which dephosphorylates activated PDGFR, thus attenuating downstream kinase cascades including PI 3 kinase/Akt and Erk1/2 MAPK, leading to inhibition of DNA synthesis in mesangial cells. PDGF-dependent activation of PDGFR elicits the pathological manifestation of mesangioproliferative glomerulonephritis because anti-PDGF antibody, as well as inactivation of PDGFR ameliorates the disease progression in experimental models (14, 16, 19). The relatively nontoxic small molecular compound resveratrol, which targets PTP1B to inhibit activation of PDGFR, may represent an effective therapy for proliferative glomerular disorders.
Acknowledgments
We thank Dr. Brent Wagner for critically reading the manuscript. This work was supported by National Institutes of Health (NIH) grant RO1 DK 50190, the Juvenile Diabetes Research Foundation, and a VA Merit Review grant to G.G.C., who is a research career scientist in the Department of Veterans Affairs. N.G.C. is supported by NIH RO1 AR52425 and VA Merit Review and Morrison Trust grants. A.K. is a recipient of a VA VISN grant. B.S.K. is supported by grants from the NIH O’Brien Kidney Center, NIH RO1 DK 077295, the American Diabetes Association, and a VA Research Service Merit Review grant. H.E.A. is supported by NIH RO1 DK 33665 and VA Merit Review grants.
References
- Heldin C H, Eriksson U, Ostman A. New members of the platelet-derived growth factor family of mitogens. Arch Biochem Biophys. 2002;398:284–290. doi: 10.1006/abbi.2001.2707. [DOI] [PubMed] [Google Scholar]
- Fredriksson L, Li H, Eriksson U. The PDGF family: four gene products form five dimeric isoforms. Cytokine Growth Factor Rev. 2004;15:197–204. doi: 10.1016/j.cytogfr.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Hoch R V, Soriano P. Roles of PDGF in animal development. Development. 2003;130:4769–4784. doi: 10.1242/dev.00721. [DOI] [PubMed] [Google Scholar]
- Heldin C H, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- Chiara F, Bishayee S, Heldin C H, Demoulin J B. Autoinhibition of the platelet-derived growth factor beta-receptor tyrosine kinase by its C-terminal tail. J Biol Chem. 2004;279:19732–19738. doi: 10.1074/jbc.M314070200. [DOI] [PubMed] [Google Scholar]
- Tallquist M, Kazlauskas A. PDGF signaling in cells and mice. Cytokine Growth Factor Rev. 2004;15:205–213. doi: 10.1016/j.cytogfr.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Choudhury G G, Karamitsos C, Hernandez J, Gentilini A, Bardgette J, Abboud H E. PI-3-kinase and MAPK regulate mesangial cell proliferation and migration in response to PDGF. Am J Physiol Renal Physiol. 1997;273:F931–F938. doi: 10.1152/ajprenal.1997.273.6.F931. [DOI] [PubMed] [Google Scholar]
- Valius M, Kazlauskas A. Phospholipase C-gamma 1 and phosphatidylinositol 3 kinase are the downstream mediators of the PDGF receptor’s mitogenic signal. Cell. 1993;73:321–334. doi: 10.1016/0092-8674(93)90232-f. [DOI] [PubMed] [Google Scholar]
- Choudhury G G, Grandaliano G, Jin D C, Katz M S, Abboud H E. Activation of PLC and PI 3 kinase by PDGF receptor alpha is not sufficient for mitogenesis and migration in mesangial cells. Kidney Int. 2000;57:908–917. doi: 10.1046/j.1523-1755.2000.00907.x. [DOI] [PubMed] [Google Scholar]
- Abboud H E. Role of platelet-derived growth factor in renal injury. Annu Rev Physiol. 1995;57:297–309. doi: 10.1146/annurev.ph.57.030195.001501. [DOI] [PubMed] [Google Scholar]
- Jefferson J A, Johnson R J. Experimental mesangial proliferative glomerulonephritis (the anti-Thy-1.1 model) J Nephrol. 1999;12:297–307. [PubMed] [Google Scholar]
- Floege J, Eng E, Young B A, Alpers C E, Barrett T B, Bowen-Pope D F, Johnson R J. Infusion of platelet-derived growth factor or basic fibroblast growth factor induces selective glomerular mesangial cell proliferation and matrix accumulation in rats. J Clin Invest. 1993;92:2952–2962. doi: 10.1172/JCI116918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floege J, Ostendorf T, Janssen U, Burg M, Radeke H H, Vargeese C, Gill S C, Green L S, Janjic N. Novel approach to specific growth factor inhibition in vivo: antagonism of platelet-derived growth factor in glomerulonephritis by aptamers. Am J Pathol. 1999;154:169–179. doi: 10.1016/S0002-9440(10)65263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert R E, Kelly D J, McKay T, Chadban S, Hill P A, Cooper M E, Atkins R C, Nikolic-Paterson D J. PDGF signal transduction inhibition ameliorates experimental mesangial proliferative glomerulonephritis. Kidney Int. 2001;59:1324–1332. doi: 10.1046/j.1523-1755.2001.0590041324.x. [DOI] [PubMed] [Google Scholar]
- Johnson R J, Raines E W, Floege J, Yoshimura A, Pritzl P, Alpers C, Ross R. Inhibition of mesangial cell proliferation and matrix expansion in glomerulonephritis in the rat by antibody to platelet-derived growth factor. J Exp Med. 1992;175:1413–1416. doi: 10.1084/jem.175.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz P J, DiCorleto P E, Silver B J, Abboud H E. Mesangial cells express PDGF mRNAs and proliferate in response to PDGF. Am J Physiol Renal Physiol. 1988;255:F674–F684. doi: 10.1152/ajprenal.1988.255.4.F674. [DOI] [PubMed] [Google Scholar]
- Silver B J, Jaffer F E, Abboud H E. Platelet-derived growth factor synthesis in mesangial cells: induction by multiple peptide mitogens. Proc Natl Acad Sci U S A. 1989;86:1056–1060. doi: 10.1073/pnas.86.3.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Isaka Y, Tsujie M, Akagi Y, Sudo T, Ohno N, Imai E, Hori M. Electroporation-mediated PDGF receptor-IgG chimera gene transfer ameliorates experimental glomerulonephritis. Kidney Int. 2001;59:2134–2145. doi: 10.1046/j.1523-1755.2001.00728.x. [DOI] [PubMed] [Google Scholar]
- Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8:1875–1887. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- Lindahl P, Hellstrom M, Kalen M, Karlsson L, Pekny M, Pekna M, Soriano P, Betsholtz C. Paracrine PDGF-B/PDGF-Rbeta signaling controls mesangial cell development in kidney glomeruli. Development. 1998;125:3313–3322. doi: 10.1242/dev.125.17.3313. [DOI] [PubMed] [Google Scholar]
- Soriano P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev. 1994;8:1888–1896. doi: 10.1101/gad.8.16.1888. [DOI] [PubMed] [Google Scholar]
- De la Lastra C A, Villegas I. Resveratrol as an anti-inflammatory and anti-aging agent: mechanisms and clinical implications. Mol Nutr Food Res. 2005;49:405–430. doi: 10.1002/mnfr.200500022. [DOI] [PubMed] [Google Scholar]
- Fulda S, Debatin K M. Resveratrol modulation of signal transduction in apoptosis and cell survival: a mini-review. Cancer Detect Prev. 2006;30:217–223. doi: 10.1016/j.cdp.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, Rabinovitch P S. Medicine: grapes versus gluttony. Nature. 2006;444:280–281. doi: 10.1038/nature05308. [DOI] [PubMed] [Google Scholar]
- Howitz K T, Bitterman K J, Cohen H Y, Lamming D W, Lavu S, Wood J G, Zipkin R E, Chung P, Kisielewski A, Zhang L L, Scherer B, Sinclair D A. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Wood J G, Rogina B, Lavu S, Howitz K, Helfand S L, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Baur J A, Pearson K J, Price N L, Jamieson H A, Lerin C, Kalra A, Prabhu V V, Allard J S, Lopez-Lluch G, Lewis K, Pistell P J, Poosala S, Becker K G, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein K W, Spencer R G, Lakatta E G, Le Couteur D, Shaw R J, Navas P, Puigserver P, Ingram D K, de Cabo R, Sinclair D A. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang M, Xu S, Maitland-Toolan K A, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren T J, Cohen R A. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55:2180–2191. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- Godichaud S, Si-Tayeb K, Auge N, Desmouliere A, Balabaud C, Payrastre B, Negre-Salvayre A, Rosenbaum J. The grape-derived polyphenol resveratrol differentially affects epidermal and platelet-derived growth factor signaling in human liver myofibroblasts. Int J Biochem Cell Biol. 2006;38:629–637. doi: 10.1016/j.biocel.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Ahmad N, Adhami V M, Afaq F, Feyes D K, Mukhtar H. Resveratrol causes WAF-1/p21-mediated G(1)-phase arrest of cell cycle and induction of apoptosis in human epidermoid carcinoma A431 cells. Clin Cancer Res. 2001;7:1466–1473. [PubMed] [Google Scholar]
- Banerjee S, Bueso-Ramos C, Aggarwal B B. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: role of nuclear factor-κB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 2002;62:4945–4954. [PubMed] [Google Scholar]
- Dorrie J, Gerauer H, Wachter Y, Zunino S J. Resveratrol induces extensive apoptosis by depolarizing mitochondrial membranes and activating caspase-9 in acute lymphoblastic leukemia cells. Cancer Res. 2001;61:4731–4739. [PubMed] [Google Scholar]
- Jang M, Cai L, Udeani G O, Slowing K V, Thomas C F, Beecher C W, Fong H H, Farnsworth N R, Kinghorn A D, Mehta R G, Moon R C, Pezzuto J M. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- Mgbonyebi O P, Russo J, Russo I H. Antiproliferative effect of synthetic resveratrol on human breast epithelial cells. Int J Oncol. 1998;12:865–869. [PubMed] [Google Scholar]
- Brunet A, Sweeney L B, Sturgill J F, Chua K F, Greer P L, Lin Y, Tran H, Ross S E, Mostoslavsky R, Cohen H Y, Hu L S, Cheng H L, Jedrychowski M P, Gygi S P, Sinclair D A, Alt F W, Greenberg M E. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Choudhury G G. Akt serine threonine kinase regulates platelet-derived growth factor-induced DNA synthesis in glomerular mesangial cells: regulation of c-fos AND p27(kip1) gene expression. J Biol Chem. 2001;276:35636–35643. doi: 10.1074/jbc.M100946200. [DOI] [PubMed] [Google Scholar]
- Choudhury G G, Biswas P, Grandaliano G, Fouqueray B, Harvey S A, Abboud H E. PDGF-mediated activation of phosphatidylinositol 3 kinase in human mesangial cells. Kidney Int. 1994;46:37–47. doi: 10.1038/ki.1994.242. [DOI] [PubMed] [Google Scholar]
- Venkatesan B, Mahimainathan L, Das F, Ghosh-Choudhury N, Ghosh Choudhury G. Downregulation of catalase by reactive oxygen species via PI 3 kinase/Akt signaling in mesangial cells. J Cell Physiol. 2007;211:457–467. doi: 10.1002/jcp.20953. [DOI] [PubMed] [Google Scholar]
- Venkatesan B A, Mahimainathan L, Ghosh-Choudhury N, Gorin Y, Bhandari B, Valente A J, Abboud H E, Choudhury G G. PI 3 kinase-dependent Akt kinase and PKCepsilon independently regulate interferon-γ-induced STAT1alpha serine phosphorylation to induce monocyte chemotactic protein-1 expression. Cell Signal. 2006;18:508–518. doi: 10.1016/j.cellsig.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Das F, Ghosh-Choudhury N, Mahimainathan L, Venkatesan B, Feliers D, Riley D J, Kasinath B S, Choudhury G G. Raptor-rictor axis in TGFβ-induced protein synthesis. Cell Signal. 2008;20:409–423. doi: 10.1016/j.cellsig.2007.10.027. [DOI] [PubMed] [Google Scholar]
- Choudhury G G, Mahimainathan L, Das F, Venkatesan B, Ghosh-Choudhury N. c-Src couples PI 3 kinase/Akt and MAPK signaling to PDGF-induced DNA synthesis in mesangial cells. Cell Signal. 2006;18:1854–1864. doi: 10.1016/j.cellsig.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Mahimainathan L, Ghosh-Choudhury N, Venkatesan B A, Danda R S, Choudhury G G. EGF stimulates mesangial cell mitogenesis via PI3-kinase-mediated MAPK-dependent and AKT kinase-independent manner: involvement of c-fos and p27Kip1. Am J Physiol Renal Physiol. 2005;289:F72–F82. doi: 10.1152/ajprenal.00277.2004. [DOI] [PubMed] [Google Scholar]
- Das F, Mahimainathan L, Ghosh-Choudhury N, Venkatesan B, Kasinath B S, Abboud H E, Ghosh Choudhury G. TGFβ intercepts nuclear glycogen synthase kinase 3β to inhibit PDGF-induced DNA synthesis in mesangial cells. FEBS Lett. 2007;581:5259–5267. doi: 10.1016/j.febslet.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Ghosh Choudhury G, Lenin M, Calhaun C, Zhang J H, Abboud H E. PDGF inactivates forkhead family transcription factor by activation of Akt in glomerular mesangial cells. Cell Signal. 2003;15:161–170. doi: 10.1016/s0898-6568(02)00057-8. [DOI] [PubMed] [Google Scholar]
- De Boer V C, de Goffau M C, Arts I C, Hollman P C, Keijer J. SIRT1 stimulation by polyphenols is affected by their stability and metabolism. Mech Ageing Dev. 2006;127:618–627. doi: 10.1016/j.mad.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Choudhury G G, Ghosh-Choudhury N, Abboud H E. Association and direct activation of signal transducer and activator of transcription1α by platelet-derived growth factor receptor. J Clin Invest. 1998;101:2751–2760. doi: 10.1172/JCI1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury G G, Wang L M, Pierce J, Harvey S A, Sakaguchi A Y. A mutational analysis of phosphatidylinositol-3-kinase activation by human colony-stimulating factor-1 receptor. J Biol Chem. 1991;266:8068–8072. [PubMed] [Google Scholar]
- Wenzel U O, Fouqueray B, Biswas P, Grandaliano G, Choudhury G G, Abboud H E. Activation of mesangial cells by the phosphatase inhibitor vanadate. Potential implications for diabetic nephropathy. J Clin Invest. 1995;95:1244–1252. doi: 10.1172/JCI117774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyomoto H, Fouqueray B, Abboud H E, Choudhury G G. Phorbol 12-myristate 13-acetic acid inhibits PTP1B activity in human mesangial cells. A possible mechanism of enhanced tyrosine phosphorylation. FEBS Lett. 1994;353:217–220. doi: 10.1016/0014-5793(94)01039-0. [DOI] [PubMed] [Google Scholar]
- Ghosh Choudhury G, Kim Y S, Simon M, Wozney J, Harris S, Ghosh-Choudhury N, Abboud H E. Bone morphogenetic protein 2 inhibits platelet-derived growth factor-induced c-fos gene transcription and DNA synthesis in mesangial cells. Involvement of mitogen-activated protein kinase. J Biol Chem. 1999;274:10897–10902. doi: 10.1074/jbc.274.16.10897. [DOI] [PubMed] [Google Scholar]
- Rahman I, Biswas S K, Kirkham P A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol. 2006;72:1439–1452. doi: 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Ahmad K A, Harris N H, Johnson A D, Lindvall H C, Wang G, Ahmed K. Protein kinase CK2 modulates apoptosis induced by resveratrol and epigallocatechin-3-gallate in prostate cancer cells. Mol Cancer Ther. 2007;6:1006–1012. doi: 10.1158/1535-7163.MCT-06-0491. [DOI] [PubMed] [Google Scholar]
- Koopman G, Reutelingsperger C P, Kuijten G A, Keehnen R M, Pals S T, van Oers M H. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–1420. [PubMed] [Google Scholar]
- Borra M T, Smith B C, Denu J M. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- Leibiger I B, Berggren P O. Sirt1: a metabolic master switch that modulates lifespan. Nat Med. 2006;12:34–36; discussion 36. doi: 10.1038/nm0106-34. [DOI] [PubMed] [Google Scholar]
- Bitterman K J, Anderson R M, Cohen H Y, Latorre-Esteves M, Sinclair D A. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J Biol Chem. 2002;277:45099–45107. doi: 10.1074/jbc.M205670200. [DOI] [PubMed] [Google Scholar]
- Grozinger C M, Chao E D, Blackwell H E, Moazed D, Schreiber S L. Identification of a class of small molecule inhibitors of the sirtuin family of NAD-dependent deacetylases by phenotypic screening. J Biol Chem. 2001;276:38837–38843. doi: 10.1074/jbc.M106779200. [DOI] [PubMed] [Google Scholar]
- Paternot S, Arsenijevic T, Coulonval K, Bockstaele L, Dumont J E, Roger P P. Distinct specificities of pRb phosphorylation by CDK4 activated by cyclin D1 or cyclin D3: differential involvement in the distinct mitogenic modes of thyroid epithelial cells. Cell Cycle. 2006;5:61–70. doi: 10.4161/cc.5.1.2265. [DOI] [PubMed] [Google Scholar]
- Sherr C J, Roberts J M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Dube N, Tremblay M L. Involvement of the small protein tyrosine phosphatases TC-PTP and PTP1B in signal transduction and diseases: from diabetes, obesity to cell cycle, and cancer. Biochim Biophys Acta. 2005;1754:108–117. doi: 10.1016/j.bbapap.2005.07.030. [DOI] [PubMed] [Google Scholar]
- Tonks N K. PTP1B: from the sidelines to the front lines! FEBS Lett. 2003;546:140–148. doi: 10.1016/s0014-5793(03)00603-3. [DOI] [PubMed] [Google Scholar]
- Aziz M H, Nihal M, Fu V X, Jarrard D F, Ahmad N. Resveratrol-caused apoptosis of human prostate carcinoma LNCaP cells is mediated via modulation of phosphatidylinositol 3′-kinase/Akt pathway and Bcl-2 family proteins. Mol Cancer Ther. 2006;5:1335–1341. doi: 10.1158/1535-7163.MCT-05-0526. [DOI] [PubMed] [Google Scholar]
- Zhang W, Fei Z, Zhen H N, Zhang J N, Zhang X. Resveratrol inhibits cell growth and induces apoptosis of rat C6 glioma cells. J Neurooncol. 2007;81:231–240. doi: 10.1007/s11060-006-9226-x. [DOI] [PubMed] [Google Scholar]
- Kuwajerwala N, Cifuentes E, Gautam S, Menon M, Barrack E R, Reddy G P. Resveratrol induces prostate cancer cell entry into S phase and inhibits DNA synthesis. Cancer Res. 2002;62:2488–2492. [PubMed] [Google Scholar]
- Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- Sinclair D A. Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev. 2005;126:987–1002. doi: 10.1016/j.mad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Cohen H Y, Miller C, Bitterman K J, Wall N R, Hekking B, Kessler B, Howitz K T, Gorospe M, de Cabo R, Sinclair D A. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- Fu M, Liu M, Sauve A A, Jiao X, Zhang X, Wu X, Powell M J, Yang T, Gu W, Avantaggiati M L, Pattabiraman N, Pestell T G, Wang F, Quong A A, Wang C, Pestell R G. Hormonal control of androgen receptor function through SIRT1. Mol Cell Biol. 2006;26:8122–8135. doi: 10.1128/MCB.00289-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta M C, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- Rodgers J T, Lerin C, Haas W, Gygi S P, Spiegelman B M, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Yang Y, Fu W, Chen J, Olashaw N, Zhang X, Nicosia S V, Bhalla K, Bai W. SIRT1 sumoylation regulates its deacetylase activity and cellular response to genotoxic stress. Nat Cell Biol. 2007;9:1253–1262. doi: 10.1038/ncb1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung F, Hoberg J E, Ramsey C S, Keller M D, Jones D R, Frye R A, Mayo M W. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Kennedy B K. Does resveratrol activate yeast Sir2 in vivo? Aging Cell. 2007;6:415–416. doi: 10.1111/j.1474-9726.2007.00314.x. [DOI] [PubMed] [Google Scholar]
- Geng Y, Whoriskey W, Park M Y, Bronson R T, Medema R H, Li T, Weinberg R A, Sicinski P. Rescue of cyclin D1 deficiency by knockin cyclin E. Cell. 1999;97:767–777. doi: 10.1016/s0092-8674(00)80788-6. [DOI] [PubMed] [Google Scholar]
- Alkhalaf M. Resveratrol-induced apoptosis is associated with activation of p53 and inhibition of protein translation in T47D human breast cancer cells. Pharmacology. 2007;80:134–143. doi: 10.1159/000103253. [DOI] [PubMed] [Google Scholar]
- Alkhalaf M, Jaffal S. Potent antiproliferative effects of resveratrol on human osteosarcoma SJSA1 cells: Novel cellular mechanisms involving the ERKs/p53 cascade. Free Radic Biol Med. 2006;41:318–325. doi: 10.1016/j.freeradbiomed.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Kim A L, Zhu Y, Zhu H, Han L, Kopelovich L, Bickers D R, Athar M. Resveratrol inhibits proliferation of human epidermoid carcinoma A431 cells by modulating MEK1 and AP-1 signalling pathways. Exp Dermatol. 2006;15:538–546. doi: 10.1111/j.1600-0625.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu G. Isorhapontigenin and resveratrol suppress oxLDL-induced proliferation and activation of ERK1/2 mitogen-activated protein kinases of bovine aortic smooth muscle cells. Biochem Pharmacol. 2004;67:777–785. doi: 10.1016/j.bcp.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Olson E R, Naugle J E, Zhang X, Bomser J A, Meszaros J G. Inhibition of cardiac fibroblast proliferation and myofibroblast differentiation by resveratrol. Am J Physiol Heart Circ Physiol. 2005;288:H1131–H1138. doi: 10.1152/ajpheart.00763.2004. [DOI] [PubMed] [Google Scholar]
- Arvidsson A K, Rupp E, Nanberg E, Downward J, Ronnstrand L, Wennstrom S, Schlessinger J, Heldin C H, Claesson-Welsh L. Tyr-716 in the platelet-derived growth factor beta-receptor kinase insert is involved in GRB2 binding and Ras activation. Mol Cell Biol. 1994;14:6715–6726. doi: 10.1128/mcb.14.10.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo-Guisado E, Lorenzo-Benayas M J, Fernandez-Salguero P M. Resveratrol modulates the phosphoinositide 3-kinase pathway through an estrogen receptor alpha-dependent mechanism: relevance in cell proliferation. Int J Cancer. 2004;109:167–173. doi: 10.1002/ijc.11720. [DOI] [PubMed] [Google Scholar]
- Frojdo S, Cozzone D, Vidal H, Pirola L. Resveratrol is a class IA phosphoinositide 3-kinase inhibitor. Biochem J. 2007;406:511–518. doi: 10.1042/BJ20070236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdeau A, Dube N, Tremblay M L. Cytoplasmic protein tyrosine phosphatases, regulation and function: the roles of PTP1B and TC-PTP. Curr Opin Cell Biol. 2005;17:203–209. doi: 10.1016/j.ceb.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy A L, Normandin D, Cheng A, Himms-Hagen J, Chan C C, Ramachandran C, Gresser M J, Tremblay M L, Kennedy B P. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- Klaman L D, Boss O, Peroni O D, Kim J K, Martino J L, Zabolotny J M, Moghal N, Lubkin M, Kim Y B, Sharpe A H, Stricker-Krongrad A, Shulman G I, Neel B G, Kahn B B. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol. 2000;20:5479–5489. doi: 10.1128/mcb.20.15.5479-5489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007;6:307–319. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Zabolotny J M, Kim Y B. Silencing insulin resistance through SIRT1. Cell Metab. 2007;6:247–249. doi: 10.1016/j.cmet.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Haj F G, Verveer P J, Squire A, Neel B G, Bastiaens P I. Imaging sites of receptor dephosphorylation by PTP1B on the surface of the endoplasmic reticulum. Science. 2002;295:1708–1711. doi: 10.1126/science.1067566. [DOI] [PubMed] [Google Scholar]
- Haj F G, Markova B, Klaman L D, Bohmer F D, Neel B G. Regulation of receptor tyrosine kinase signaling by protein tyrosine phosphatase-1B. J Biol Chem. 2003;278:739–744. doi: 10.1074/jbc.M210194200. [DOI] [PubMed] [Google Scholar]
- Chang Y, Ceacareanu B, Zhuang D, Zhang C, Pu Q, Ceacareanu A C, Hassid A. Counter-regulatory function of protein tyrosine phosphatase 1B in platelet-derived growth factor- or fibroblast growth factor-induced motility and proliferation of cultured smooth muscle cells and in neointima formation. Arterioscler Thromb Vasc Biol. 2006;26:501–507. doi: 10.1161/01.ATV.0000201070.71787.b8. [DOI] [PubMed] [Google Scholar]
- Egawa K, Maegawa H, Shimizu S, Morino K, Nishio Y, Bryer-Ash M, Cheung A T, Kolls J K, Kikkawa R, Kashiwagi A. Protein-tyrosine phosphatase-1B negatively regulates insulin signaling in l6 myocytes and Fao hepatoma cells. J Biol Chem. 2001;276:10207–10211. doi: 10.1074/jbc.M009489200. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Maegawa H, Egawa K, Shi K, Bryer-Ash M, Kashiwagi A. Mechanism for differential effect of protein-tyrosine phosphatase 1B on Akt versus mitogen-activated protein kinase in 3T3–L1 adipocytes. Endocrinology. 2002;143:4563–4569. doi: 10.1210/en.2002-220517. [DOI] [PubMed] [Google Scholar]