Abstract
Adenosine is an immunosuppressive nucleoside, and adenosine A2A receptors inhibit T-cell activation. We investigated the role of A2A receptors in regulating T helper (Th)1- and Th2-cell development and effector function. A2A-receptor stimulation suppressed the development of T-cell receptor (TCR) -stimulated naive T cells into both Th1 and Th2 cells, as indicated by decreased IFN-γ production by cells developed under Th1-skewing conditions and decreased interleukin (IL) -4, IL-5, and IL-10 production by cells developed under Th2-skewing conditions. Using A2A receptor-deficient mice, we demonstrate that A2A receptor activation inhibits Th1- and Th2-cell development by decreasing the proliferation and IL-2 production of naive T cells, irrespective of whether the cells are expanded under Th1- or Th2-skewing environment. Using in vivo established Th1 and Th2 cells, we further demonstrate the nonselective nature of A2A receptor-mediated immunosuppressive effects, because A2A receptor activation decreased IFN-γ and IL-4 secretion and mRNA level of TCR-stimulated effector Th1 and Th2 cells, respectively. A2A receptor mRNA expression in both Th1 and Th2 effector cells increased following TCR stimulation. In summary, these data demonstrate that A2A receptor activation has strong inhibitory actions during early developmental, as well as late effector, stages of Th1- and Th2-cell responses.—Csóka, B., Himer, L., Selmeczy, Z., Vizi, E. S., Pacher, P., Ledent, C., Deitch, E. A., Spolarics, Z., Németh, Z. H., Haskó, G. Adenosine A2A receptor activation inhibits T helper 1 and T helper 2 cell development and effector function.
Keywords: autoimmunity, allergy, inflammation, rheumatoid arthritis, spleen and lymph nodes
Adenosine is a purine nucleoside signaling molecule that accumulates extracellularly at sites of hypoxia, ischemia, and immune response/inflammation (1,2,3,4). Adenosine controls multiple physiological responses by engaging G protein-coupled cell surface receptors, which are the A1, A2A, A2B, and A3 receptors (5, 6). Adenosine regulates the function of both the innate and adaptive immune systems through targeting virtually every cell type that is involved in orchestrating an immune response: adenosine receptors are expressed on monocytes and macrophages (7, 8), dendritic cells (9, 10), mast cells (11,12,13,14), neutrophils (15,16,17), endothelial cells (18,19,20), eosinophils (21, 22), and epithelial cells (23, 24), as well as lymphocytes (25,26,27), natural killer (NK) cells (28,29,30), and NKT cells (31). Consistent with its multifaceted regulatory action on immune cells, adenosine has been shown to alter the course of a wide spectrum of infectious, allergic, and autoimmune diseases (1,2,3,4).
One paradigm that has been at the forefront of immunological research and that can explain susceptibility or resistance to immune-mediated disease is the T helper (Th)1/Th2 hypothesis (32). In response to microbial pathogens, CD4+ T cells differentiate into Th1 or Th2 cells; each of these subsets is responsible for activating immune responses adapted to the type of infectious agent. Th1 cells secrete IFN-γ and induce B cells to produce antibodies of the immunoglobulin G2 isotype, which are responsible for phagocyte activation and antibody-dependent cellular cytotoxicity and important for defense against intracellular pathogens (33). Th2 cells produce interleukin (IL) -4, IL-5, IL-10, and IL-13 and induce production of immunoglobulin E antibodies, which are responsible for immunity against parasitic infections (33). These mechanisms that are utilized to protect the host by activating a Th1- or Th2-type antimicrobial response can also be the source of significant tissue injury that is associated with autoimmune or allergic diseases.
Despite the importance of the Th1/Th2 paradigm as an overarching hypothesis that is currently driving our understanding and exploration of tissue injury that is associated with an immune response, there is a substantial gap in our knowledge of the role that adenosine and adenosine receptors have in governing Th1- and Th2-cell development. Recent studies utilizing naive mouse T cells stimulated short-term (up to 24 h) via the T-cell receptor (TCR) in the absence of antigen-presenting cells (APCs) or polarizing stimuli have demonstrated that adenosine receptor activation decreases the production of both Th1 and Th2 cytokines, effects that were mediated by the A2A receptor (34, 35). This is consistent with the predominant expression of A2A receptors on murine T lymphocytes (25,26,27). Nevertheless, the fact that the secretion of Th1 and Th2 cytokines by naive T cells was inhibited by A2A receptor activation in short-term (24 h) cultures cannot be interpreted as demonstration that Th1- and Th2-cell development was decreased, because a true Th1/Th2 polarization requires several cell cycles (4–5 days) and polarizing conditions to occur (36).
A further relevant question concerns the effect of adenosine receptor activation on effector Th1 and Th2 cells. This is important, because in addition to local adenosine levels being increased in response to the insult initiating the immune response, such as after an acute episode of Escherichia coli infection (37), adenosine concentrations can remain elevated during later phases of immune responses, even after the development of Th1 or Th2 effector cells (1, 12). For example, transgenic overexpression of the Th2 cytokine IL-13 in the lung results in chronic lung disease resembling the symptoms of chronic obstructive pulmonary disease, and there is a gradual and chronic increase in lung adenosine levels as the disease process progresses (38). In turn, increased levels of adenosine can promote IL-13 production, leading to an autoinductive cycle that amplifies and perpetuates inflammation.
In the current study, we addressed the hypothesis that A2A receptor activation can alter Th1- or Th2-cell differentiation in a model, in which naive murine CD4+ cells were stimulated for 5 days via the TCR and under truly polarizing conditions. In addition, we explored the effect of A2A receptor stimulation on the activation of Th1 and Th2 effector cell clones that were developed by in vivo immunization of mice.
MATERIALS AND METHODS
Experimental animals and drugs
Male BALB/c mice were purchased from Charles River Laboratories (Bar Harbor, ME, USA) or the National Institute of Oncology (Budapest, Hungary). A2A receptor knockout (KO) mice and their wild-type (WT) littermates (both on the CD-1 background) were bred in a specific pathogen-free facility, using founder heterozygous male and female mice (39, 40). All mice were maintained in accordance with the recommendations of the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the experiments were approved by the New Jersey Medical School Animal Care Committee or the Animal Care Committee of the Hungarian Academy of Sciences. The selective A2A receptor agonist 2-p-(2-carboxyethyl)phenethyl-amino-5′-N-ethyl-carboxamidoadenosine (CGS21680) was purchased from Sigma-Aldrich (St. Louis, MO, USA). The selective A2A receptor antagonist 4-(2-[7-amino-2-(2-furyl) [1.2.4]triazolo[2.3-a][1.3.5]triazin-5-ylamino] ethyl) phenol (ZM241385) was purchased from Tocris Cookson (Ellisville, MO, USA). Stock solutions of the various agents were prepared using dimethyl sulfoxide.
Isolation of CD4+ cells and in vitro development of Th1/Th2 cells
Splenocytes were harvested from 8- to 12 wk-old BALB/c or A2A adenosine receptor KO and WT littermate mice. Purified CD4+ cells were obtained by positive selection using magnetic beads coated with anti-CD4 Ab (Miltenyi Biotech, Auburn, CA, USA), according to the manufacturer’s protocol. This procedure yielded >98% pure CD4+ T cells (data not shown), as tested by flow cytometry using an anti-CD4+ Ab (BD Biosciences, San Jose, CA, USA). Purified CD4+ cells were resuspended in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% heat-inactivated FBS (Invitrogen), 100 U/ml penicillin/100 μg/ml streptomycin (Cellgro; Mediatech, Herndon, VA, USA), 50 mM HEPES (Invitrogen), and 50 μM β-mercaptoethanol (Sigma-Aldrich). Purified CD4+ T cells were activated in 24-well plates (106 cells/well) with soluble anti-CD3 Ab (3 μg/ml; BD Pharmingen, San Diego, CA, USA) and anti-CD28 Ab (2 μg/ml; BD Pharmingen). For Th1-polarizing conditions, 20 ng/ml IL-12 (PeproTech, Rocky Hill, NJ, USA) and 10 μg/ml anti-IL-4 Ab (BD Pharmingen) were added to the cultures, whereas 40 ng/ml IL-4 (PeproTech) and 10 μg/ml anti-IFN-γ Ab (BD Pharmingen) were added to achieve Th2-skewing conditions. CGS21680 (0.01–10 μM) or its vehicle was added to the cells 30 min before activation with anti-CD3/anti-CD28. After 5 days, the cells were washed and restimulated with plate-bound anti-CD3 Ab (3 μg/ml) and anti-CD28 Ab (2 μg/ml) for 24 h, following which supernatants were collected. In other experiments, IL-2 concentrations were measured from the supernatants at 0–24 h.
Measurement of cytokine levels
Supernatants were tested for IFN-γ, IL-2, IL-4, IL-5, and IL-10 protein content using ELISA Duoset kits (R&D Systems), according to the manufacturer’s instructions.
5-Carboxyfluorescein diacetate succinimidyl ester (CFSE) proliferation assay
Purified CD4+ T cells were resuspended at 10 × 106 cells/ml in PBS/0.1% BSA and labeled with CFSE (Molecular Probes, Eugene, OR, USA) by incubation for 10 min in 37°C/5% CO2 at a final concentration of 5 μM. Labeling was quenched with 5× volume ice-cold RPMI 1640 medium for 5 min, and the cells were washed twice before culturing on 24-well plates under Th1 or Th2-polarizing conditions, as described above. After 3 days, flow cytometry analysis was performed using a FACSCalibur flow cytometer (BD Biosciences).
Preparation of Th1 and Th2 hybridomas
Eight- to 12-wk-old female BALB/c mice were immunized by injecting them with 200 μg of hapten-carrier antigen FITC-KLH (Keyhole limpet hemocyanin from Megathura crenulata conjugated with fluorescein-5-isothyocianate; Sigma) emulsified 1:1 in CFA containing 1 mg/ml Mycobacterium tuberculosis (H37RA; heat-killed and dried; Sigma) into each hind footpad (50–50 μl), tail base (100 μl), and intraperitoneally (800 μl), as we have described previously (41).
At 11 days after immunization, the inguinal and popliteal lymph nodes were harvested, and erythrocytes were removed with ACK lysis buffer (0.15 M NH4Cl, 1 mM KHCO3, and 0.1 mM EDTA disodium salt, pH 7.2). Lymph node cells (5×106/ml) were differentiated into Th1 cells by activation with 25 μg/ml FITC-KLH and 1 μg/ml FITC-LPS in the presence of 0.2 μg/ml IFN-γ and 5 μg/ml anti-IL-4 or Th2 cells by incubation with FITC-KLH and FITC-LPS in the presence of 0.2 μg/ml IL-4 and 5 μg/ml anti-IFN-γ. After 3 days, CD8+ and FcγRII/III+ cells were removed from the mixture of cells by panning: the cells were incubated with rat anti-CD8 and rat anti-FcγR, and then placed onto goat anti-rat antibody-coated petri dish. Unbound CD4+ cells were collected by carefully removing them from the dish. All monoclonal antibodies and cytokines were purchased from R&D Systems (Minneapolis, MN, USA).
BWαβ− thymoma cells [American Type Culture Collection (ATCC), Manassas, VA, USA] were added to CD4+ cells at a ratio of 1:4 and incubated with fusion agent polyethylene-glycol (PEG; Hybri-Max ready-to-use solution; Sigma) for a few minutes, as we have previously described (41). Agglutinated cells were then subjected to limiting dilution by carefully adding GKN buffer (11 mM d-glucose, 5.5 mM KCl, 137 mM NaCl, 25 mM Na2HPO4, and 5.5 mM NaH2PO4·2H2O, pH 7.4). Diluted cells were cultured with feeder thymocytes in 96-well plates in HAT (hypoxanthine-aminopterin-thymidine-containing RPMI 1640 medium, Sigma) selection medium. After 10–12 days, when both the nonfused cells and feeder thymocytes died, surviving fused hybridoma cells were placed in normal RPMI 1640 medium, which contained 10% FCS. Clones that were positive for both CD3 and CD4, as assessed using flow cytometry, were used in later experiments. Hybridomas that produced primarily IFN-γ were designated as Th1 clones, and hybridomas producing high concentrations of IL-4 were designated as Th2 clones.
Determination of cytokine production by Th1 and Th2 hybridomas
To determine the role of A2A receptors in regulating cytokine production by Th1 or Th2 cells, 5 × 104 cells/well were placed in 96-well plates in l-glutamine-containing RPMI 1640 medium supplemented with 10% fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin (Life Technologies, San Diego, CA, USA). Cells were then treated with increasing concentrations of CGS21680 followed by stimulation with 5 μg/ml anti-CD3 and 2.5 × 104 cells/well LK35 B-lymphoma cells (ATCC) 30 min later. In antagonist experiments, cells were treated with increasing concentrations of ZM241385 (1–1000 nM) and 30 min later with 100 nM CGS21680. The cells were then activated 30 min later by treatment with 5 μg/ml anti-CD3 and 2.5 × 104 cells/well LK35 B-lymphoma cells. The cells were incubated at 37°C in a humidified atmosphere of 5% CO2 and 95% air for 16–18 h. Thereafter, the cells were centrifuged, and supernatants were collected for measuring IFN-γ or IL-4 levels using ELISA.
Real-time polymerase chain reaction (PCR) analysis of IFN-γ, IL-4, and adenosine receptor mRNA levels in Th1 and Th2 hybridomas
Cells were treated in the same way as described for cytokine production, and at the end of the incubation period, RNA was extracted using TRIzol (Invitrogen), as we have described before (42). Five micrograms of appropriately diluted RNA per sample was reverse transcribed into cDNA using 0.5 μl of oligo (dT)18 primer (0.5 μg/μl), 2 μl of 10× RT-PCR buffer, 2 μl of 25 mM MgCl2, 4 μl of 5 mM dNTP and 0.3 μl of Omniscript reverse transcriptase. This reaction mixture was supplemented with diethyl pyrocarbonate (DEPC) water to 20 μl final volume and heated to 99°C for 5 min, and then synthesis was continued for 15 min at 42°C using Eppendorf Mastercycler (Eppendorf North America, Westbury, NY, USA). cDNA was stored at −20°C. Real-time PCR was performed according to standard protocols using the LightCycler DNA Master SYBR Green I Kit (Roche, Indianapolis, IN, USA). cDNA samples were used as template, and data were normalized for 18S (endogenous housekeeping gene) levels. The following primers were used for cytokine mRNA detection: IL-4: 5′-CGA AGA ACA CCA CAG AGA GTG AGC T-3′ (forward), 5′-GAC TCA TTC ATG GTG CAG CTT ATC G-3′ (reverse); IFN-γ: 5′-AGC GGC TGA CTG AAC TCA GAT TGT AG-3′ (forward), 5′-GTC ACA GTT TTC AGC TGT ATA GGG-3′ (reverse); and 18S: 5′-GTA ACC CGT TGA ACC CCA TT-3′ (forward), 5′-CCA TCC AAT CGG TAG TAG CG-3′ (reverse). Primers for adenosine receptor mRNA detection were described previously (41). PCR conditions were optimized for primers, templates, and MgCl2.
Statistical analysis
Values in the figures are expressed as means ± se of n observations. Statistical analysis of the data was performed by Student’s t test or one-way analysis of variance followed by Dunnett’s test, as appropriate.
RESULTS
A2A receptor activation inhibits the development of Th1 and Th2 cells
CD4+ T cells obtained from BALB/c mice grown under Th1-polarizing conditions produced high amounts of IFN-γ on restimulation (1052±201 ng/ml; n=4), whereas IFN-γ production by Th2-differentiated restimulated cells was negligible (68±6 ng/ml; n=4). In contrast, CD4+ T cells cultured under Th2-polarizing conditions secreted high quantities of IL-4 (1032±23 ng/ml; n=4), IL-5 (349±18 ng/ml; n=4), and IL-10 (162±11 ng/ml; n=4), but Th1-differentiated cells released low levels of these cytokines (IL-4: 0.2±0.02 ng/ml; IL-5: 1.17±0.24 ng/ml; IL-10: 11.7±1.87 ng/ml; n=4). Differentiating BALB/c Th1 cells in the presence of increasing concentrations of the selective A2A receptor agonist CGS21680 inhibited, in a concentration-dependent manner, IFN-γ production by these cells when measured 24 h after restimulation (Fig. 1). Cells that were polarized toward the Th2 direction and exposed to CGS21680 throughout the differentiation period produced less IL-4, IL-5, and IL-10 on restimulation than vehicle-treated cells (Fig. 1). Again, the effect of CGS21680 was concentration dependent; however, CGS21680 was less efficacious in suppressing Th2-cell than Th1-cell cytokine production. These effects of CGS21680 on both Th1 and Th2 cytokines were reproduced using cells isolated from CD-1 mice (data not shown). We conclude that A2A receptor stimulation inhibits both Th1- and Th2-cell development.
Figure 1.
A2A receptor activation with CGS21680 decreases Th1- and Th2-cell development, as indicated by decreased production of IFN-γ (A) by restimulated Th1 cells and decreased production of IL-4 (B), IL-5 (C), and IL-10 (D) by restimulated Th2 cells. Th1-cell development of naive BALB/c splenic CD4+ cells was induced by TCR activation using anti-CD3/anti-CD28 in the presence of exogenous IL-12 and anti-IL-4 Ab, and Th2-cell development was elicited by TCR stimulation in the presence of exogenous IL-4 and anti-IFN-γ Ab. CGS21680 or its vehicle was added to the cells 30 min before TCR activation prior to inducing Th1- or Th2-cell development. After 5 days, the cells were washed and restimulated with anti-CD3/anti-CD28 for 24 h, following which supernatants were collected, and cytokine concentrations were measured using ELISA. Results are means ± se from one experiment representative of three separate experiments; n = 4 wells/group. *P < 0.05; **P < 0.01.
A2A receptor activation inhibits the proliferation of CD4+ T cells during development toward Th1 or Th2 direction
Because CGS21680 decreased the number of both Th1 and Th2 cells when counted at the end of the 5-day differentiation period (data not shown) and because CGS21680 has been shown to decrease the proliferation rate of naive CD4+ T cells (43), we next addressed the hypothesis that CGS21680 would suppress Th1/Th2 development by decreasing the proliferation of cells irrespective of the nature of the polarizing condition. Using CFSE staining of naive CD4+ T cells isolated from A2A receptor WT mice that were subsequently polarized toward a Th1 or Th2 profile, we found that CGS21680 decreased the proliferation rate of cells developing toward both Th1 and Th2 directions, as determined using flow cytometry (Fig. 2). We confirmed that CGS21680 acted on A2A receptors in decreasing both Th1- and Th2-cell proliferation, because CGS21680 failed to inhibit the proliferation of both Th1 and Th2 cells that were developed from naive CD4+ cells isolated from A2A receptor KO mice (Fig. 2).
Figure 2.
CGS21680 (CGS) decreases the proliferation of TCR-activated CD4+ T cells developing toward Th1 (A) and Th2 (B) directions in A2A receptor WT but not KO mice, as assessed using CFSE staining and flow cytometry. Th1- and Th2-cell development was induced as described in Fig. 1, and proliferation was examined 3 days after commencement of Th1 or Th2 development. CGS (100 nM) or its vehicle (veh) was added to the cells 30 min before TCR activation prior to inducing Th1- or Th2-cell development. Results are means ± se from one experiment representative of three separate experiments; n = 4 wells/group. **P < 0.01.
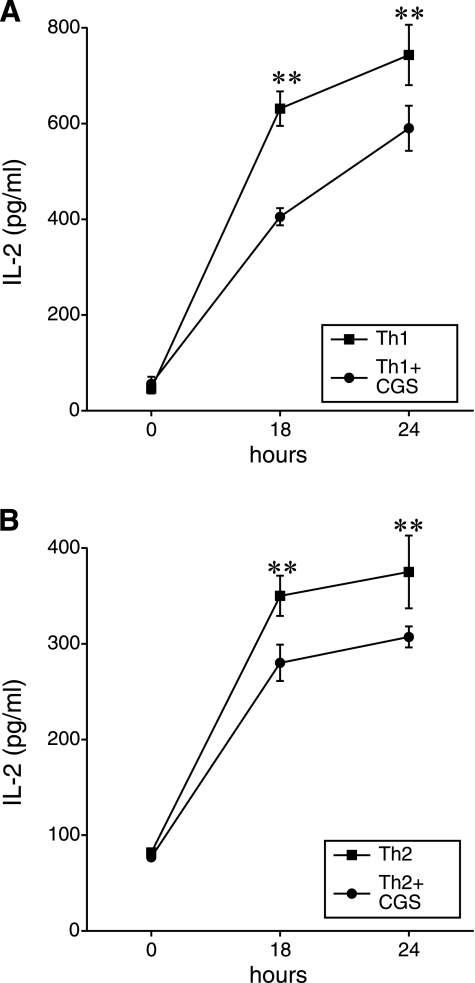
A2A receptor activation inhibits IL-2 production by developing Th1 or Th2 cells
Since IL-2 is a pivotal autocrine mediator in inducing proliferation of activated T cells, we next investigated the possibility that A2A receptor activation decreases Th1- and Th2-cell proliferation by reducing IL-2 production by developing Th1 and Th2 cells. Treatment of TCR-stimulated lymphocytes undergoing differentiation into Th1 or Th2 cells with CGS21680 markedly decreased IL-2 production by both developing Th1 and Th2 cells (Fig. 3).
Figure 3.
CGS21680 (CGS) decreases IL-2 production by TCR-activated CD4+ T cells developing toward Th1 (A) and Th2 (B) directions, as assessed using ELISA. Th1- and Th2-cell development was induced as described in Fig. 1, and IL-2 levels in cell-free supernatants were measured 0–24 h after commencement of Th1 or Th2 development. CGS (100 nM) or its vehicle (veh) was added to the cells 30 min before TCR activation prior to inducing Th1- or Th2-cell development. Results are means ± se from one experiment representative of three separate experiments; n = 4 wells/group. **P < 0.01.
A2A receptor activation inhibits IFN-γ and IL-4 production by established Th1- and Th2-cell clones, respectively
Having established that A2A receptor activation decreases Th1- and Th2-cell development, a process that occurs at the initial phases of an immune response, we next investigated the effect of A2A receptor activation on responses of established Th1 and Th2 cells, representing later stages of the immune response. We first examined the effect of CGS21680 on cytokine production by established Th1- or Th2-cell clones (41). Stimulation of the Th1 or Th2 clone with anti-CD3 and LK35 B-lymphoma APCs resulted in a predominant IFN-γ or IL-4 response, respectively. Stimulated Th1 cells produced 302 ± 40 pg/ml IFN-γ and 65 ± 4 pg/ml IL-4 (n=5), whereas nonstimulated cells produced less than 10 pg/ml of both cytokines. Stimulated Th2 cells produced 540 ± 12 pg/ml IL-4 and 60 ± 5 pg/ml IFN-γ (n=5). CGS21680 almost completely abolished the production of IFN-γ by the Th1 clone, with a half-maximal inhibitory concentration of ∼10 nM (Fig. 4A). CGS21680 also decreased IL-4 production, although with a lower potency, by the Th2 clone (Fig. 4B). The effect of CGS21680 on both IFN-γ and IL-4 production was completely prevented by the selective A2A antagonist ZM241385 (Fig. 4C, D), confirming involvement of A2A receptors. Finally, none of the agonists or antagonists had any toxic effect on either clone as determined using the MTT assay (data not shown).
Figure 4.
The A2A receptor agonist CGS21680 (CGS) decreases IFN-γ production by stimulated Th1 hybridoma cells (A) and IL-4 production by stimulated Th2 hybridoma cells (B). Basal IFN-γ production by Th1 cells was 3.4 ± 0.07 pg/ml (n=5) and basal IL-4 production by Th2 cells was 3.2 ± 0.27 pg/ml (n=5). Pretreatment of the cells with the A2A receptor antagonist ZM241385 (ZM) 30 min before adding CGS (100 nM) prevents the inhibitory effect of CGS on IFN-γ (C) and IL-4 (D) production. Cells were stimulated with anti-CD3 Ab and LK35 B-lymphoma cells (APCs) for 16–18 h in the presence or absence of CGS. CGS21680 or its vehicle was added to the cells 30 min before stimulation. Cytokine levels were determined from the supernatants using ELISA. Results are means ± se from three separate experiments; n = 6 wells/group. **P < 0.01 vs. 0 μM CGS21680; #P < 0.05 vs. vehicle+CGS; ##P < 0.01 vs. vehicle+CGS.
A2A receptor activation decreases IFN-γ and IL-4 mRNA accumulation in established Th1- and Th2-cell clones, respectively
To begin to understand the intracellular mechanism of the inhibitory effect of A2A receptor stimulation on cytokine production by established Th1 and Th2 cells, we evaluated the effect of CGS21680 on steady-state levels of IFN-γ and IL-4 mRNA in Th1 and Th2 cells, respectively. Figure 5 shows that pretreatment of Th1 and Th2 cells with CGS21680 decreased the steady-state levels of IFN-γ and IL-4 mRNA, respectively.
Figure 5.
The A2A receptor agonist CGS21680 (CGS) decreases IFN-γ mRNA levels in stimulated Th1 hybridoma cells (A) and IL-4 mRNA levels in stimulated Th2 hybridoma cells (B). Cells were stimulated with anti-CD3 Ab and LK35 B-lymphoma cells (APCs) for 16–18 h in the presence or absence of CGS (100 nM). CGS or its vehicle was added to the cells 30 min before stimulation. IFN-γ and IL-4 mRNA levels were determined using real-time PCR. Results are means ± se of n = 3 samples/group of one representative experiment from three separate experiments. **P < 0.01.
Stimulation using anti-CD3 and LK35 cells augments A2A mRNA accumulation in established Th1 and Th2 cells
We then sought to determine the expression level of A2A receptor mRNA in Th1 and Th2 cells both before and after stimulation with anti-CD3 and LK35 cells. Our results revealed that A2A receptor mRNA levels increase markedly at 4 h after stimulation in both Th1 and Th2 cells and remain elevated in Th2 but not Th1 cells at 8 h following stimulation (Tables 1 and 2). In addition, the level of A2B and A3 mRNA increases markedly following 8 h of stimulation in both Th1 and Th2 cells (Tables 1 and 2).
TABLE 1.
mRNA expression of adenosine receptors in a Th1 hybridoma cell line
| Receptor | 0 h | 4 h | 8 h | 16 h |
|---|---|---|---|---|
| A1 | ||||
| Control | 141 ± 22.7 | 111 ± 10.6 | 141 ± 9.7 | 190 ± 43 |
| Activated | 119 ± 19 | 86.2 ± 7.8 | 236 ± 40.9* | 132 ± 13.4 |
| A2A | ||||
| Control | 13366 ± 887 | 10809 ± 1000 | 6851 ± 892 | 4648 ± 1564 |
| Activated | 14598 ± 2777 | 43527 ± 5640** | 8710 ± 1837 | 2213 ± 537 |
| A2B | ||||
| Control | 2442 ± 572 | 3126 ± 401 | 1730 ± 159 | 2424 ± 666 |
| Activated | 2075 ± 139 | 2857 ± 271 | 4984 ± 1030* | 4311 ± 1044 |
| A3 | ||||
| Control | 8005 ± 644 | 7585 ± 884 | 5350 ± 479 | 3614 ± 904 |
| Activated | 7233 ± 717 | 6353 ± 663 | 12998 ± 2571** | 3213 ± 259 |
mRNA levels of adenosine receptors and housekeeping gene 18S were determined using real-time PCR. Data are expressed as arbitrary units of ratio of expression of adenosine receptors and 18S. Results are expressed as means ± se (n=12–14 from 3 separate experiments).
P < 0.05,
P < 0.01 vs. control.
TABLE 2.
mRNA expression of adenosine receptors in a Th2 hybridoma cell line
| Receptor | 0 h | 4 h | 8 h | 16 h |
|---|---|---|---|---|
| A1 | ||||
| Control | 97.1 ± 18.6 | 132 ± 18.7 | 128 ± 16 | 133 ± 16.3 |
| Activated | 107 ± 18 | 136 ± 21.9 | 130 ± 13.9 | 144 ± 13.7 |
| A2A | ||||
| Control | 11209 ± 2314 | 9006 ± 1406 | 5602 ± 621 | 9930 ± 1154 |
| Activated | 10464 ± 1519 | 44249 ± 7401** | 30292 ± 5157** | 3765 ± 547 |
| A2B | ||||
| Control | 1260 ± 344 | 1201 ± 166 | 1732 ± 235 | 2706 ± 718 |
| Activated | 1714 ± 291 | 1644 ± 126 | 5154 ± 447** | 4547 ± 782 |
| A3 | ||||
| Control | 5701 ± 148 | 5755 ± 631 | 5671 ± 531 | 6950 ± 183 |
| Activated | 5169 ± 745 | 5152 ± 714 | 10312 ± 838** | 7075 ± 769 |
mRNA levels of adenosine receptors and housekeeping gene 18S were determined using real-time PCR. Data are expressed as arbitrary units of ratio of expression of adenosine receptors and 18S. Results are expressed as means ± se (n=12–14 from 3 separate experiments).
P < 0.01 vs. control.
DISCUSSION
Prior to the current study, the direct effect of A2A receptor activation on Th1/Th2 responses had not been addressed in detail. Adenosine via A2A receptors had been shown to diminish IFN-γ production by naive (nonpolarized) murine CD4+ T cells (34), and we previously documented that inosine, a purine able to activate adenosine receptors, suppresses IFN-γ production by anti-CD3-stimulated splenocytes, under nonpolarizing conditions (44). Also, A2A receptor activation was recently shown to limit IL-4 and IL-10 secretion by naive murine CD4+ T cells (35). In addition, Sitkovsky and coworkers detected decreased IFN-γ and IL-4 expression in A2A receptor-expressing human Th cells that were stimulated with phorbol 12-myristate 13-acetate when compared to stimulated T cells that did not express A2A receptors (25), suggesting an A2A receptor-mediated down-regulation of cytokine production. However, because in these previous studies, the role of adenosine was studied in short-term cultures (24 h), in which T-cell receptor stimulation by anti-CD3 Ab induces both Th1 and Th2 cytokine production, and T-cell responses are not polarized toward secretion of only Th1 or Th2 cytokines, a clear role for adenosine in regulating Th1- and Th2-cell development could not be established.
In the current study, we polarized naive T cells for 5 days in the presence of polarizing stimuli (IL-12 for Th1 and IL-4 for Th2 development), a procedure that clearly established Th1 or Th2 responses, where Th1 cells produced only IFN-γ and Th2 cells only IL-4. Our results using this truly polarizing system demonstrate that direct activation of A2A receptors on CD4+ lymphocytes uniformly blocks the in vitro development of both Th1 and Th2 cells, and this effect is achieved by preventing expansion of naive T cells, irrespective of whether they are exposed to Th1- or Th2-polarizing conditions. Previous studies have indicated that adenosine inhibits TCR- or IL-2-triggered lymphocyte proliferation (43, 45, 46). The current results extend these observations by providing genetic evidence that A2A receptor activation can potently down-regulate CD4+ lymphocyte expansion: CGS21680 inhibited the proliferation of CD4+ cells that were isolated from A2A receptor WT mice but failed to block the proliferation of cells obtained from A2A KO mice. We also found that A2A receptor activation suppressed IL-2 production by T cells, again, independently of whether the cells were expanded under Th1- or Th2-polarizing conditions. Because IL-2 is critical for TCR-induced T-cell proliferation (45), the early inhibition of T-cell IL-2 production provides mechanistic insight, explaining the antiproliferative action of CGS21680.
Although our data suggest that A2A receptor activation on T lymphocytes decreases both Th1- and Th2-cell development, A2A receptor activation can, indirectly, skew Th1/Th2 development toward a Th2 phenotype. Specifically, stimulating A2A receptors on dendritic cells has recently been shown to drive a predominantly Th2-type response, because naive T cells primed with dendritic cells that had been exposed to adenosine produced decreased amounts of IFN-γ and increased amounts of IL-5 (10), indicating that adenosine can indirectly affect Th1/Th2 development by interfering with APC function. On the basis of these results, it was suggested that this preferential Th2-cell-development skewing effect of adenosine was secondary to A2A receptor-mediated suppression of the production of IL-12, a major Th1-inducing cytokine, by dendritic cells.
In addition to demonstrating a negative impact of A2A receptor activation on the development of Th1 and Th2 cells, our results also revealed that A2A receptor activation reduces IFN-γ and IL-4 production by in vivo established Th1 and Th2 cells, respectively. It is noteworthy that although ZM241385 completely reversed the suppressive effect of CGS21680 on both IL-4 and IFN-γ production, clearly indicating a role for A2A receptors, CGS21680 was less efficacious in inhibiting IL-4 production. Supporting a predominant role for A2A receptors in mediating the suppressive effect of CGS21680 on cytokine production is the observation that A2A receptor expression is highest when compared to all other adenosine receptors. In addition, A2A receptor expression rapidly increases following both Th1- and Th2-cell stimulation, while A2B and A3 receptor expression increases to a lesser degree and in a more delayed fashion. One caveat with these results is that we only measured receptor expression at the mRNA level and these mRNA levels may not reflect the expression of functional receptors on the cell membrane. Nevertheless, our demonstration of increased A2A mRNA expression following T-cell stimulation is in agreement with results of previous studies (34, 47).
The mechanism behind these inhibitory actions of A2A receptors on IL-4 and IFN-γ production appears to be pretranslational, as A2A receptor activation reduced mRNA levels of IFN-γ and IL-4 in Th1 and Th2 cells, respectively. A recent study showed that adenosine inhibited cytokine production by Th1 and Th2 effector cells (48), but A2A receptors were only marginally implicated. Because the suppressive activity of adenosine on IFN-γ production by Th1 cells was abrogated by an A2A receptor antagonist, it was proposed that the effect of adenosine on IFN-γ production by Th1 cells was mediated by A2A receptors (48); however, the role of A2A receptors in producing the effect of adenosine on IL-4 production by Th2 cells was not investigated.
In summary, these data demonstrate that A2A receptor activation has strong inhibitory actions on both Th1 and Th2 cells during early and late stages of lymphocyte activation. These inhibitory effects of A2A receptor activation on Th1- and Th2-cell activation can also be extended to another inflammation-inducing Th subset, Th17 cells (47). In contrast, A2A receptor activation increases the induction of various subsets of anti-inflammatory T-regulatory cells (47), indicating that A2A-receptor activation promotes an overall anti-inflammatory T-cell environment. Further advances in understanding the immunosuppressive effects of A2A-receptor agonists may help develop therapeutic approaches to target inflammatory, autoimmune, and neoplastic disorders (1,2,3,4, 49).
Acknowledgments
This work was supported by U.S. National Institutes of Health (NIH) grant R01 GM66189 and the Intramural Research Program of NIH, National Institute on Alcohol Abuse and Alcoholism, as well as Hungarian Research Fund OTKA (T 049537) and Hungarian National R&D Programme 1A/036/2004.
References
- Hasko G, Cronstein B N. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Sitkovsky M V, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, Thiel M. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- Bours M J, Swennen E L, Di Virgilio F, Cronstein B N, Dagnelie P C. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Fredholm B B, Ijzerman A P, Jacobson K A, Klotz K N, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Hasko G, Pacher P, Deitch E A, Vizi E S. Shaping of monocyte and macrophage function by adenosine receptors. Pharmacol Ther. 2007;113:264–275. doi: 10.1016/j.pharmthera.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoa N D, Montesinos M C, Reiss A B, Delano D, Awadallah N, Cronstein B N. Inflammatory cytokines regulate function and expression of adenosine A2A receptors in human monocytic THP-1 cells. J Immunol. 2001;167:4026–4032. doi: 10.4049/jimmunol.167.7.4026. [DOI] [PubMed] [Google Scholar]
- Panther E, Idzko M, Herouy Y, Rheinen H, Gebicke-Haerter P J, Mrowietz U, Dichmann S, Norgauer J. Expression and function of adenosine receptors in human dendritic cells. FASEB J. 2001;15:1963–1970. doi: 10.1096/fj.01-0169com. [DOI] [PubMed] [Google Scholar]
- Panther E, Corinti S, Idzko M, Herouy Y, Napp M, la Sala A, Girolomoni G, Norgauer J. Adenosine affects expression of membrane molecules, cytokine and chemokine release, and the T-cell stimulatory capacity of human dendritic cells. Blood. 2003;101:3985–3990. doi: 10.1182/blood-2002-07-2113. [DOI] [PubMed] [Google Scholar]
- Feoktistov I, Biaggioni I. Adenosine A2B receptors. Pharmacol Rev. 1997;49:381–402. [PubMed] [Google Scholar]
- Blackburn M R. Too much of a good thing: adenosine overload in adenosine-deaminase-deficient mice. Trends Pharmacol Sci. 2003;24:66–70. doi: 10.1016/S0165-6147(02)00045-7. [DOI] [PubMed] [Google Scholar]
- Hua X, Kovarova M, Chason K D, Nguyen M, Koller B H, Tilley S L. Enhanced mast cell activation in mice deficient in the A2b adenosine receptor. J Exp Med. 2007;204:117–128. doi: 10.1084/jem.20061372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozard J R. The case for a role for adenosine in asthma: almost convincing? Curr Opin Pharmacol. 2003;3:264–269. doi: 10.1016/s1471-4892(03)00039-0. [DOI] [PubMed] [Google Scholar]
- Cronstein B N, Daguma L, Nichols D, Hutchison A J, Williams M. The adenosine/neutrophil paradox resolved: human neutrophils possess both A1 and A2 receptors that promote chemotaxis and inhibit O2 generation, respectively. J Clin Invest. 1990;85:1150–1157. doi: 10.1172/JCI114547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan G W, Linden J, Buster B L, Scheld W M. Neutrophil A2A adenosine receptor inhibits inflammation in a rat model of meningitis: synergy with the type IV phosphodiesterase inhibitor, rolipram. J Infect Dis. 1999;180:1550–1560. doi: 10.1086/315084. [DOI] [PubMed] [Google Scholar]
- Fortin A, Harbour D, Fernandes M, Borgeat P, Bourgoin S. Differential expression of adenosine receptors in human neutrophils: up-regulation by specific Th1 cytokines and lipopolysaccharide. J Leukoc Biol. 2006;79:574–585. doi: 10.1189/jlb.0505249. [DOI] [PubMed] [Google Scholar]
- Eltzschig H K, Thompson L F, Karhausen J, Cotta R J, Ibla J C, Robson S C, Colgan S P. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism. Blood. 2004;104:3986–3992. doi: 10.1182/blood-2004-06-2066. [DOI] [PubMed] [Google Scholar]
- Thompson L F, Eltzschig H K, Ibla J C, Van De Wiele C J, Resta R, Morote-Garcia J C, Colgan S P. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A, Victor-Vega C, Gadangi S, Montesinos M C, Chu C C, Cronstein B N. Adenosine A2A receptor stimulation increases angiogenesis by down-regulating production of the antiangiogenic matrix protein thrombospondin 1. Mol Pharmacol. 2005;67:1406–1413. doi: 10.1124/mol.104.007807. [DOI] [PubMed] [Google Scholar]
- Kohno Y, Ji X, Mawhorter S D, Koshiba M, Jacobson K A. Activation of A3 adenosine receptors on human eosinophils elevates intracellular calcium. Blood. 1996;88:3569–3574. [PMC free article] [PubMed] [Google Scholar]
- Knight D, Zheng X, Rocchini C, Jacobson M, Bai T, Walker B. Adenosine A3 receptor stimulation inhibits migration of human eosinophils. J Leukoc Biol. 1997;62:465–468. doi: 10.1002/jlb.62.4.465. [DOI] [PubMed] [Google Scholar]
- Sitaraman S V, Merlin D, Wang L, Wong M, Gewirtz A T, Si-Tahar M, Madara J L. Neutrophil-epithelial crosstalk at the intestinal lumenal surface mediated by reciprocal secretion of adenosine and IL-6. J Clin Invest. 2001;107:861–869. doi: 10.1172/JCI11783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Wu Y, Belardinelli L, Zeng D. A2B adenosine receptors induce IL-19 from bronchial epithelial cells, resulting in TNF-α increase. Am J Respir Cell Mol Biol. 2006;35:587–592. doi: 10.1165/rcmb.2005-0476OC. [DOI] [PubMed] [Google Scholar]
- Koshiba M, Rosin D L, Hayashi N, Linden J, Sitkovsky M V. Patterns of A2A extracellular adenosine receptor expression in different functional subsets of human peripheral T cells. Flow cytometry studies with anti-A2A receptor monoclonal antibodies. Mol Pharmacol. 1999;55:614–624. [PubMed] [Google Scholar]
- Apasov S, Chen J F, Smith P, Sitkovsky M. A2A receptor dependent and A(2A) receptor independent effects of extracellular adenosine on murine thymocytes in conditions of adenosine deaminase deficiency. Blood. 2000;95:3859–3867. [PubMed] [Google Scholar]
- Armstrong J M, Chen J F, Schwarzschild M A, Apasov S, Smith P T, Caldwell C, Chen P, Figler H, Sullivan G, Fink S, Linden J, Sitkovsky M. Gene dose effect reveals no Gs-coupled A2A adenosine receptor reserve in murine T-lymphocytes: studies of cells from A2A-receptor-gene-deficient mice. Biochem J. 2001;354:123–130. doi: 10.1042/0264-6021:3540123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B A, Blay J, Hoskin D W. 2-chloroadenosine stimulates granule exocytosis from mouse natural killer cells: evidence for signal transduction through a novel extracellular receptor. Exp Cell Res. 1997;233:187–197. doi: 10.1006/excr.1997.3530. [DOI] [PubMed] [Google Scholar]
- Harish A, Hohana G, Fishman P, Arnon O, Bar-Yehuda S. A3 adenosine receptor agonist potentiates natural killer cell activity. Int J Oncol. 2003;23:1245–1249. [PubMed] [Google Scholar]
- Raskovalova T, Huang X, Sitkovsky M, Zacharia L C, Jackson E K, Gorelik E. Gs protein-coupled adenosine receptor signaling and lytic function of activated NK cells. J Immunol. 2005;175:4383–4391. doi: 10.4049/jimmunol.175.7.4383. [DOI] [PubMed] [Google Scholar]
- Lappas C M, Day Y J, Marshall M A, Engelhard V H, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T R, Cherwinski H, Bond M W, Giedlin M A, Coffman R L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- Mosmann T R, Coffman R L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Lappas C M, Rieger J M, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J Immunol. 2005;174:1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- Naganuma M, Wiznerowicz E B, Lappas C M, Linden J, Worthington M T, Ernst P B. Cutting edge: Critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J Immunol. 2006;177:2765–2769. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- Bird J J, Brown D R, Mullen A C, Moskowitz N H, Mahowald M A, Sider J R, Gajewski T F, Wang C R, Reiner S L. Helper T cell differentiation is controlled by the cell cycle. Immunity. 1998;9:229–237. doi: 10.1016/s1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]
- Rogachev B, Ziv N Y, Mazar J, Nakav S, Chaimovitz C, Zlotnik M, Douvdevani A. Adenosine is upregulated during peritonitis and is involved in downregulation of inflammation. Kidney Int. 2006;70:675–681. doi: 10.1038/sj.ki.5001609. [DOI] [PubMed] [Google Scholar]
- Blackburn M R, Lee C G, Young H W, Zhu Z, Chunn J L, Kang M J, Banerjee S K, Elias J A. Adenosine mediates IL-13-induced inflammation and remodeling in the lung and interacts in an IL-13-adenosine amplification pathway. J Clin Invest. 2003;112:332–344. doi: 10.1172/JCI16815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledent C, Vaugeois J M, Schiffmann S N, Pedrazzini T, El Yacoubi M, Vanderhaeghen J J, Costentin J, Heath J K, Vassart G, Parmentier M. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature. 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- Nemeth Z H, Csoka B, Wilmanski J, Xu D, Lu Q, Ledent C, Deitch E A, Pacher P, Spolarics Z, Hasko G. Adenosine A2A receptor inactivation increases survival in polymicrobial sepsis. J Immunol. 2006;176:5616–5626. doi: 10.4049/jimmunol.176.9.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth Z H, Bleich D, Csoka B, Pacher P, Mabley J G, Himer L, Vizi E S, Deitch E A, Szabo C, Cronstein B N, Hasko G. Adenosine receptor activation ameliorates type 1 diabetes. FASEB J. 2007;21:2379–2388. doi: 10.1096/fj.07-8213com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth Z H, Lutz C S, Csoka B, Deitch E A, Leibovich S J, Gause W C, Tone M, Pacher P, Vizi E S, Hasko G. Adenosine augments IL-10 production by macrophages through an A2B receptor-mediated posttranscriptional mechanism. J Immunol. 2005;175:8260–8270. doi: 10.4049/jimmunol.175.12.8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Apasov S, Koshiba M, Sitkovsky M. Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood. 1997;90:1600–1610. [PubMed] [Google Scholar]
- Hasko G, Kuhel D G, Nemeth Z H, Mabley J G, Stachlewitz R F, Virag L, Lohinai Z, Southan G J, Salzman A L, Szabo C. Inosine inhibits inflammatory cytokine production by a posttranscriptional mechanism and protects against endotoxin-induced shock. J Immunol. 2000;164:1013–1019. doi: 10.4049/jimmunol.164.2.1013. [DOI] [PubMed] [Google Scholar]
- Zhang H, Conrad D M, Butler J J, Zhao C, Blay J, Hoskin D W. Adenosine acts through A2 receptors to inhibit IL-2-induced tyrosine phosphorylation of STAT5 in T lymphocytes: role of cyclic adenosine 3′,5′-monophosphate and phosphatases. J Immunol. 2004;173:932–944. doi: 10.4049/jimmunol.173.2.932. [DOI] [PubMed] [Google Scholar]
- Erdmann A A, Gao Z G, Jung U, Foley J, Borenstein T, Jacobson K A, Fowler D H. Activation of Th1 and Tc1 cell adenosine A2A receptors directly inhibits IL-2 secretion in vitro and IL-2-driven expansion in vivo. Blood. 2005;105:4707–4714. doi: 10.1182/blood-2004-04-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarek P E, Huang C T, Lutz E R, Kowalski J, Horton M R, Linden J, Drake C G, Powell J D. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111:251–259. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobie J J, Shah P R, Yang L, Rebhahn J A, Fowell D J, Mosmann T R. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′-adenosine monophosphate to adenosine. J Immunol. 2006;177:6780–6786. doi: 10.4049/jimmunol.177.10.6780. [DOI] [PubMed] [Google Scholar]
- Ohta A, Gorelik E, Prasad S J, Ronchese F, Lukashev D, Wong M K, Huang X, Caldwell S, Liu K, Smith P, Chen J F, Jackson E K, Apasov S, Abrams S, Sitkovsky M. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]