Abstract
Toll-like receptors (TLRs) are among the fundamental molecules that alert the immune system to the presence of an infection by recognizing pathogen-associated molecules. Much of our understanding regarding TLR function stems from the study of innate immune cells. Recent studies by several groups, including ours, have shown that TLRs can function as costimulatory receptors for antigen-specific T cells, resulting in enhanced T-cell survival and increased expression of effector molecules. We report that the ligation of the TLR1/2 heterodimer on OT-1 cytotoxic T-lymphocytes (CTL) but not TLR2–/–OT-1 T cells increased cytolytic activity in vitro and in vivo. On the basis of these data, we tested the hypothesis that TLR1/2 stimulation on CTLs would enhance antitumor activity in a therapeutic model of B16-Ova melanoma. Adoptive OT-1 T-cell transfer into wild-type and MyD88–/– mice, followed by injection with TLR1/2 ligand, resulted in a synergistic antitumor effect, which correlated with the induction of CD8 T cells specific to various tumor antigens. In contrast, mice receiving TLR2–/–OT-1 T cells and TLR1/2 ligand showed minimal therapeutic efficacy. These findings emphasize the physiological significance of TLR2 engagement on CTLs and could make possible new approaches for the development of effective immunotherapies by manipulating TLR signaling within CTLs.—Asprodites, N., Zheng, L., Geng, D., Velasco-Gonzalez, C., Sanchez-Perez, L., Davila, E. Engagement of Toll-like receptor-2 on cytotoxic T-lymphocytes occurs in vivo and augments antitumor activity.
Keywords: T cell activation, costimulation, immunotherapy
The use of toll-like receptor (TLR) agonists for the development of potent antitumor T-cell responses has been a subject of major focus in recent times (1,2,3). TLRs belong to a family of host defense pattern recognition receptors that alert the immune system to an infection by recognizing pathogen-associated molecular patterns derived from various microorganisms (2, 4). TLRs are expressed primarily on cells of the innate immune system, such as dendritic cells (DCs) and macrophages (5). The engagement of TLRs on these antigen presenting cells (APCs) induces the expression of costimulatory molecules [i.e., cluster of differentiation (CD) 80 and CD86], as well as inflammatory cytokines [i.e., interleukin (IL) -12, IFN-α, and IFN-γ] and chemokines [i.e., macrophage inflammatory protein (MIP) -1β and RANTES (regulated on activation normal T-cell expressed and secreted)] (2, 6) necessary for optimal T-cell activation. In addition, TLR stimulation prolongs APC survival by enhancing the expression of antiapoptotic molecules (2, 4, 7,8,9,10) Clearly, the function of TLRs on cells of the innate immune system is important for generating potent cell-mediated immune responses. However, the biological significance of TLR stimulation on CTLs is poorly understood.
Studies conducted by several groups, including our own, have shown that CD4 and CD8 T cells express functional TLRs. For example, the engagement of TLR1/2, TLR5, TLR7/8, and TLR9 directly on CD4 T-helper cells enhanced cytokine (IL-2) production, maintained CD25 (IL-2Rα chain) expression (11), and consequently augmented proliferation in vitro (12,13,14,15). The stimulation of TLR9 on CD4 T cells has been reported to bypass CD28 signals (12), further highlighting the costimulatory effects of these receptors. We and others recently demonstrated that the engagement of TLR3 or TLR9 on CD4 T-helper cells promoted T-cell survival by increasing the expression of antiapoptotic molecules such as bcl-xL and bcl-2 (16,17,18). The effects of TLR stimulation on CTLs are less characterized. However, Cottalorda et al. (19) recently demonstrated that the engagement of TLR2 on CTLs in vitro augmented proliferation and increased the expression of the antiapoptotic molecule A1. The ligation of TLR2 or TLR3 on CD8 T cells has been reported to enhance the expression of the effector molecules IFN-γ and granzyme B (19, 20). Altogether, these studies suggest that TLR agonists may influence immunity by directly stimulating TLRs on T cells. However, because the majority of these studies were conducted in vitro and have focused primarily on CD4 T cells, the physiological significance and potential costimulatory effects of TLR engagement on CD8 T cells are unknown.
We sought to understand the adjuvant effect of TLR1/2 engagement directly on CD8 T cells in vivo. TLR2 is a transmembrane protein receptor whose expression (in T cells) is up-regulated following T-cell activation (14, 19, 21, 22). TLR2 forms a homodimer or heterodimer with TLR1 or TLR6. The TLR1/2 complex binds with bacterial lipopeptides as well as the synthetic ligand [tripalmitoyl-S-(bis(palmitoyloxy)propyl)-Cys-Ser-(Lys)3-Lys]. Genetic analysis and functional studies revealed that TLR2 is required for lipoprotein/peptide recognition, making this receptor indispensable for TLR1/2 signaling (23, 24). Four adapter proteins have been shown to convey TLR-mediated signals, and central to TLR2 signaling is the myeloid differentiation factor-88, MyD88 molecule (25, 26).
We report that the engagement of TLR1/2 on ovalbumin-specific OT-1 CTLs increased proliferation and the expression of various effector molecules. In vivo and in vitro studies showed that TLR1/2 ligation on CTLs, but not TLR2–/– CTLs, increased cytotoxicity. We tested the hypothesis that TLR1/2 engagement on CTLs would augment antitumor activity against established B16 melanoma tumors, which express the Ova antigen (B16-Ova). Wild-type (WT) and MyD88–/– mice receiving OT-1 CTLs plus TLR1/2 agonists exhibited significant tumor reduction, whereas mice receiving TLR2–/–OT-1 T cells plus TLR1/2 agonists succumbed to tumor challenge. The enhanced antitumor activity of TLR1/2-stimulated CTLs was associated with the priming of naive T cells specific for various B16 tumor antigens. These findings indicate that TLR1/2 ligation on CTLs occurs in vivo and that this interaction intensifies antitumor responses by augmenting effector function and by facilitating the de novo generation of tumor-specific T cells.
MATERIALS AND METHODS
Mice
All studies have been reviewed and approved by the LSUHSC Institutional Animal Care and Use Committee. Wild-type C57BL6 mice were obtained from Charles River Laboratories (Wilmington, MA, USA), MyD88–/– mice were a kind gift from Dr. Douglas Golenbock (Boston University, Boston, MA, USA), B6.129-TLR2tm1kir/J (TLR2–/–) mice, and OT-1 mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). TLR2–/–OT-1 mice were generated by crossing TLR2–/– with the OT-1 mice. TLR2–/–OT-1 mice were used from the fifth or later generation. Genotypes of OT-1 and TLR2-deficient OT-1 mice were determined by polymerase chain reaction (PCR) on tail DNA as recommended by the Jackson Laboratory. The percentage of OT-1 cells of the total CD8 T cell population routinely exceeded 90%, as determined by flow cytometry.
T-cell sorting, dendritic cell generation, and T-cell proliferation assays
CD8 T cells were purified by negative selection (Stemcell Technology, Vancouver, BC, Canada) followed by positive selection (Miltenyi Biotec, Auburn, CA, USA). Flow cytometry was used to determine T-cell purity, which routinely exceeded 99%. WT or TLR2–/– DCs were generated from the bone marrow using a standard protocol. Briefly, adherent bone marrow cells (2×106 cells/ml) were cultured in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal calf serum (FCS; low endotoxin, Sigma-Aldrich, St. Louis, MO, USA), l-glutamine, gentamicin (Invitrogen), and β-ME (Sigma-Aldrich) and cultured for 10 days in the presence of 500 U/ml IL-4 and 800 U/ml granulocyte-macrophage colony-stimulating factor (GM-CSF) at 37°C 5% CO2. For maturation, DCs were activated with 50 ng/ml of LPS (Salmonella Minnesota; Sigma-Aldrich) for 12–24 h and extensively washed prior to using DCs in proliferation or activation assays. DCs were pulsed with the Ova258–265 peptide (1.0 μg/ml, SIINFKEL) at 37°C and extensively washed after 2 h. Purified OT-1 or TLR2–/–OT-1 T cells (3×104/well) were cultured together with 3 × 103 peptide-pulsed or unpulsed DCs in the presence or absence of TLR2 agonist (10 μg/ml). For proliferation assays, cells were pulsed with [3H] thymidine (0.5 μCi/well) for 16 h prior to measuring thymidine uptake using a Packard direct β-counter (Packard, Meriden, CT, USA). In some experiments, T-cell proliferation was measured by labeling cells with 5 μM 5-carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen) and analyzed by flow cytometry following T-cell activation with plate-bound anti-CD3 antibodies.
Quantitative real-time PCR (RT-PCR)
The number of RNA transcripts was determined by quantitative RT-PCR, as we described previously (27). Briefly, RNA was extracted from purified OT-1 T cells following activation, as described in Fig. 1. All PCR primer sets were purchased from SuperArray Bioscience Corporation (Frederick, MD, USA). Gene quantification was determined using the ABI 7900HT Sequence Detection System from Applied Biosystems (Foster City, CA, USA). Serial dilutions (106 to 0 transcripts) of all transcripts were used to generate the standard curve for each transcript and then normalized to β-actin levels. All RT-PCR reactions were performed in triplicate with 0.2 μM gene-specific primer and 1× RT2 SYBR® Green with ROX (SuperArray Bioscience). Thermal cycling conditions were 95°C for 10 min followed by 40 cycles of 95°C, 15 s for denaturation, and 60°C 1 min for annealing and extension.
Figure 1.
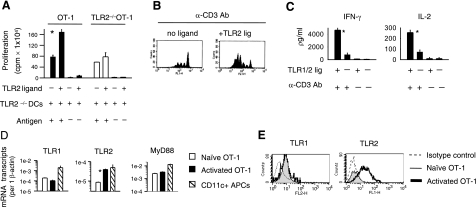
TLR1/2 engagement costimulates CTL responses. A) OT-1 T cells were purified by negative selection followed by positive selection using magnetic beads. OT-1 cells (3×104) were stimulated in vitro with 3 × 103 antigen-pulsed (SIINFEKL) H2-Kb TLR2–/– DCs in the presence or absence of 10 μg/ml TLR1/2 ligand. T-cell proliferation was measured at the end of 5 days. Values represent average ± sd of replicate samples. B) OT-1 T cells were labeled with CFSE and stimulated with plate-bound anti-CD3 antibody (1.0 μg/ml) for 5 days in the presence or absence of TLR1/2 ligand (10 μg/ml). C) OT-1 T cells were activated with-plate bound anti-CD3 (2.5 μg/ml) antibody for 72 h in the absence or presence of TLR1/2 ligand. The amount of cytokines secreted by cells was determined by ELISA. D) After 24 h of activation (black bar) or not (white bar), T cells were purified by positive selection using magnetic beads, and the expression of the indicated mRNA transcripts was determined using RT-PCR. For control, CD11+ APCs were sorted by magnetic beads (slashed bar) from spleens of WT mice and the level of transcripts was determined via RT-PCR. E) Alternatively, the surface expression of TLR1 and TLR2 proteins on nonactivated (gray fill) or activated OT-1 T cells (bold line) was evaluated using flow cytometry. All data are representative of at least 3 independent experiments, each yielding similar results. *P < 0.05; Student’s t test.
Flow cytometry
The surface expression of TLR1 and TLR2 OT-1 T cells was determined using flow cytometry. Cells were stained with 2 μg/106 cells/100 μl PE-conjugated anti-TLR1 or FITC-conjugated anti-TLR2 (eBioscience, San Diego, CA, USA) antibodies for 45 min at 4°C, washed, and analyzed on an FACScalibur (BD Pharmingen, San Jose, CA, USA). The frequency of Ova-specific, Trp-2-, and gp100-specific CD8 T cells was determined by staining lymph node cells with Ova-pentamer, Trp-2-pentamer (Pro-Immune, Bradenton, FL, USA) or mgp100-tetramer (Beckman Coulter, Fullerton, CA, USA), and CD8 antibody (BD Pharmingen). In some experiments, the levels of IFN-γ, granzyme B, perforin, and Fas ligand were determined by flow cytometry using BD Pharmingen’s intracellular staining kit. Alternatively, cytokine secretion was examined using BD Pharmingen’s OptEIA ELISA kit following T-cell activation with plate-bound antibodies.
In vitro and in vivo cytotoxicity assays
In vitro cytotoxicity assays were conducted as we previously described (28). Briefly, OT-1 T cells were purified as described above and activated using antigen-pulsed TLR2–/–APCs in the absence or presence of the TLR1/2 agonist Pam3 (10 μg/ml). After 5 days, cytolytic activity against EL4, peptide-pulsed EL4, or B16-Ova target cells was measured at different effectors to target ratios in a 4-h 51Cr release assay (28). For in vivo cytotoxicity assays, OT-1 T cells were activated in vitro using antigen-pulsed APCs, as described in Fig. 1. Two days later, 1 × 106 purified OT-1 or TLR2–/–OT-1 T cells were injected (i.v.) into MyD88–/– mice. Five days after T-cell transfer, two groups of MyD88–/– splenocytes served as target cells in an in vivo cytotoxicity assay. Target cells were divided into two groups. One group was labeled with a high concentration of the CFSE dye CFSEhigh (5 μM) and pulsed with the an irrelevant H-2Kb-restricted CTL epitope (SV40 T Ag, VVYDFLKC; 1 μg/ml per 1×106 cells in 0.1 ml at 37°C for 1 h). The other group was labeled with CFSElow (0.5 μM) and pulsed with the SIINFEKL peptide. CFSEhigh and CFSElow target cells were mixed at a 1:1 ratio and injected i.v. (2×107 total cells) in the absence and presence of TLR1/2 agonist (50 μg). The percentage of target cells recovered 28 h after adoptive transfer (AT) was evaluated by FACS analysis.
Tumor challenge and measurement of tumor-specific T-lymphocytes
MyD88–/– or WT mice were injected (s.c.) with 25 × 103 B16-Ova tumor cells in the rear leg flank. When tumors reached a size of ∼5 mm2, mice were injected (i.v.) with 2.5 × 106 OT-1 or TLR2–/–OT-1 T cells. Prior to AT, OT-1 T cells were activated in vitro for 48 h using antigen-pulsed APCs. Mice were injected (s.c.) with TLR1/2 ligand (50 μg) or control PBS peritumorally every 5 days. Tumor sizes (mm2) were calculated by perpendicular measurement by longitudinal diameter. Statistical analyses were performed using the MIXED procedure of SAS v9.13 (SAS Institute, Cary, NC, USA), and tumor volume kinetics were assessed by fitting quadratic random coefficient models. Group averages were compared further if any or both of the interaction terms (linear, quadratic) in the model showed a value of P ≤ 0.05. In some experiments, the frequency of in vivo-primed CTLs was quantified by flow cytometry. Between 30 and 35 days after tumor challenge, the spleens and lymph nodes were pooled from the indicated groups. CD8 T cells were purified by negative selection and then restimulated with IFN-γ-treated (100 U/ml), irradiated B16 tumor cells for 5 days in the presence of IL-2 (100 U/ml). T-cell cytotoxicity was determined in a 4 h 51Cr-release cytotoxicity assay against the following target cells: B16-Ova, B16, EL4, EL4 + Trp-2180–188, EL4 + mgp-10025–33, and EL4 + Ova257–264.
RESULTS
TLR1/2 engagement on CTLs augments proliferation and cytokine production
Previous reports have indicated that the ligation of various TLRs on a heterogeneous population of T cells enhanced their responses in vitro (13, 15,16,17). We examined the costimulatory effects of TLR1/2 ligation on a monoclonal population of CD8 T cells. OT-1 T cells were primed with antigen-pulsed TLR2–/–DCs in the absence or presence of the TLR1/2 ligand. The addition of TLR1/2 ligand increased OT-1 T-cell proliferation significantly (Fig. 1A; P<0.004), as compared with OT-1 cells stimulated with antigen alone. To verify that the effects of TLR1/2 ligand occurred through the stimulation of TLR2 on CTLs, we generated TLR2–/–OT-1 mice and examined T-cell proliferation. Antigenic stimulation of TLR2–/–OT-1 T cells induced proliferation to similar levels as OT-1 T cells. However, TLR1/2 ligand had little effect on TLR2–/–OT-1 T-cell division. TLR1/2 ligand by itself did not induce T-cell proliferation without concomitant TCR stimulation (Fig. 1A). These findings are consistent with previous reports showing that TLR2 (or TLR9) engagement costimulates T-cell responses, specifically following T-cell activation (16, 19).
To determine whether TLR1/2 ligation costimulated T-cell responses in the absence of APCs, we examined T-cell proliferation and cytokine production following activation with plate-bound anti-CD3 antibody. The engagement of TLR1/2 on OT-1 T cells increased proliferation, as determined by CFSE dilution (Fig. 1B). In addition, TLR1/2-stimulated CTLs produced 6-fold more IFN-γ and 4-fold more IL-2 than non-TLR-stimulated OT-1 T cells, as measured by ELISA (Fig. 1C). Notably, TLR1/2 engagement on CTLs increased the mean fluorescence intensity and the percentage of cytokine-producing cells, indicating that TLR stimulation induced higher cytokine levels on a per-cell basis (not shown). These data confirm that TLR1/2 ligands costimulate OT-1 T-cell responses, resulting in enhanced proliferation and cytokine production.
The costimulatory effects of TLR1/2 stimulation depended on T-cell activation. Therefore, we examined whether TCR stimulation increased the expression levels of TLR1, TLR2, and MyD88. Purified OT-1 T cells were or were not activated using Ova peptide-pulsed APCs and were sorted and purified prior to collecting RNA. We observed that TLR2 mRNA transcript levels were significantly increased in activated CTLs relative to nonactivated cells (Fig. 1D; P<0.0001), as determined by RT-PCR. However, both naive and activated OT-1 T cells expressed similar levels of TLR1 and MyD88. The levels of TLR1, TLR2, and MyD88 were higher in CD11+ APCs. Consistent with the higher expression levels of TLRs and MyD88, CD11+ APCs showed greater sensitivity to TLR1/2 stimulation than activated CTLs (Supplemental Fig. 1). In agreement with the PCR data, TLR2 protein expression increased on T-cell activation, whereas TLR1 levels remained similar in activated and naive OT-1 T cells (Fig. 1E).
TLR1/2 engagement on cytotoxic T-lymphocytes augments the expression levels of cytolytic molecules and enhances cytolytic activity in vitro and in vivo
On the basis of the enhanced production of IFN-γ by TLR1/2-stimulated OT-1 T cells, we examined the expression of various effector molecules in response to TLR1/2-stimulation. We quantified IFN-γ, Fas ligand, perforin, and granzyme B gene expression levels in TLR1/2-stimulated and non-TLR-stimulated OT-1 CTLs, using RT-PCR. Figure 2A is a representative graph of an RT-PCR reaction showing that although the levels of β-actin in TLR1/2-stimulated and non-TLR-stimulated CTLs are similar, TLR1/2 ligation on CTLs significantly (P<0.0001) increased IFN-γ mRNA transcripts, as represented by lower cycle values. Likewise, the numbers of granzyme B, Fas ligand, and to some extent perforin mRNA transcripts were higher in TLR1/2-stimulated OT-1 T cells (Fig. 2A). Consistent with the RT-PCR data, IFN-γ, granzyme B, and Fas ligand protein levels increased in response to TLR1/2 stimulation, as determined by flow cytometry (Fig. 2B). These data indicated that TLR1/2 ligation on CTLs increases the expression levels of effector molecules at both the RNA and protein levels.
Figure 2.
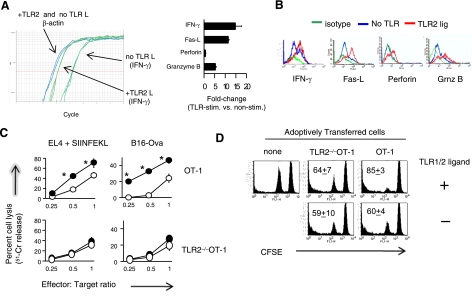
TLR1/2 engagement on OT-1 CTLs enhances the expression of effector molecules and augments cytolytic function. A) OT-1 T cells were purified and stimulated with peptide-pulsed TLR2–/– DCs, in the absence or presence of TLR1/2 ligand. After 24 h of activation, T cells were sorted, and the fold changes in gene expression in TLR1/2-stimulated and non-TLR-stimulated cells were determined using RT-PCR. B) Alternatively, the intracellular levels of granzyme-B, perforin, IFN-γ, and the surface expression of Fas ligand were determined by flow cytometry 72 h after activation. C) Purified OT-1 T cells were stimulated with peptide-pulsed TLR2–/– DCs in the absence (open circles) or presence (filled circles) of TLR1/2 ligand. After 5 days, OT-1 and TLR2–/–OT-1 T-cell cytoxicity was tested against peptide-pulsed EL4 or B16-Ova target cells at the indicated effector to target ratios using a standard 4-h 51Cr-release assay. D) OT-1 T cells were stimulated in vitro for 48 h and injected (i.v.) into naive MyD88–/– mice (n=3) 2 days later. Five days after AT, MyD88–/– splenocytes (target cells) were labeled with CFSEhigh and pulsed with an irrelevant H-2Kb-restricted CTL epitope (VVYDFLKC), and the other group was labeled with CFSElow and pulsed with the Ova257–264 (SIINFEKL) peptide. CFSEhigh and CFSElow target cells were mixed at a 1:1 ratio and injected (i.v.) with or without TLR1/2 agonist. The percentage of target cells was determined by flow cytometry. Graphs show average ± sd percentage lysis; 3 mice/group. Data in A, B are representative of 3 independent experiments; data in C, D are representative of 2 experiments, each showing identical trends. *P ≤ 0.02; ANOVA.
Because the primary function of a CTL is to kill infected or malignant cells and because TLR1/2 ligation on CTLs increased the expression levels of effector molecules, we examined the cytolytic activity of TLR1/2-stimulated cells. Purified OT-1 T cells were activated using antigen-pulsed TLR2–/– DCs in the absence or presence of TLR1/2 ligand. After 5 days, CD8 T cells were enriched via negative selection, and CTL cytoxiticy was examined in a standard 4 h chromium-release assay, as we have previously described (28, 29). TLR1/2-ligated OT-1 T cells but not TLR2–/–OT-1 T cells showed enhanced cytotoxicity against peptide-pulsed EL4 and B16-Ova melanoma cells (Fig. 2C). In the absence of TLR1/2 ligand, OT-1 and TLR2–/–OT-1 CTLs demonstrated similar levels of cytotoxicity (Fig. 2C). TLR1/2-stimulated OT-1 T cells did not lyse nonpulsed EL-4 target cells (data not shown), indicating that TLR1/2 ligation alone did not enhance nonspecific killing.
We examined the biological significance of TLR1/2-engaged CTLs on cytolytic activity in an in vivo cytotoxicity assay. After 2 days of activation in vitro, OT-1 or TLR2–/–OT-1 T cells were injected (i.v.) into MyD88–/– mice, which do not respond to TLR2 signals. Five days after T-cell transfer, two groups of MyD88–/– splenocytes, which served as target cells, were labeled with 5 μM CFSE (CFSEhigh) and pulsed with an irrelevant H-2Kb-restricted CTL epitope, and the other group was labeled with 0.5 μM CFSE and pulsed with the SIINFEKL peptide. CFSEhigh and CFSElow target cells were mixed at a 1:1 ratio and injected (i.v.) with or without the TLR1/2 agonist. In mice injected with OT-1 CD8 T cells plus TLR2 ligand, the vast majority of target cells were eliminated, as compared with target cells from mice that received OT-1 T cells alone (Fig. 2D). In contrast, the injection of TLR1/2 agonist did not increase TLR2–/–OT-1 T-cell cytotoxicity. Injection with TLR1/2 ligand alone did not have a cytotoxic effect on the target population. These findings indicate that TLR1/2 engagement on CD8 T cells occurs in vivo and that this interaction increases their cytolytic potential in an antigen-specific manner.
TLR1/2 engagement on OT-1 T cells in vivo reduces tumor growth in a therapeutic model of melanoma
Based on the enhanced killing activity of TLR1/2-ligated CTLs, we examined whether TLR1/2 ligation on CTLs in vivo augmented antitumor activity in a therapeutic model of B16-Ova melanoma. As depicted in Fig. 3A, mice were injected (s.c.) in the rear flank with B16-Ova melanoma cells. B16-Ova melanoma cells express the chicken ovalbumin antigen in the context of the H-2Kb major histocompatibility complex (MHC) I molecule, recognized by OT-1 CTLs. When tumors reached a size of 5 mm2, mice were injected (i.v.) with OT-1 or TLR2–/–OT-1 CTLs. Prior to AT, OT-1 T cells were activated in vitro for 5 days using peptide-pulsed APCs. Following AT, mice were injected peritumorally (s.c.) with TLR1/2 ligand or control PBS every 5 days.
Figure 3.
TLR1/2 engagement on OT-1 T cells in vivo enhances antitumor activity against established B16-Ova melanoma tumors. A) Model depicting experimental design. B–G) WT (B–D) or MyD88–/– mice (E–G) were injected (s.c.) with 25 × 103 B16-Ova tumor cells. When tumors reached a size of 5 mm2, mice were injected (i.v.) with 2.5 × 106 OT-1 or TLR2–/–OT-1 T cells. Prior to AT, CTLs were activated in vitro for 48 h, as described in Materials and Methods. Mice were injected peritumorally with TLR1/2 ligand (bold line) or PBS (light line) every 5 days, starting on the same day that mice received T cells. Tumor sizes (mm2) were calculated by measuring perpendicular by longitudinal diameter. Data are compiled from 3 independent experiments. Each experiment yielded similar trends. Statistical analyses were performed using the MIXED procedure of SAS v9.13, and tumor volume kinetics were assessed by fitting quadratic random coefficient models; *P < 0.001.
Tumor growth was similar in TLR1/2 ligand-treated and untreated WT mice (Fig. 3B), indicating that the stimulation of TLR1/2 on APCs was insufficient to suppress tumor development. However, adoptive T-cell transfer of TLR2–/–OT-1 cells plus injections with the TLR1/2 agonist delayed tumor growth in WT mice, as compared with mice receiving TLR2–/–OT-1 T cells alone (Fig. 3C). These data suggested that TLR1/2-stimulated APCs enhanced the antitumor effects of TLR2–/–OT-1 T cells. However, these mice succumbed to tumor challenge by day 35. The decreased antitumor activity observed in WT mice receiving TLR2–/–OT-1 T cells alone was unlikely due to decreased TLR2–/–OT-1 cytotoxicity, as these cells showed similar cytotoxicity to OT-1 T cells alone in vitro (Fig. 2C) and in vivo (Fig. 2D). In sharp contrast, the majority of WT mice receiving OT-1 T cells plus TLR1/2 agonist exhibited significant tumor reduction, and 5 of 14 mice eradicated tumor (Fig. 3D). Altogether, these data suggested that TLR1/2 ligation on OT-1 CTLs augmented antitumor activity. However, TLR1/2-stimulated APCs may have contributed to the enhanced antitumor WT mice.
To determine whether TLR1/2 stimulation directly on CTLs influenced antitumor activity, we conducted similar studies to those described above in tumor-bearing MyD88–/– mice. Injections with TLR1/2 ligand did not alter tumor growth kinetics, as compared with PBS-treated mice (Fig. 3E). In contrast to WT mice, MyD88–/– mice receiving TLR2–/–OT-1 T cell and TLR1/2 ligand did not show a difference in tumor growth kinetics as compared with mice injected with cells alone (Fig. 3F). These data indicated that the enhanced antitumor effects observed in WT mice receiving TLR2–/–OT-1 and TLR2 ligand was likely due to the activation of APCs. Notably, cells from MyD88–/– mice did not respond to multiple injections of TLR1/2 ligand, confirming that MyD88 is required to transduce TLR2 signals (Supplemental Fig. 2). In contrast, AT of OT-1 T cells plus TLR1/2 agonists decreased tumor growth in 7 of 11 MyD88–/– mice, as compared with mice that received OT-1 T cells plus PBS (Fig. 3G); 4 of these mice showed tumor eradication. Notably, on rechallenge with an i.v. injection of B16 cells, 50 days after a primary challenge with B16-Ova cells, fewer pulmonary metastases were observed as compared with naive mice, indicating that surviving mice developed some level of immunity against B16 tumor cells (not shown). Together, these data indicated that in addition to stimulating TLRs on APCs, TLR1/2 stimulation directly on CTLs enhanced antitumor effects.
TLR1/2-stimulated CTLs promote the priming of CD8 T cells to B16 tumor antigens
We previously documented that peptide vaccination administered together with the TLR9 agonist augmented the generation of tumor-specific T cells, specifically in tumor-bearing mice (28, 29). On the basis of those studies, we postulated that the enhanced antitumor responses could have resulted from the generation of antitumor CTLs via antigen cross-priming. Here, we examined whether mice receiving TLR1/2 agonists and OT-1 T cells generated CTL responses to bona fide B16 antigens. The frequencies of Ova-, Trp-2-, and mgp100-specific CD8 T cells in the lymph nodes were examined using MHC I tetramers or pentamers and subsequently analyzed by flow cytometry. Unlike the Ova antigen, Trp-2(180–188) and gp100(25–33) epitopes are genuine tumor antigens expressed on the Kb or Db MHC molecules of B16 melanoma cells (30).
Figure 4A shows representative dot plots of tetramer-stained CD8 T cells obtained from the draining lymph nodes of MyD88–/– tumor-bearing mice. Mice receiving OT-1 T cells and TLR2 ligand showed significantly higher numbers (and percentages) of Ova-specific T cells than mice receiving TLR2–/–OT-1 T cells plus TLR1/2 ligands or mice that received OT-1 T cells alone (Figs. 4A). Similarly, gp100- and Trp2-specific CTLs were detected at higher frequencies in mice receiving OT-1 T cells and TLR1/2 ligand than in mice injected with TLR2–/–OT-1 T cells and TLR1/2 ligand. However, neither gp100- nor Trp2-specific CTLs were detected in mice receiving OT-1 CTLs alone or in mice receiving TLR1/2 injections alone. These results indicated that TLR1/2 engagement on OT-1 T cells helped maintain elevated numbers of transferred cells and contributed to the priming of CTLs specific for tumor antigens.
Figure 4.
TLR1/2-engaged CTLs promote antigen-cross presentation. Graphs are representative dot plots of tetramer-stained CD8 T cells obtained from mice that received OT-1, TLR2–/–OT-1, or no T cells in the absence or presence of TLR1/2 ligand, as in Fig. 3A. Mice were sacrificed or died 30–35 days after tumor challenge; percentage of Ova257–264-, Trp-2-, or mouse gp100-specific CD8+ T cells in the draining lymph nodes was determined by staining cells with anti-CD8 antibody and the different tetramers or pentamers. Data represent average ± sd percentage of tetramer-positive CD8 T cells; 3–4 mice/group.
We next examined the ability of CD8 T cells obtained from tumor-bearing mice to lyse B16-Ova and B16 melanoma cells. Because the frequency of tumor-specific CTLs was less than 3% of the total CD8 T-cell population, CD8 T cells were initially enriched from the spleen and lymph nodes of tumor-bearing mice and then restimulated with irradiated, IFN-γ-treated, B16-Ova tumor cells, in the presence of IL-2. Five days after in vitro stimulation, CD8 T-cell cytotoxicity was measured. Figure 5A shows that CD8 T cells obtained from mice injected with OT-1 T cells and TLR2 ligand killed B16-Ova cells at higher levels than did CD8 T cells obtained from mice receiving TLR2–/–OT-1 T cells and TLR1/2 ligand. CD8 T cells obtained from tumor-bearing mice that did not receive T cells did not lyse B16-Ova cells. Further, CD8 T cells obtained from B16-Ova tumor-bearing mice, injected with OT-1 T cells and TLR1/2 ligand, lysed B16 tumor cells (Fig. 5A). CD8 T-cell specificity to Ova and tumor antigens was confirmed further using a cold target cytotoxicity assay, in which B16-Ova cells served as competitor target cells (Supplemental Fig. 3). In contrast, injection with TLR2–/–OT-1 T cells and TLR1/2 ligand did not induce B16-specific T cells. Because B16 cells do not express ovalbumin and, therefore, are not a direct target for OT-1 T cells, these data indicated that a subpopulation of CD8 T cells recognized tumor-associated antigens on B16 melanoma cells.
Figure 5.
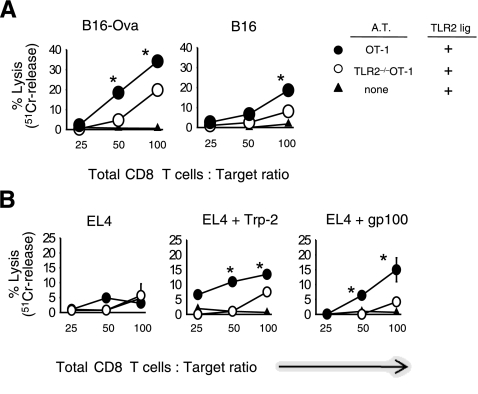
Endogenous CD8 T cells show cytolytic activity against B16 melanoma tumor antigens. WT mice were injected with B16-Ova tumor cells followed by AT of OT-1 or TLR2–/–OT-1 T cells, as described in Fig. 3. All mice were injected with TLR1/2 ligand every 5 days, starting on the same day that tumor-bearing mice received CTLs. Between 30 and 35 days after tumor challenge, the spleens and lymph nodes were pooled from the indicated groups. CD8 T cells were purified by negative selection. To expand tumor-specific CTLs, purified CD8 T cells were restimulated with IFN-γ-treated, irradiated B16 tumor cells for 5 days in the presence of IL-2 (100 U/ml). T-cell cytotoxicity was determined in a 4 h 51Cr-release cytotoxicity assay against the indicated target cells. All experimental determinations were performed in triplicate; averages ± sd were consistently within 15% of the mean. Error bars represent mean ± sd of triplicate samples. *P ≤ 0.03; ANOVA.
To monitor the antigen specificity to B16 tumors, we examined CD8 T-cell responses against the tumor-specific antigens Trp2180–88 and mouse gp10025–33. CD8 T cells obtained from B16-Ova tumor-bearing mice, injected with OT-1 T cells and TLR1/2 ligand, killed Trp2180–88, gp10025–33-pulsed (Fig. 5B) and Ova-pulsed (Supplemental Fig. 3) EL4 target cells. In contrast, CD8 T cells isolated from tumor-bearing mice injected with TLR2–/–OT-1 T cells and TLR1/2 ligand showed minimal reactivity to Trp2180–88 and gp10025–33-pulsed EL4 target cells. CD8 T cells isolated from mice injected with TLR2 ligand and OT-1 showed higher killing activity against the Ova target cells than did mice injected with TLR2–/–OT-1 and TLR2 ligand. CD8 T cells from mice that did not receive T cells did not lyse peptide-pulsed target cells. In all groups, CD8 T cells failed to lyse nonpulsed EL-4 cells, demonstrating that CD8 T cells do not kill in a nonspecific manner. Although the percentage lysis of target cells was <20%, we highlight that the overall frequency of Trp2180–88- and gp10025–33-specificCTLs was <3%. Therefore, although the initial effector (total CD8 T cells) to target ratio was conducted at 100:1, the actual percentage of tumor-specific CTLs was considerably lower than the starting E:T ratios. We conclude that TLR1/2-stimulated OT-1 T cells facilitate the priming of tumor-specific CTLs and that these responses could have contributed to the enhanced antitumor activity observed in Fig. 3.
DISCUSSION
Here we show that TLR1/2 engagement on cytotoxic T-lymphocytes occurs in vivo and that this interaction enhances T-cell cytolytic activity. The ligation of TLR1/2 on OT-1 T cells increased the expression of cytolytic molecules at the RNA and protein levels, which correlated with improved cytotoxic activity. WT and MyD88–/– mice that received OT-1 T cells (but not TLR2–/–OT-1 T cells) and TLR1/2 ligand demonstrated significant antitumor responses against an established melanoma tumor. This antitumor activity was associated with the induction of CD8 T cells specific to the tumor antigens Trp-2 and gp100. We conclude that the adjuvant effects of certain TLR agonists, such as TLR1/2 ligands, may occur through the engagement of TLRs on both APCs and on T cells.
TLR1/2 engagement on CTLs has a profound influence on both the cytolytic function and expression of effector molecules. The studies presented here (Figs. 1 and 2) as well as those conducted by various groups (6, 14, 19, 21, 31,32,33) showed that TLR engagement on CTLs significantly enhanced IFN-γ production, a hallmark of T-cell activation. Recently, Cottalorda et al. (19) showed that costimulation with TLR1/2 increased granzyme B secretion. Here, we demonstrated that the effects of Pam3Cys on CTLs occurred via TLR2, as TLR2–/–OT-1 CTL showed little increased cytolytic activity following addition of TLR1/2 ligand. Moreover, TLR1/2 ligation significantly enhanced the expression of Fas ligand, granzyme B, perforin, Fas-L, and IFN-γ at the level of mRNA, indicating that TLR1/2-mediated signals influence the transcriptional mechanisms that regulate effector gene expression. The tissue-specific T-BOX (TBX) transcription factor (T-bet) and eomesodermin (EOMES) are key transcription factors known to regulate the transcription of perforin, granzyme B, and IFN-γ (34). Our ongoing studies indicate that TLR2 signals on CTLs may regulate effector gene transcription by modulating T-bet and/or EOMES activity. In support of this assertion, recent reports indicate that engagement of certain TLRs on monocytes and DCs rapidly induces T-bet expression (35,36,37). Alternatively, TLR engagement on CTLs may have induced the expression of EOMES or promoted the interaction between EOMES and T-bet. Identifying the signaling pathways and the molecules (i.e., TLR agonists) that regulate T-cell activation and the expression of cytolytic molecules is important for the strategic manipulation of more effective vaccines.
An important feature of vaccines is the ability to induce T-cell proliferation and survival. The biological importance of TLR stimulation (on CTLs) on proliferation or survival has not been addressed formally. However, in the current study, we observed that OT-1 T cells outnumbered TLR2–/–OT-1 T cells, specifically in mice injected with TLR1/2 ligand but not PBS (Fig. 4). These data suggested that TLR engagement on T cells contributed to increased numbers. In support of this assertion, TLR1/2 engagement has been shown to augment CD8 T-cell proliferation in vitro (19) (Fig. 1). Further, TLR1/2 engagement on CTLs has been shown to enhance cell survival through the increased expression of antiapoptotic proteins such as A1 (19). Gelman et al. (16, 17) reported that the ligation of TLR3 or TLR9 on CD4 T cells enhanced cell survival, which correlated with increased expression of bcl-xL in vitro. Similarly, we recently documented that TLR9 engagement on CD4 T cells enhanced the expression levels of bcl-2 and bcl-xL and improved cell survival following exposure to genotoxic stress (18). These studies suggest that the ligation of certain TLRs on T cells may influence the duration and extent of T-cell response by regulating apoptosis and/or cell division, a hypothesis we are currently testing.
In our current model, repeated peritumoral injections of TLR1/2 ligand were required to induce potent antitumor T-cell responses (Fig. 3). Neither a single peritumoral injection nor repeated injections (s.c.) at distal sites generated potent antitumor responses. These data indicated that the TLR1/2 agonist was required to be present near the tumor site in order to potentiate antitumor function. In addition to augmenting CTL cytolytic activity, TLR ligands may have enhanced CTL activity by promoting tumor infiltration. In support of this hypothesis, Zanin-Zhorov et al. (22) showed that TLR2 ligands recruited and helped maintain T cells near the site of infection by altering the expression of adhesion molecules on T cells. Also, in models of chronic inflammatory joint disease, it has been shown that TLR ligands can costimulate T cells of multiple specificities, including self-antigens, and this interaction results in enhanced cytolytic function and IFN-γ production (38,39,40,41). Therefore, we propose that in addition to augmenting the effector function through increased expression of effector molecules, peritumoral injection of TLR1/2 ligands served to recruit and maintain infiltrating T cells at the tumor site.
The efficient cross-priming of tumor antigen-specific T cells is another significant characteristic sought after in developing of effective vaccines. In the current studies, TLR1/2-stimulated OT-1 T cells promoted the priming of T cells specific for Trp-2 and gp100 tumor antigens (Figs. 4 and 5). Although the factors that influence cross-presentation have not been entirely defined, evidence suggests that antigens released at high concentrations due to cellular destruction are most efficiently cross-presented (42). We postulate that the enhanced antitumor activity observed in mice receiving TLR1/2 agonist and OT-1 CTLs (Fig. 3) resulted in the release of vast amounts of tumor antigens and subsequently presented to naive CD8 T cells.
The studies presented here offer novel insights into the physiological significance of TLR stimulation on CD8 T cells and may pose a significant impact on the development of effective vaccines and cell-mediated tumor therapies. For example, the results to clinical trials using T-cell-based therapies have shown promise in cancer treatment (43,44,45,46). However, therapeutic efficacy is often limited by poor T-cell survival and/or inadequate effector function (44, 45, 47,48,49). In this respect, it would be beneficial to incorporate TLR agonists that can activate both APCs and T cells in order to generate and maintain high numbers of cytolytic T cells. Understanding the molecular signaling pathways through which TLR signals costimulate T-cell responses will provide novel opportunities for increasing the efficiency with which tumor-specific effector cells are generated and boosting cytolytic activity by manipulating TLR signaling in T cells.
In summary, the adjuvant effects of certain TLR agonists may extend farther than solely activating innate immune cells. The data presented here indicate that TLR ligation on CTLs occurs in vivo and that this interaction leads to heightened T-cell responses with potent effector function.
Supplementary Material
Acknowledgments
Research was supported by a National Institutes of Health Center for Biomedical Research Center Excellence grant (1P20 RR021970) and the Louisiana Cancer Research Consortium. The authors declare no competing financial interests.
References
- Krieg A M. Development of TLR9 agonists for cancer therapy. J Clin Invest. 2007;117:1184–1194. doi: 10.1172/JCI31414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- Krieg A M. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Ghosh T K, Mickelson D J, Fink J, Solberg J C, Inglefield J R, Hook D, Gupta S K, Gibson S, Alkan S S. Toll-like receptor (TLR) 2–9 agonists-induced cytokines and chemokines: I. Comparison with T cell receptor-induced responses. Cell Immunol. 2006;243:48–57. doi: 10.1016/j.cellimm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Davila E, Velez M G, Heppelmann C J, Celis E. Creating space: an antigen-independent, CpG-induced peripheral expansion of naive and memory T lymphocytes in a full T cell compartment. Blood. 2002;100:2537–2545. doi: 10.1182/blood-2002-02-0401. [DOI] [PubMed] [Google Scholar]
- Jahrsdorfer B, Jox R, Muhlenhoff L, Tschoep K, Krug A, Rothenfusser S, Meinhardt G, Emmerich B, Endres S, Hartmann G. Modulation of malignant B cell activation and apoptosis by bcl-2 antisense ODN and immunostimulatory CpG ODN. J Leukoc Biol. 2002;72:83–92. [PubMed] [Google Scholar]
- Yi A K, Hornbeck P, Lafrenz D E, Krieg A M. CpG DNA rescue of murine B lymphoma cells from anti-IgM-induced growth arrest and programmed cell death is associated with increased expression of c-myc and bcl-xL. J Immunol. 1996;157:4918–4925. [PubMed] [Google Scholar]
- Prins R M, Craft N, Bruhn K W, Khan-Farooqi H, Koya R C, Stripecke R, Miller J F, Liau L M. The TLR-7 agonist, imiquimod, enhances dendritic cell survival and promotes tumor antigen-specific T cell priming: relation to central nervous system antitumor immunity. J Immunol. 2006;176:157–164. doi: 10.4049/jimmunol.176.1.157. [DOI] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll-dependent control mechanisms of CD4 T cell activation. Immunity. 2004;21:733–741. doi: 10.1016/j.immuni.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Bendigs S, Salzer U, Lipford G B, Wagner H, Heeg K. CpG-oligodeoxynucleotides co-stimulate primary T cells in the absence of antigen-presenting cells. Eur J Immunol. 1999;29:1209–1218. doi: 10.1002/(SICI)1521-4141(199904)29:04<1209::AID-IMMU1209>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Caron G, Duluc D, Fremaux I, Jeannin P, David C, Gascan H, Delneste Y. Direct stimulation of human T cells via TLR5 and TLR7/8: flagellin and R-848 up-regulate proliferation and IFN-gamma production by memory CD4+ T cells. J Immunol. 2005;175:1551–1557. doi: 10.4049/jimmunol.175.3.1551. [DOI] [PubMed] [Google Scholar]
- Komai-Koma M, Jones L, Ogg G S, Xu D, Liew F Y. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc Natl Acad Sci U S A. 2004;101:3029–3034. doi: 10.1073/pnas.0400171101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuguchi T, Takagi K, Musikacharoen T, Yoshikai Y. Gene expressions of lipopolysaccharide receptors, toll-like receptors 2 and 4, are differently regulated in mouse T lymphocytes. Blood. 2000;95:1378–1385. [PubMed] [Google Scholar]
- Gelman A E, Zhang J, Choi Y, Turka L A. Toll-like receptor ligands directly promote activated CD4+ T cell survival. J Immunol. 2004;172:6065–6073. doi: 10.4049/jimmunol.172.10.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman A E, LaRosa D F, Zhang J, Walsh P T, Choi Y, Sunyer J O, Turka L A. The adaptor molecule MyD88 activates PI-3 kinase signaling in CD4+ T cells and enables CpG oligodeoxynucleotide-mediated costimulation. Immunity. 2006;25:783–793. doi: 10.1016/j.immuni.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Asprodites N, Keene A H, Rodriguez P, Brown K D, Davila E. TLR9 engagement on CD4 T lymphocytes represses γ-radiation-induced apoptosis through activation of checkpoint kinase response elements. Blood. 2008;111:2704–2713. doi: 10.1182/blood-2007-07-104141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottalorda A, Verschelde C, Marcais A, Tomkowiak M, Musette P, Uematsu S, Akira S, Marvel J, Bonnefoy-Berard N. TLR2 engagement on CD8 T cells lowers the threshold for optimal antigen-induced T cell activation. Eur J Immunol. 2006;36:1684–1693. doi: 10.1002/eji.200636181. [DOI] [PubMed] [Google Scholar]
- Tabiasco J, Devevre E, Rufer N, Salaun B, Cerottini J C, Speiser D, Romero P. Human effector CD8+ T lymphocytes express TLR3 as a functional coreceptor. J Immunol. 2006;177:8708–8713. doi: 10.4049/jimmunol.177.12.8708. [DOI] [PubMed] [Google Scholar]
- Mokuno Y, Matsuguchi T, Takano M, Nishimura H, Washizu J, Ogawa T, Takeuchi O, Akira S, Nimura Y, Yoshikai Y. Expression of toll-like receptor 2 on gamma delta T cells bearing invariant V gamma 6/V delta 1 induced by Escherichia coli infection in mice. J Immunol. 2000;165:931–940. doi: 10.4049/jimmunol.165.2.931. [DOI] [PubMed] [Google Scholar]
- Zanin-Zhorov A, Nussbaum G, Franitza S, Cohen I R, Lider O. T cells respond to heat shock protein 60 via TLR2: activation of adhesion and inhibition of chemokine receptors. FASEB J. 2003;17:1567–1569. doi: 10.1096/fj.02-1139fje. [DOI] [PubMed] [Google Scholar]
- Ingalls R R, Lien E, Golenbock D T. Differential roles of TLR2 and TLR4 in the host response to Gram-negative bacteria: lessons from a lipopolysaccharide-deficient mutant of Neisseria meningitidis. J Endotoxin Res. 2000;6:411–415. [PubMed] [Google Scholar]
- Buwitt-Beckmann U, Heine H, Wiesmuller K H, Jung G, Brock R, Akira S, Ulmer A J. J. Biol Chem. 2006;281:9049–9057. doi: 10.1074/jbc.M512525200. [DOI] [PubMed] [Google Scholar]
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- Fitzgerald K A, Palsson E M, Bowie-McDermott A G, Jefferies CA, Mansell A S, Brady G, Brint E, Dunne A, Gray P, Harte M T, McMurray D, Smith D E, Sims J E, Bird T A, O'Neill L A. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413:78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- Davila E, Kang Y M, Park Y W, Sawai H, He X, Pryshchep S, Goronzy J J, Weyand C M. Cell-based immunotherapy with suppressor CD8+ T cells in rheumatoid arthritis. J Immunol. 2005;174:7292–7301. doi: 10.4049/jimmunol.174.11.7292. [DOI] [PubMed] [Google Scholar]
- Davila E, Celis E. Repeated administration of cytosine-phosphorothiolated guanine-containing oligonucleotides together with peptide/protein immunization results in enhanced CTL responses with anti-tumor activity. J Immunol. 2000;165:539–547. doi: 10.4049/jimmunol.165.1.539. [DOI] [PubMed] [Google Scholar]
- Davila E, Kennedy R, Celis E. Generation of antitumor immunity by cytotoxic T lymphocyte epitope peptide vaccination, CpG-oligodeoxynucleotide adjuvant, and CTLA-4 blockade. Cancer Res. 2003;63:3281–3288. [PubMed] [Google Scholar]
- Overwijk W W, Tsung A, Irvine K R, Parkhurst M R, Goletz T J, Tsung K, Carroll M W, Liu C, Moss B, Rosenberg S A, Restifo N P. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188:277–286. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabelitz D. Expression and function of Toll-like receptors in T lymphocytes. Curr Opin Immunol. 2007;19:39–45. doi: 10.1016/j.coi.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Schwarz K, Storni T, Manolova V, Didierlaurent A, Sirard J C, Rothlisberger P, Bachmann M F. Role of Toll-like receptors in costimulating cytotoxic T cell responses. Eur J Immunol. 2003;33:1465–1470. doi: 10.1002/eji.200323919. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- Glimcher L H, Townsend M J, Sullivan B M, Lord G M. Recent developments in the transcriptional regulation of cytolytic effector cells. Nat Rev Immunol. 2004;4:900–911. doi: 10.1038/nri1490. [DOI] [PubMed] [Google Scholar]
- Lugo-Villarino G, Ito S, Klinman D M, Glimcher L H. The adjuvant activity of CpG DNA requires T-bet expression in dendritic cells. Proc Natl Acad Sci U S A. 2005;102:13248–13253. doi: 10.1073/pnas.0506638102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighvani A A, Frucht D M, Jankovic D, Yamane H, Aliberti J, Hissong B D, Nguyen BV, Gadina M, Sher A, Paul W E, O'Shea J J. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc Natl Acad Sci U S A. 2001;98:15137–15142. doi: 10.1073/pnas.261570598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo-Villarino G, Maldonado-Lopez R, Possemato R, Penaranda C, Glimcher L H. T-bet is required for optimal production of IFN-gamma and antigen-specific T cell activation by dendritic cells. Proc Natl Acad Sci U S A. 2003;100:7749–7754. doi: 10.1073/pnas.1332767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobek V, Birkner N, Falk I, Wurch A, Kirschning C J, Wagner H, Wallich R, Lamers M C, Simon M M. Direct Toll-like receptor 2 mediated co-stimulation of T cells in the mouse system as a basis for chronic inflammatory joint disease. Arthritis Res Ther. 2004;6:R433–R446. doi: 10.1186/ar1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaghy A, Prakken B J, Takabayashi K, Firestein G S, Boyle D, Zvailfler N J, Roord S T, Albani S, Carson D A, Raz E. Immunostimulatory DNA sequences influence the course of adjuvant arthritis. J Immunol. 2002;168:51–56. doi: 10.4049/jimmunol.168.1.51. [DOI] [PubMed] [Google Scholar]
- Terato K, Harper D S, Griffiths M M, Hasty D L, Ye X J, Cremer M A, Seyer J M. Collagen-induced arthritis in mice: synergistic effect of E. coli lipopolysaccharide bypasses epitope specificity in the induction of arthritis with monoclonal antibodies to type II collagen. Autoimmunity. 1995;22:137–147. doi: 10.3109/08916939508995311. [DOI] [PubMed] [Google Scholar]
- Van der Heijden I M, Wilbrink B, Tchetverikov I, Schrijver I A, Schouls L M, Hazenberg M P, Breedveld F C, Tak P P. Presence of bacterial DNA and bacterial peptidoglycans in joints of patients with rheumatoid arthritis and other arthritides. Arthritis Rheum. 2000;43:593–598. doi: 10.1002/1529-0131(200003)43:3<593::AID-ANR16>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Kurts C, Miller J F, Subramaniam R M, Carbone F R, Heath W R. Major histocompatibility complex class I-restricted cross-presentation is biased towards high dose antigens and those released during cellular destruction. J Exp Med. 1998;188:409–414. doi: 10.1084/jem.188.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June C H. Principles of adoptive T cell cancer therapy. J Clin Invest. 2007;117:1204–1212. doi: 10.1172/JCI31446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leen A M, Rooney C M, Foster A E. Improving T cell therapy for cancer. Annu Rev Immunol. 2007;25:243–265. doi: 10.1146/annurev.immunol.25.022106.141527. [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Klebanoff C A, Palmer D C, Wrzesinski C, Kerstann K, Yu Z, Finkelstein S E, Theoret M R, Rosenberg S A, Restifo N P. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulos C M, Wrzesinski C, Kaiser A, Hinrichs C S, Chieppa M, Cassard L, Palmer D C, Boni A, Muranski P, Yu Z, Gattinoni L, Antony P A, Rosenberg S A, Restifo N P. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest. 2007;117:2197–2204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley M E, Wunderlich J, Nishimura M I, Yu D, Yang J C, Topalian S L, Schwartzentruber D J, Hwu P, Marincola F M, Sherry R, Leitman S F, Rosenberg S A. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother. 2001;24:363–373. doi: 10.1097/00002371-200107000-00012. [DOI] [PubMed] [Google Scholar]
- Dudley M E, Rosenberg S A. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, He X, Tsang T C, Harris D T. SING: a novel strategy for identifying tumor-specific, CTL-recognized tumor antigens. FASEB J. 2004;18:600–602. doi: 10.1096/fj.03-0881fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.