Abstract
Degradation of the cartilage proteoglycan aggrecan is a key early event in the development of osteoarthritis. Adamalysin with thrombospondin motifs (ADAMTS) -4 and ADAMTS-5 are considered to be the main enzymes responsible for aggrecan breakdown, making them attractive drugs targets. Here we show that calcium pentosan polysulfate (CaPPS), a chemically sulfated xylanopyranose from beechwood, is a multifaceted exosite inhibitor of the aggrecanases and protects cartilage against aggrecan degradation. CaPPS interacts with the noncatalytic spacer domain of ADAMTS-4 and the cysteine-rich domain of ADAMTS-5, blocking activity against their natural substrate aggrecan with inhibitory concentration 50 values of 10–40 nM but only weakly inhibiting hydrolysis of a nonglycosylated recombinant protein substrate. In addition, CaPPS increased cartilage levels of tissue inhibitor of metalloproteinases-3 (TIMP-3), an endogenous inhibitor of ADAMTS-4 and -5. This was due to the ability of CaPPS to block endocytosis of TIMP-3 mediated by low-density lipoprotein receptor-related protein. CaPPS also increased the affinity of TIMP-3 for ADAMTS-4 and -5 by more than 100-fold, improving the efficacy of TIMP-3 as an aggrecanase inhibitor. Studies with TIMP-3-null mouse cartilage indicated that CaPPS inhibition of aggrecan degradation is TIMP-3 dependent. These unique properties make CaPPS a prototypic disease-modifying agent for osteoarthritis.—Troeberg, L., Fushimi, K., Khokha, R., Emonard, H., Ghosh, P., Nagase, H. Calcium pentosan polysulfate is a multifaceted exosite inhibitor of aggrecanases.
Keywords: ADAMTS, LRP, osteoarthritis, cartilage destruction, proteoglycan
Osteoarthritisc (OA) is characterized by destruction of the cartilage matrix, which results in impairment of articular joint function. Degradation of the matrix proteoglycan aggrecan is an early event, crucial for the development of OA lesions (1), and inhibitors of aggrecan breakdown therefore have therapeutic potential as disease-modifying agents for OA (2). Analysis of synovial fluids and cartilage lesions of patients with OA indicates that matrix metalloproteinases (MMPs) and aggrecanases, which are members of the adamalysin with thrombospondin motifs (ADAMTS) family, are the main aggrecan-degrading enzymes (3,4,5). Among them, ADAMTS-5 (aggrecanase 2) appears to be a major aggrecanase in mice (6, 7), but both ADAMTS-4 (aggrecanase 1) and ADAMTS-5 are considered to play a role in human disease (8). Thus interest has been growing in the development of inhibitors for ADAMTS-4 and -5 (9,10,11), mirroring the development of MMP inhibitors for the treatment of diseases associated with elevated MMP activity. Numerous MMP inhibitors have been developed, and several were clinically tested in patients with cancer (12) and rheumatoid arthritis (13), but they failed to show efficacy and exhibited side effects such as musculoskeletal pain and mild thrombocytopenia (12). These failures are thought to be due to the lack of selectivity of the inhibitors and inhibition of biologically important off-target metalloproteinases and other effects. Selectivity is thus a prerequisite for nontoxic therapeutic inhibitors.
One way to increase selectivity against specific metalloproteinases is to generate exosite or allosteric inhibitors. MMPs and ADAMTSs are multidomain proteins, and in many cases their noncatalytic domains play an important role in recognition and effective cleavage of their natural extracellular matrix (ECM) substrates. For example, collagenases (MMP-1, MMP-8, and MMP-13) require their hemopexin domain to cleave triple helical collagen (14), and the noncatalytic ancillary domains of ADAMTS-4 and -5 are essential for cleaving aggrecan core protein (15,16,17). Molecules that bind to an enzyme exosite block interaction with natural ECM substrates and are an attractive alternative to active site-directed inhibitors because they can be highly specific and effectively block hydrolysis only of the target substrate, thus minimizing in vivo side effects.
Calcium pentosan polysulfate (CaPPS) is a calcium salt form of chemically sulfated beechwood xylosan, with a molecular mass of 4000–6000 Da. It has previously been shown to be an effective antiarthritic agent in various animal models and in some human OA trials (18,19,20). Although the mechanism of its action was not fully understood, CaPPS has been shown to block aggrecan breakdown (21). It was also reported that CaPPS increases production of the endogenous aggrecanase inhibitor tissue inhibitor of metalloproteinases (TIMP)-3 by rheumatoid synovial (22) and gingival fibroblasts (23). At about that time, we found that TIMP-3 is a potent inhibitor of ADAMTS-4 and -5 (24) and showed that TIMP-3 effectively blocks aggrecan breakdown in interleukin (IL) -1α-stimulated cartilage in culture (25). From these observations we postulated that CaPPS blocks aggrecan breakdown by increasing TIMP-3 levels in the cartilage. In this report we show that CaPPS indeed increases TIMP-3 levels in the cartilage without changing its mRNA levels. We found that this increase is due to blockade of TIMP-3 endocytosis, which is mediated by low-density lipoprotein receptor-related protein (LRP). In addition, CaPPS directly inhibits the aggrecanase activity of ADAMTS-4 and -5 as an exosite inhibitor. Furthermore, CaPPS greatly enhances TIMP-3 affinity for ADAMTS-4 and -5 but not for MMPs. These unique properties of CaPPS provide a new paradigm for the development of novel types of antiosteoarthritic agents.
MATERIALS AND METHODS
Reagents and antibodies
CaPPS was from Arthropharm (Sydney, Australia). Porcine heparin, desulfated porcine heparin, human hyaluronan, and type 1A bacterial collagenase were from Sigma-Aldrich (Dorset, UK). Bovine chondroitin-4-sulfate and all-trans retinoic acid were from Fluka (Seelze, Germany). Porcine dermatan sulfate was from Calbiochem (San Diego, CA, USA). Sucrose octasulfate was from Toronto Research Chemicals (North York, ON, Canada). Chondroitinase ABC and keratanase were from Seikagaku (Tokyo, Japan). Recombinant human IL-1α was kindly provided by Prof. J. Saklatvala (Kennedy Institute of Rheumatology, London, UK). Bovine nasal aggrecan (26), human receptor-associated protein (RAP) (27), and human ADAMTS-4 and -5 (15,16,17) were prepared as described previously. Recombinant human TIMP-3 was expressed in HEK293 using a pCEP4-based expression vector (Invitrogen, Paisley, UK) kindly provided by Prof. M. Seiki (University of Tokyo, Japan). TIMP-3-containing medium was prepared by incubating cells with 250 nM RAP in serum-free Dulbecco modified Eagle medium (DMEM) for 36 h and concentrating the medium 10-fold. TIMP-3 concentration was determined by titration against a known concentration of the catalytic domain of MMP-1 using a fluorescent substrate enzyme assay. A recombinant interglobular domain of human aggrecan (330YTGED to HLPGG458) tagged with glutathione S-transferase and FLAG was expressed in Escherichia coli (unpublished results). The 2-B-6 antibody that recognizes deglycosylated aggrecan fragments (28), BC-3 antibody that recognizes the N-terminal ARGSV generated by aggrecanase cleavage of the bovine aggrecan core protein at the Glu373-Ala374 bond (29), and BC-14 antibody that recognizes the N-terminal FFGVG generated by MMP cleavage of bovine aggrecan at Ser341-Phe342 (30) were kind gifts from Prof. B. Caterson and Dr. C. Hughes (University of Cardiff, Cardiff, UK). The anti-GELE antibody that recognizes the C-terminal GELE generated by aggrecanase cleavage of bovine aggrecan at Glu1480-Gly1481 has been previously described (16). The rabbit anti-AGEG antibody that recognizes the N-terminal AGEG generated by aggrecanase cleavage of bovine aggrecan at Glu1771-Ala1772 was raised in a rabbit against the aggrecan peptide sequence AGEGPSGGC (amino acids 1772 to 1778, amino acids in italics not part of the aggrecan sequence but added to link the peptide to keyhole limpet hemocyanin). Mouse anti-TIMP-3 antibodies were from Daiichi Fine Chemicals (136–13H4, Toyama, Japan; used at 1:1000 for all experiments except cartilage extraction) and R&D Systems (MAB9731, Minneapolis, MN, USA; used at 1:1000 to analyze cartilage extracts). Sheep anti-TIMP-1 and anti-TIMP-2 have been previously described (31, 32).
Human and porcine cartilage explant cultures
Human osteoarthritic cartilage was obtained from patients undergoing joint replacement surgery at Heatherwood and Wexham Park Hospitals (Slough, UK) and the Orthopaedic Surgery Department of Charing Cross Hospital (London, UK) with the consent of patients and the East Berkshire and Riverside Research Ethics Committees. Human and porcine (25) cartilage explants were grown (5% CO2, 37°C) in serum-free DMEM for 3 d with 1 μM all-trans retinoic acid or IL-1α (10 ng/ml), respectively, either with or without CaPPS. Glycosaminoglycan released into the medium was measured using the dimethylenemethylene blue assay (33). Aggrecan fragments in the medium were deglycosylated overnight with chondroitinase ABC and keratanase (0.01 U each/10 μg dry weight of aggrecan) and analyzed by Western blotting.
Extraction of TIMP-3 from cartilage matrix
Cartilage explants (4 g cartilage/treatment) were dissected from porcine metacarpophalangeal joints and treated with IL-1α (10 ng/ml) with or without CaPPS (50 μg/ml) in 20 ml of serum-free DMEM for 3 d. The conditioned media were concentrated by precipitation with 5% trichloroacetic acid. The cartilage was extracted for 4 d at 4°C with 10 ml of 4 M guanidine hydrochloride, 50 mM sodium acetate (pH 5.8) with 5 mM EDTA, 1 mM p-aminoethylbenzene sulfonyl fluoride, and 10 μM E-64. The extracts were passed through a cell strainer, CsCl was added to give a final density of 1.5 g/ml, and the mixture was ultracentrifuged (26). After centrifugation, the top 3/5 of the gradient was dialyzed against 50 mM Tris HCl (pH 7.5) with 150 mM NaCl, and proteins were precipitated with trichloroacetic acid. Equivalent volumes of conditioned medium and cartilage extract were analyzed by Western blotting using anti-TIMP-1, anti-TIMP-2, and anti-TIMP-3 antibodies.
Chondrocyte and HTB94 chondrosarcoma cell culture
Primary porcine chondrocytes were prepared by digesting cartilage with type 1A bacterial collagenase. Chondrocytes were passed through a cell strainer, washed twice, and plated in DMEM with 10% fetal calf serum. HTB94 human chondrosarcoma cells (American Culture Type Collection, Manassas, VA) were maintained in DMEM with 10% fetal calf serum, 100 U/ml penicillin, and 100 U/ml streptomycin. After treatment of cells with various concentrations of glycosaminoglycans for 8 to 36 h, conditioned media were concentrated 50-fold by trichloroacetic acid precipitation and analyzed by Western blotting.
To determine the amount of matrix-associated TIMP-3, HTB94 cells were treated with 100 μg/ml CaPPS or heparin in serum-free DMEM for 36 h, and conditioned media were concentrated by trichloroacetic acid precipitation. Cells attached to the culture flask were dissociated with EDTA. The ECM remaining in the culture flask was solubilized with reducing SDS sample buffer. TIMP-3 levels in equivalent volumes of each sample were analyzed by Western blotting.
Analysis of mRNA levels by quantitative reverse transcriptase-polymerase chain reaction (RT-PCR)
mRNAs from HTB94 cells and HTB94 cells transfected with TIMP-3/pCEP4 plasmid were isolated using a QIAamp RNA Blood Minikit (Qiagen Ltd, Crawley, UK). mRNAs were extracted from cartilage samples using a BioPulverizer (BioSpec Products, Bartlesville, IA, USA) and TRI Reagent® (Helena Biosciences, Gateshead, UK). Levels of TIMP-3 and 18S rRNA were quantified (n=3) by quantitative RT-PCR on a Rotor-Gene 6000 (Corbett Research, Cambridge, UK) using a SuperScript III Platinum One-Step qRT-PCR System (Invitrogen) with validated 6-carboxyfluorescein (FAM) -labeled primers for human TIMP-3 (TaqMan Gene Expression Assay; Applied Biosystems, Foster City, CA, USA) and 18S rRNA (LUX primer set, Invitrogen). A standard curve was constructed relating nanograms of RNA to CT values. Standardized CT values (ng TIMP-3 mRNA/ng 18S rRNA) are given relative to control untreated HTB94 cells (defined as 1) and were analyzed using a paired t test.
Assays for ADAMTS-4 and -5 and MMP activity
The activities of ADAMTS-4 and -5 were measured against aggrecan and the recombinant aggrecan IGD domain flanked by glutathione S-transferase at the N terminus and FLAG peptide at the C terminus (gst-IGD-flag) as described previously (17). Briefly, aggrecan (50 μg) was incubated with various isoforms of ADAMTS-4 or -5, and the reaction was terminated by 50 mM EDTA. The products were deglycosylated overnight with chondroitinase ABC and keratanase and analyzed by Western blotting using the BC-3 antibody. ADAMTS-4 or -5 activities were also measured using 17 μM gst-IGD-flag. The reactions were terminated with 20 mM EDTA, and products were analyzed by densitometric analysis of SDS-PAGE gels. Activities of MMP-2 and -13 were measured using the fluorescent quenched peptide substrate (7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-(3-[2,4-dinitrophenyl]-l- 2,3-diaminopropionyl)-Ala-Arg-NH2 (34).
Effect of CaPPS on TIMP-3 inhibition of aggrecanases
Ki(app) values of TIMP-3 for various isoforms of ADAMTS-4 and -5 were determined under equilibrium kinetic conditions (35). The effect of CaPPS on TIMP-3 inhibition of ADAMTS-4 and -5 was measured by incubating the enzymes with various concentrations of TIMP-3 in the presence or absence of 0.05 μg/ml CaPPS for 1 h at 37°C and residual activity against gst-IGD-flag determined. Ki(app) values of TIMP-3 for MMP-2 and -13 were similarly determined under equilibrium kinetic conditions using the fluorescent peptide substrate (34).
Timp3-null mouse cartilage explant culture
Femoral head cartilage from 3-wk-old wild-type or Timp3-null mice (36) was stimulated with 10 ng/ml IL-1α with or without CaPPS (50 or 100 μg/ml) in serum-free DMEM for 3 d. Aggrecan breakdown was monitored by Western blotting with the 2-B-6 antibody.
RESULTS
CaPPS inhibits aggrecanase activity in porcine and human cartilage
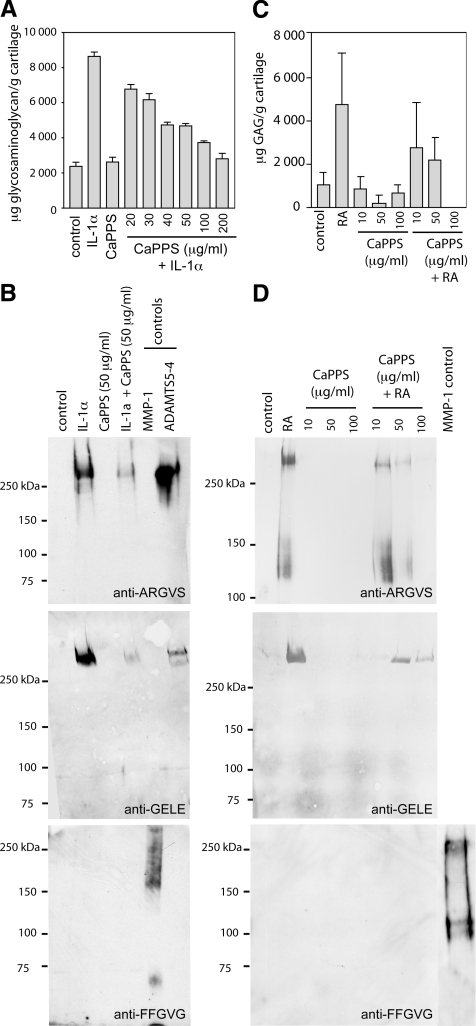
CaPPS has been reported to protect bovine and human cartilage against aggrecan breakdown (21). We tested whether this inhibition is due to suppression of MMP and/or ADAMTS activities. Treatment of cultured porcine cartilage explants with IL-1α caused a 4-fold increase in glycosaminoglycan release into the medium, and this increase was dose-dependently inhibited by CaPPS (Fig. 1A). Further analysis of aggrecan breakdown products with neoepitope antibodies that recognize aggrecanase-dependent cleavage of the Glu373-Ala374 bond (anti-ARGSV) and the Glu1480-Gly1481 bond (anti-GELE), as well as a neoepitope antibody recognizing MMP-dependent cleavage at the Ser341-Phe342 bond (anti-FFGVG), indicated that aggrecan was primarily degraded by aggrecanases and that these were effectively inhibited by CaPPS (Fig. 1B). CaPPS similarly blocked glycosaminoglycan release in explants of human OA cartilage stimulated with retinoic acid (Fig. 1C). This protection was also due to inhibition of aggrecanase activity (Fig. 1D). Similar protection was seen with cartilage from 3 other OA patients.
Figure 1.
CaPPS protects porcine and human cartilage against IL-1α- or retinoic acid-induced aggrecan breakdown. A, B) Porcine cartilage explants were cultured for 3 d in serum-free DMEM in the presence of 10 ng/ml IL-1α and/or 20–200 (A) or 50 μg/ml CaPPS (B). Bovine aggrecan digested with recombinant MMP-1 (500 nM) or ADAMTS5–4 (20 nM) for 18 h at 37°C is shown for comparison. Release of aggrecan fragments into the conditioned medium was assessed by the dimethylenemethylene blue assay (A) and by Western blotting using neoepitope antibodies that recognize aggrecan fragments generated by aggrecanases (anti-ARGV and anti-GELE) and MMPs (anti-FFGVG) (B). C, D) Human (female, 74-yr-old) osteoarthritic cartilage explants were cultured for 3 d in serum-free DMEM in the presence of 1 μM all-trans retinoic acid (RA) and/or 10–100 μg/ml CaPPS. Release of aggrecan fragments into the conditioned medium was assessed by the dimethylenemethylene blue assay (shown as the average±sd of triplicate assays, n=3) (C) and aggrecan neoepitope antibodies (D).
CaPPS increases TIMP-3 levels in cartilage and in conditioned medium of cultured cells
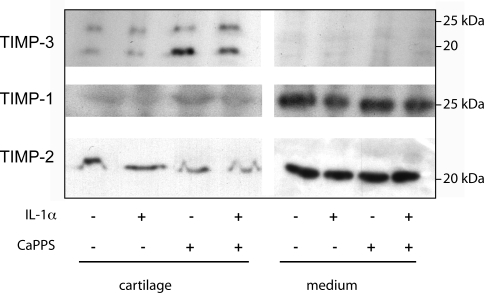
Because the major aggrecanases, ADAMTS-4 and -5, are inhibited by TIMP-3 and not the other TIMPs (24), we tested our hypothesis that CaPPS inhibition of aggrecanase activity in cartilage is due to an increase of TIMP-3 in the tissue. Porcine cartilage explants in culture were treated with IL-1α and/or CaPPS for 3 d and the levels of TIMP-1, -2, and -3 in the cartilage and conditioned medium examined. TIMP-3 was present almost exclusively within the cartilage explants. Treatment with IL-1α did not alter the level of TIMP-3, but treatment with CaPPS increased TIMP-3 levels ∼3.5-fold in the cartilage, estimated from pixel density (Fig. 2). TIMP-1 and -2 were present primarily in the conditioned medium, and their levels were unaffected by IL-1α or CaPPS. We were unable to determine the effects of CaPPS on production of ADAMTS-4 and -5 because their levels in our cartilage extracts were too low to detect by Western blot.
Figure 2.
Effects of CaPPS on cartilage TIMP-3. Porcine cartilage explants were cultured for 3 d in serum-free DMEM with IL-1α and/or 50 μg/ml CaPPS. Conditioned media were concentrated by trichloroacetic acid precipitation. Cartilage extracts were prepared using guanidine hydrochloride and gradient density centrifugation. TIMP levels in equivalent volumes of medium and cartilage extracts were analyzed by Western blotting.
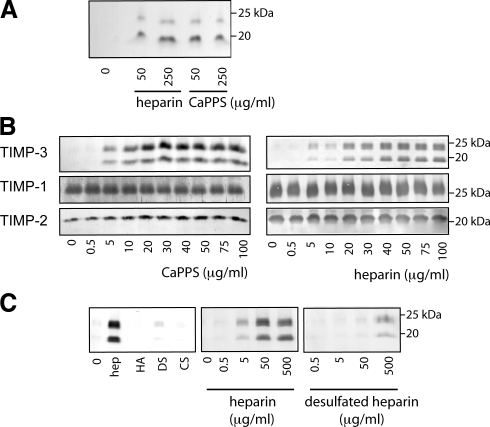
To investigate the mechanism underlying the increase in TIMP-3 protein levels in cartilage, we examined the production of TIMP-3 by cultured primary porcine chondrocytes. TIMP-3 was not detectable in the conditioned medium of untreated chondrocytes (Fig. 3A), but addition of CaPPS caused an increase in TIMP-3 levels in the medium. Addition of heparin to chondrocytes also resulted in an accumulation of TIMP-3 in the medium (Fig. 3A). Similar results were observed with HTB94 chondrosarcoma cells; CaPPS and heparin dose-dependently increased TIMP-3 in the medium, with CaPPS and heparin having half maximal effective concentrations (EC50) of 10 and 25 μg/ml, respectively (Fig. 3B). TIMP-3 was detectable in the conditioned medium 2–4 h after addition of CaPPS or heparin, with levels increasing to a maximum by 18 h (data not shown). TIMP-3 accumulation was reversible; after removal of CaPPS or heparin from the medium, TIMP-3 was undetectable within 24–48 h (data not shown). When heparin-Sepharose beads were added to cells, TIMP-3 accumulated on the beads (data not shown), indicating that it interacts directly with sulfated glycosaminoglycans. Glycosaminoglycans with low levels of sulfation (chondroitin or dermatan sulfate) or no sulfation (hyaluronan) did not support TIMP-3 accumulation, and desulfation of heparin greatly reduced TIMP-3 accumulation (Fig. 3C). Levels of TIMP-1 and -2 were not affected by CaPPS or heparin treatment.
Figure 3.
Effects of CaPPS on TIMP-3 in the medium of pig chondrocytes and HTB94 chondrosarcoma. A) Primary pig chondrocytes were cultured for 36 h in the presence of CaPPS or heparin; the conditioned media were concentrated and analyzed for TIMP-3 by Western blotting. B) Human HTB94 chondrosarcoma cells were cultured for 36 h in the presence of CaPPS or heparin; the conditioned media were concentrated and analyzed for TIMP-1, -2, and -3 by Western blotting. C) HTB94 chondrosarcoma cells were cultured for 36 h in the presence of 100 μg/ml of hyaluronan (HA), dermatan sulfate (DS), or chondroitin sulfate (CS), 0–500 μg/ml of heparin or desulfated heparin. Conditioned media were concentrated and analyzed for TIMP-3 by Western blotting.
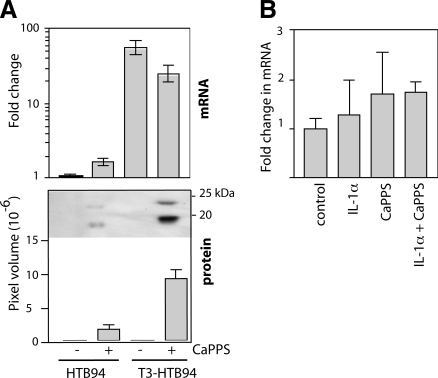
We then examined whether the increase in TIMP-3 levels in the cartilage and medium was due to increased transcription. Quantitative reverse transcription PCR indicated that treatment of HTB94 cells with CaPPS (50 μg/ml) had no effect on TIMP-3 mRNA levels (paired t test, P=0.0603, Fig. 4A). When these cells were transfected with a vector to overexpress TIMP-3, TIMP-3 mRNA levels increased 50-fold (P=0.02). Addition of CaPPS to transfected cells did not significantly affect TIMP-3 mRNA levels (P=0.1295), but TIMP-3 protein was detected in the medium only when CaPPS was added. CaPPS also had no effect on TIMP-3 mRNA levels in cartilage explants, either alone or in combination with IL-1α (P>0.35 for all, Fig. 4B).
Figure 4.
CaPPS does not increase TIMP-3 transcription. A) HTB94 cells were transfected with either mock or TIMP-3-overexpressing plasmid (T3-HTB94) and grown for 36 h in serum-free DMEM in the presence or absence of 50 μg/ml CaPPS. RNA was extracted from the cells, and levels of TIMP-3 mRNA were quantified relative to GAPDH mRNA by quantitative RT-PCR. Conditioned media were concentrated and analyzed for TIMP-3 by Western blotting. B) Porcine cartilage explants were grown for 36 h in serum-free DMEM in the presence of IL-1α and/or 50 μg/ml CaPPS. RNA was extracted from the cartilage, and TIMP-3 mRNA was quantified relative to GAPDH mRNA by quantitative RT-PCR.
LRP-mediated endocytosis of TIMP-3
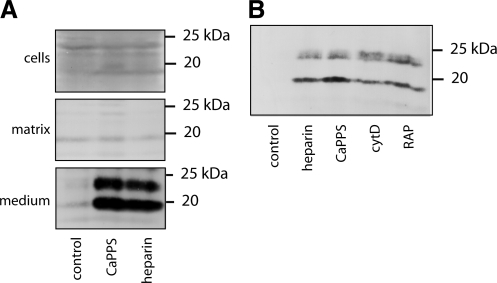
Heparin has been reported to solubilize TIMP-3 from the ECM (37), so we tested whether the observed accumulation of TIMP-3 in the HTB94 medium was due to solubilization of matrix-bound material. Without heparin or CaPPS treatment, very little TIMP-3 was detectable, either in the matrix, within cells, or in their conditioned medium (Fig. 5A). Addition of 100 μg/ml CaPPS or heparin for 36 h resulted in a marked accumulation of TIMP-3 in the medium, but no change in TIMP-3 levels in the cells or matrix. These results, together with CaPPS having no effect on TIMP-3 mRNA levels, suggested that newly synthesized and secreted TIMP-3 is rapidly endocytosed by the cell and that CaPPS and heparin sequester TIMP-3 in the medium by blocking this process.
Figure 5.
CaPPS blocks LRP-mediated TIMP-3 endocytosis. A) HTB94 cells were cultured for 36 h in the presence of 100 μg/ml CaPPS or heparin, and equivalent volumes of cells, matrix, and medium were analyzed for TIMP-3 by Western blotting. B) HTB94 cells were cultured for 8 h with 100 μg/ml heparin or CaPPS, cytochalasin D (cytD, 1 μM), or RAP (750 nM). Conditioned media were concentrated and analyzed for TIMP-3 by Western blotting.
To test the possibility of TIMP-3 endocytosis, we first used cytochalasin D, which blocks the pathway of endocytosis by inhibiting actin polymerization. This treatment caused an accumulation of TIMP-3 in the medium of HTB94 (Fig. 5B), suggesting that TIMP-3 is normally internalized by the cell after secretion. We postulated that this process is mediated by LRP, a scavenger receptor that has been shown to internalize a number of proteinases and their inhibitors (38). We therefore tested whether an antagonist of ligand binding to members of the LRP family, RAP, causes an increase in TIMP-3 in the medium. As shown in Fig. 5B, TIMP-3 was readily detected in the medium in the presence of RAP. These results indicate that TIMP-3 is normally internalized into cells by a member of the LRP family and that CaPPS and heparin block TIMP-3 binding to LRP and thus sequester TIMP-3 in the extracellular milieu.
CaPPS directly inhibits ADAMTS-4 and -5 as an exosite inhibitor
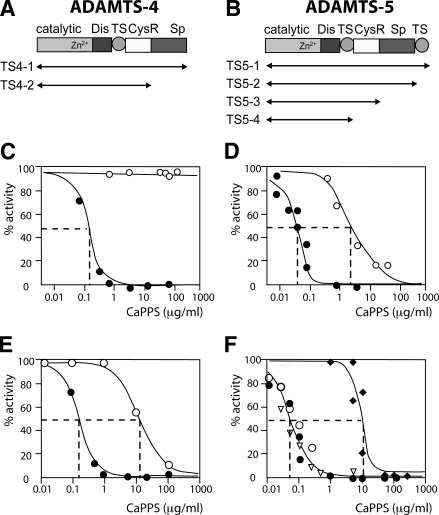
We next tested whether CaPPS directly inhibits ADAMTS-4 and -5 activity, first using recombinant aggrecan interglobular domain (IGD) expressed in E. coli as a substrate to detect inhibition. As shown in Fig. 6C, no inhibition of full-length ADAMTS-4 (TS4–1) was observed, even at 500 μg/ml CaPPS. In contrast, when hydrolysis of native aggrecan at the Glu373-Ala374 site in the interglobular domain was monitored, ADAMTS-4 (TS4–1) was inhibited by CaPPS, with an inhibitory concentration 50 (IC50) value of 0.2 μg/ml (40 nM) (Fig. 6C). Similarly, only weak inhibition of full-length ADAMTS-5 (TS5–1) by CaPPS was observed with the recombinant IGD substrate (IC50 value 3 μg/ml; 600 nM), but inhibition was greatly improved when the interglobular domain of aggrecan was used as a substrate, with an IC50 value of 0.05 μg/ml (10 nM) (Fig. 6D). CaPPS also blocked aggrecanase activity in the chondroitin sulfate-rich region of aggrecan, with an IC50 value of 0.05 μg/ml for inhibition of ADAMTS5–1 hydrolysis at the Glu1771-Ala1772 site (data not shown). We postulated that aggrecan substrate-specific inhibition of the aggrecanases by CaPPS is due to interaction of CaPPS with the noncatalytic domains of the ADAMTSs, preventing recognition of aggrecan by these domains. We have previously shown that the spacer (Sp) domain of ADAMTS-4 (16) and the cysteine-rich (CysR) domain of ADAMTS-5 (15) are the primary sites that interact with the sulfated polysaccharide chains of aggrecan. We therefore tested whether deletion of the C-terminal domains of the aggrecanases reduces the inhibitory ability of CaPPS. As shown in Fig. 6E, the IC50 value of ADAMTS4–2, which lacks the Sp domain, was 15 μg/ml (3 μM), compared to 0.2 μg/ml (40 nM) for ADAMTS4–1. For ADAMTS-5, deletion of the second thrombospondin and Sp domains had little effect on CaPPS inhibition, but deletion of the CysR domain increased the IC50 from 0.05 μg/ml (10 nM) to 15 μg/ml (3 μM) (Fig. 6F). These results indicate that CaPPS specifically interacts with the Sp domain of ADAMTS-4 and with the CysR domain of ADAMTS-5 and as such is the first example for an exosite inhibitor of this class of enzymes.
Figure 6.
CaPPS is an exosite inhibitor of ADAMTS-4 and -5). A, B) Schematic representation of forms of ADAMTS-4 (A) and ADAMTS-5 used (B). C, D) Substrate-dependent inhibition of full-length ADAMTS-4 (TS4–1, 5 nM) (C) and ADAMTS-5 (TS5–1, 0.5 nM) (D) by CaPPS. The enzymes were preincubated with CaPPS for 1 h at 37°C, and residual activity against a recombinant gst-IGD-flag substrate (○) or aggrecan (•) was measured. IC50 values for ADAMTS-4 and -5 using gst-IGD-flag were >1000 and 3 μg/ml, respectively. IC50 values for ADAMTS-4 and -5 using native aggrecan were 0.2 μg/ml (40 nM) and 0.05 μg/ml (10 nM), respectively. E) Inhibition of ADAMTS4–1 (5 nM, •) and TS4–2 (10 nM, ○) by CaPPS, measured using aggrecan as a substrate. IC50 values were 0.2 and 15 μg/ml, respectively. F) Inhibition of ADAMTS5–1 (0.5 nM, •), TS5–2 (0.5 nM, ○), TS5–3 (0.5 nM, ▿), and TS5–4 (50 nM, ♦) by CaPPS measured using aggrecan. IC50 values for the first 3 forms of ADAMTS-5 were 0.05 μg/ml but increased 300-fold for TS5–4 (IC50=15 μg/ml).
We then tested whether other sulfated glycosaminoglycans have a similar exosite inhibitor activity for ADAMTS-4 and -5. Among the compounds we tested, heparin weakly inhibited full-length ADAMTS-5 activity against aggrecan, but it was 400-fold weaker than CaPPS, with an IC50 of 20 μg/ml (Table 1). Glycosaminoglycans with lower levels of sulfation (chondroitin-4-sulfate, dermatan sulfate) or no sulfation (hyaluronan) were ineffective. The highly sulfated disaccharide sucrose octasulfate was also not an effective inhibitor, indicating that high levels of sulfation alone were not sufficient for inhibitory activity. These results indicate that interaction of CaPPS with the noncatalytic exosites of ADAMTS-4 and -5 is specific. Furthermore, CaPPS had little effect on the activity of MMP-1, -2, or -13 against a peptide substrate, the recombinant IGD substrate or aggrecan, with IC50 values greater than 20 μg/ml (4 μM) in all cases (data not shown). This indicates that CaPPS’s inhibitory effects are not a phenomenon general to metalloproteinases.
TABLE 1.
Inhibition of ADAMTS5–1 hydrolysis of the interglobular domain of aggrecan by various sulfated polysaccharides
| Polysaccharide | IC50 (μg/ml) |
|---|---|
| Heparin | 20 |
| CaPPS | 0.05 |
| Chondroitin-4-sulfate | >50 |
| Hyaluronan | >50 |
| Dermatan sulfate | >50 |
| Sucrose octasulfate | >50 |
CaPPS enhances TIMP-3 affinity for full-length ADAMTS-4 and -5
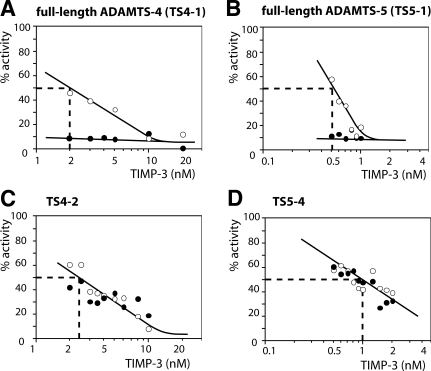
Because our data indicated that CaPPS interacts with both TIMP-3 and ADAMTS-4 and -5, we investigated whether CaPPS altered the affinity between TIMP-3 and aggrecanases. In this case we used the E. coli-expressed IGD substrate to measure enzyme activity. In the absence of CaPPS, Ki(app) values of TIMP-3 for full-length ADAMTS-4 and -5 were 2 and 0.5 nM, respectively (Fig. 7A, B). In the presence of 0.05 μg/ml CaPPS, a concentration that does not directly inhibit ADAMTS-4 or -5 hydrolysis of the recombinant substrate used (see Fig. 6C, D), TIMP-3 affinity for both enzymes was markedly enhanced, and Ki(app) was well below the lowest concentration of TIMP-3 used. Deletion of the C-terminal domains of ADAMTS-4 and -5 abolished this effect; ADAMTS4–2 and ADAMTS5–4 exhibited the same Ki(app) values with or without 0.05 μg/ml CaPPS (Ki(app) of 2.5 and 1 nM, respectively) (Fig. 7C, D). To determine how much CaPPS improves Ki(app), the assay would have to be conducted at lower enzyme concentrations, which is not possible with currently available substrates. Lower CaPPS concentrations were also effective, with 0.5 nM ADAMTS5–1 almost completely inhibited by 0.5 nM TIMP-3 in the presence of 0.5 nM (2.5 ng/ml) CaPPS, and reducing the amount of CaPPS gave a lesser effect on the Ki(app), indicating the formation of a high affinity 1:1:1 complex. The improvement in Ki(app) is therefore more than 2 orders of magnitude. Heparin (50 ng/ml) had no effect on the interaction between TIMP-3 and aggrecanases. CaPPS (50 ng/ml) had little effect on TIMP-3 inhibition of MMP-2 or -13.
Figure 7.
Enhancement of TIMP-3 inhibition of full-length ADAMTS-4 and -5 by CaPPS. ADAMTSs were incubated with various concentrations of TIMP-3 in the presence (•) or absence (○) of CaPPS (0.05 μg/ml) for 1 h at 37°C, and residual activity against gst-IGD-flag was determined (expressed as percentage of activity without TIMP-3, either with or without CaPPS). A–D) full-length ADAMTS-4 (2 nM) (A); full-length ADAMTS-5 (0.5 nM) (B); ADAMTS4–2 (2 nM) (C); and ADAMTS5–4 (0.5 nM) (D). At the concentration used, CaPPS alone does not inhibit ADAMTS-4 or -5 activity against gst-IGD-flag.
CaPPS protects Timp3−/− cartilage less effectively
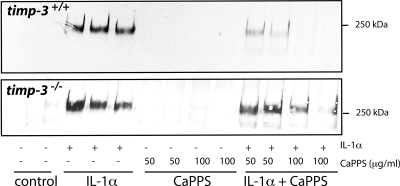
Cartilage explant cultures from Timp3-null mice (36) were examined to investigate whether CaPPS protects cartilage by acting as a direct aggrecanase inhibitor or by increasing the amount and effectiveness of TIMP-3. Stimulation of cartilage from wild-type and Timp3−/− mice with IL-1α-induced cartilage degradation and release of aggrecan fragments into the medium (Fig. 8). CaPPS protected wild-type mouse cartilage as effectively as porcine cartilage, with almost total protection at 50 μg/ml CaPPS. Timp3−/− cartilage, however, was markedly less protected by CaPPS. This indicates that effective protection against aggrecan degradation by CaPPS requires the presence of endogenous TIMP-3.
Figure 8.
CaPPS protects Timp3−/− cartilage less effectively. Femoral head cartilage explants from 3-wk-old wild-type and Timp3-null mice were cultured for 3 d in the presence of 10 ng/ml IL-1α and/or CaPPS. Aggrecan fragments released into the medium were analyzed using the 2-B-6 antibody.
DISCUSSION
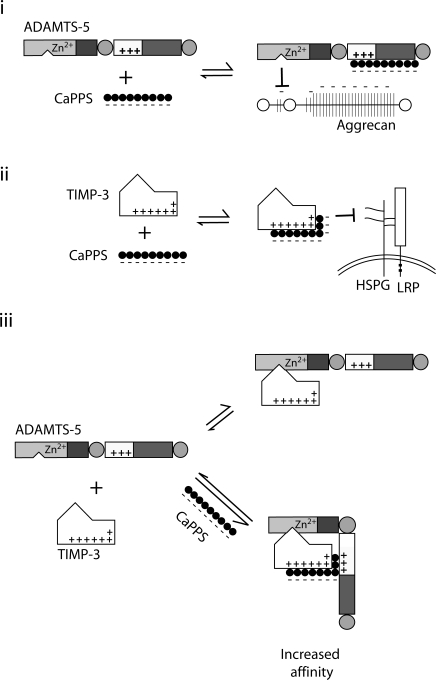
CaPPS and its sodium form have shown chondroprotective activities in various animal models of OA (20). Its medical use has been approved for treatment of canine OA in a number of countries, including the United Kingdom, Finland, Canada, Australia and New Zealand, and a limited number of clinical trials in patients with OA have shown promising outcomes (19, 20). The hallmark of OA is destruction of the cartilage matrix, primarily due to elevated activities of metalloproteinases, and therefore reduction of these proteinase activities is a potential therapeutic approach. As shown schematically in Fig. 9, we have demonstrated that CaPPS inhibits aggrecanase activity by 3 separate but interrelated modes of action.
Figure 9.
Three modes of action by which CaPPS prevents aggrecan degradation in cartilage. i) CaPPS acts as a direct inhibitor of ADAMTS-4 and -5 by interacting with their noncatalytic C-terminal domains and inhibiting their activity against aggrecan. ii) CaPPS increases extracellular levels of TIMP-3, the endogenous inhibitor of the ADAMTS-4 and -5, by blocking its interaction with cell surface heparan sulfate proteoglycans (HSPGs) and subsequent internalization through LRP. iii) CaPPS increases TIMP-3’s effectiveness as an aggrecanase inhibitor, enhancing its affinity for full-length ADAMTS-4 and -5.
In this work we first demonstrated that CaPPS allows TIMP-3 to accumulate in cartilage. TIMP-3 is an important regulator of cartilage matrix catabolism. Recent studies by Sahebjam et al. (39) showed increased collagen and proteoglycan degradation in cartilage of aging Timp3−/− mice compared with wild-type animals. Although some TIMP-3 is present in the cartilage matrix, the level is insufficient to inhibit the elevated aggrecanase activity induced by IL-1α treatment. Our studies have shown that the low level of TIMP-3 in cartilage is due to its rapid internalization by the cell via LRP-mediated endocytosis. CaPPS increases the amount of TIMP-3 in cartilage by sequestering the inhibitor extracellularly and preventing its internalization. A number of lysine and arginine residues are present in TIMP-3, mostly clustered on the opposite side from the metalloproteinase inhibitory ridge of the inhibitor. Negatively charged CaPPS most likely interacts with this patch of basic residues. LRP internalizes a large number of diverse ligands, including α2-macroglobulin-proteinase complexes, TIMP-2, MMP-2, -9, and -13, as well as several serine proteinases and their inhibitors (38) and thus regulates proteinase activity in the extracellular environment. Some LRP ligands are thought to bind initially to another cell surface receptor before being internalized by LRP. For some ligands the initial receptor is thought to be a heparan sulfate proteoglycan (38). Because both heparin and CaPPS block TIMP-3 internalization, TIMP-3 likely also binds first to such a heparan sulfate proteoglycan coreceptor before being internalized by LRP.
Our present studies have also revealed that CaPPS is an exosite inhibitor of ADAMTS-4 and -5. Domain deletion studies indicated that the effect of CaPPS is domain specific, that is, inhibition requires the Sp domain of ADAMTS-4 and the CysR domain of ADAMTS-5. Binding of CaPPS to these specific domains impairs their function by competing with their binding to aggrecan. Our previous studies showed that the Sp domain of ADAMTS-4 and the CysR domain of ADAMTS-5 play crucial roles in aggrecanolytic activity, and deletion of these domains reduces aggrecan hydrolysis by more than 95%, while having only minor effects on hydrolysis of nonaggrecan substrates such as fibromodulin and S-carboxymethylated transferrin (15, 16), implying that these domains interact with the sulfated glycosaminoglycan chains of aggrecan. Indeed, CaPPS inhibition demonstrated high substrate specificity, with the activity of the 2 aggrecanases against the E. coli-expressed recombinant IGD substrate not inhibited by CaPPS at all in the case of ADAMTS-4 or only weakly inhibited at much higher concentrations in the case of ADAMTS-5. These results confirm that CaPPS is a true exosite inhibitor of ADAMTS-4 and -5, and, as far as we are aware, it is the first example of its kind.
Another important finding in this work is that CaPPS greatly enhances the interaction of TIMP-3 with ADAMTS-4 and -5. This enhancement is so marked that we were unable to determine Ki(app) using the current assay systems. In the absence of CaPPS, TIMP-3 concentrations below the Ki(app) values inhibited equimolar concentrations of ADAMTS-4 and -5 by 30–40%. However, in the presence of CaPPS, these concentrations of TIMP-3 almost completely inhibited equimolar concentrations of ADAMTS-4 and -5, indicating that CaPPS improved the Ki by at least 2 orders of magnitude (35). Again, this effect of CaPPS was seen only for ADAMTS-4 containing the Sp domain and ADAMTS-5 containing the CysR domain. Wayne et al. (40) reported that aggrecan (10 μg/ml) increases the affinity of TIMP-3 for ADAMTS-4 4-fold, and heparin (100 μg/ml) has been shown to improve TIMP-3 inhibition of ADAMTS-2 and MMP-2 by 3- to 4-fold (41, 42). These 2 sulfated polysaccharides thus have much weaker effects on TIMP-3/ADAMTS association than CaPPS. Compared with CaPPS, heparin has only a minimal effect on aggrecanase-TIMP-3 interactions. Heparin, on the other hand, greatly improves antithrombin III inhibition of thrombin by 2 complementary mechanisms. First, heparin acts as a “template” and brings the enzyme and inhibitor into proximity, increasing the likelihood of their interaction (43). Second, heparin induces a conformational change in the inhibitor that favors enzyme inhibition (44). Electrostatic and steric constraints stagger sequential xylopyranose rings of CaPPS by 180° relative to each other, forming 2 negatively charged “faces” (20). This probably enables CaPPS to bind to 2 proteins simultaneously, a property that may underpin its ability to increase TIMP-3 interaction with ADAMTS-4 and -5.
CaPPS caused a 3.5-fold increase in TIMP-3 levels in porcine cartilage. The effect of such a relatively modest change in inhibitor concentration on net enzyme activity depends on the ratio between the enzyme concentration in the tissue and Ki (35). If the concentrations of ADAMTS-4 and -5 in cartilage are much less than the Ki value, then a 3.5-fold increase in TIMP-3 would not greatly affect aggrecanase activity. However, if the enzyme concentration is close to or higher than Ki, then modest changes in TIMP-3 concentration would substantially affect enzyme activity (35). CaPPS decreased the Ki of TIMP-3 for ADAMTS-4 and -5 by at least 2 orders of magnitude, resulting in an increase in the ratio of enzyme to Ki by at least 100-fold. This means not only that the sensitivity of the enzymes to inhibition by TIMP-3 increases, but also that small changes in TIMP-3 concentration can greatly affect enzyme activity, making CaPPS a potent enhancer of the interaction between the aggrecanases and TIMP-3. This increased affinity was not observed for MMP-1, -13, or -14. The cartilage protective effect of CaPPS is thus due both to its ability to increase the amount of TIMP-3 and to enhance its affinity for ADAMTS-4 and -5.
It is likely that inhibition of metalloproteinases by TIMP-3 takes place pericellularly as TIMP-3 is readily taken up the cell. CaPPS shifts the proteolytic balance to inhibitory by maintaining a higher concentration of extracellular TIMP-3 and by enhancing TIMP-3 affinity for the aggrecanases. Immediately after secretion, ADAMTS-4 and -5 are good targets for TIMP-3 because they still maintain their C-terminal ancillary domains. In Timp3−/− cartilage, CaPPS works only as an exosite inhibitor of aggrecanases. In the absence of TIMP-3, ADAMTS-4 and -5 are more readily processed to shorter forms by local proteolysis (45) or autolysis (46, 47). These shorter forms would be released from the pericellular region into the interterritorial region of the tissue (15, 16). Although shorter forms of ADAMTSs have less aggrecanase-degrading activity than the full-length enzymes, they are also less sensitive to inhibition by CaPPS. This may be one of the reasons why we detected aggrecan degradation in Timp3−/− cartilage even in the presence of a high concentration of CaPPS.
OA is the world’s most common musculoskeletal disease and has a substantial impact on the quality of life of an increasingly aging population, but no disease-modifying drugs are yet available. CaPPS has shown promising results in small-scale human OA trials (18, 19). In this study we have resolved that it reduces cartilage matrix breakdown by 3 concerted modes of action and alters the balance between aggrecanases and TIMP-3. CaPPS is a unique prototypic drug to protect cartilage from degradation. Future structural studies to elucidate how CaPPS interacts with TIMP-3 and aggrecanase exosites will provide new insights into designing novel types of OA disease-modifying agents.
Acknowledgments
This work was supported by Wellcome Trust grant 057508 and U.S. National Institutes of Health grant AR40994 to H.N. We thank Shi-Lu Chia (Kennedy Institute of Rheumatology) for help with mouse explant culture and Bruce Caterson and Clare Hughes of the University of Cardiff (Cardiff, UK) for provision of 2-B-6, BC3, and BC14 monoclonal antibodies. We are grateful to Jonathan Jones (Heatherwood and Wexham Park Hospitals, Slough, UK) and the Orthopaedic Surgery Department of Charing Cross Hospital (London, UK) for providing human cartilage.
References
- Mankin H J, Lippiello L. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. J Bone Joint Surg Am. 1970;52:424–434. [PubMed] [Google Scholar]
- Murphy G, Nagase H. Reappraising metalloproteinases in rheumatoid arthritis and osteoarthritis: destruction or repair? Nat Clin Pract Rheumatol. 2008;4:128–135. doi: 10.1038/ncprheum0727. [DOI] [PubMed] [Google Scholar]
- Struglics A, Larsson S, Pratta M A, Kumar S, Lark M W, Lohmander L S. Human osteoarthritis synovial fluid and joint cartilage contain both aggrecanase- and matrix metalloproteinase-generated aggrecan fragments. Osteoarthritis Cartilage. 2006;14:101–113. doi: 10.1016/j.joca.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Lark M W, Bayne E K, Flanagan J, Harper C F, Hoerrner L A, Hutchinson N I, Singer I I, Donatelli S A, Weidner J R, Williams H R, Mumford R A, Lohmander L S. Aggrecan degradation in human cartilage: evidence for both matrix metalloproteinase and aggrecanase activity in normal, osteoarthritic, and rheumatoid joints. J Clin Invest. 1997;100:93–106. doi: 10.1172/JCI119526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy J D. A contentious issue finds some clarity: on the independent and complementary roles of aggrecanase activity and MMP activity in human joint aggrecanolysis. Osteoarthritis Cartilage. 2006;14:95–100. doi: 10.1016/j.joca.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Glasson S S, Askew R, Sheppard B, Carito B, Blanchet T, Ma H L, Flannery C R, Peluso D, Kanki K, Yang Z, Majumdar M K, Morris E A. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- Stanton H, Rogerson F M, East C J, Golub S B, Lawlor K E, Meeker C T, Little C B, Last K, Farmer P J, Campbell I K, Fourie A M, Fosang A J. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434:648–652. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- Song R H, Tortorella M D, Malfait A M, Alston J T, Yang Z, Arner E C, Griggs D W. Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis Rheum. 2007;56:575–585. doi: 10.1002/art.22334. [DOI] [PubMed] [Google Scholar]
- Bursavich M G, Gilbert A M, Lombardi S, Georgiadis K E, Reifenberg E, Flannery C R, Morris E A. 5′-Phenyl-3′H-spiro[indoline-3,2′-[1,3,4]thiadiazol]-2-one inhibitors of ADAMTS-5 (aggrecanase-2) Bioorg Med Chem Lett. 2007;17:5630–5633. doi: 10.1016/j.bmcl.2007.07.048. [DOI] [PubMed] [Google Scholar]
- Bursavich M G, Gilbert A M, Lombardi S, Georgiadis K E, Reifenberg E, Flannery C R, Morris E A. Synthesis and evaluation of aryl thioxothiazolidinone inhibitors of ADAMTS-5 (Aggrecanase-2) Bioorg Med Chem Lett. 2007;17:1185–1188. doi: 10.1016/j.bmcl.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Lauer-Fields J L, Spicer T P, Chase P S, Cudic M, Burstein G D, Nagase H, Hodder P, Fields G B. Screening of potential a disintegrin and metalloproteinase with thrombospondin motifs-4 inhibitors using a collagen model fluorescence resonance energy transfer substrate. Anal Biochem. 2008;373:43–51. doi: 10.1016/j.ab.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker S, Cao J, Chen W T. Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. Oncogene. 2000;19:6642–6650. doi: 10.1038/sj.onc.1204097. [DOI] [PubMed] [Google Scholar]
- Milner J M, Cawston T E. Matrix metalloproteinase, knockout studies, and the potential use of matrix metalloproteinase inhibitors in the rheumatic diseases. Curr Drug Targets Inflamm Allergy. 2005;4:363–375. doi: 10.2174/1568010054022141. [DOI] [PubMed] [Google Scholar]
- Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Gendron C, Kashiwagi M, Lim N H, Enghild J J, Thogersen I B, Hughes C, Caterson B, Nagase H. Proteolytic activities of human ADAMTS-5: comparative studies with human ADAMTS-4. J Biol Chem. 2007;282:18294–18306. doi: 10.1074/jbc.M701523200. [DOI] [PubMed] [Google Scholar]
- Kashiwagi M, Enghild J J, Gendron C, Hughes C, Caterson B, Itoh Y, Nagase H. Altered proteolytic activities of ADAMTS-4 expressed by C-terminal processing. J Biol Chem. 2004;279:10109–10119. doi: 10.1074/jbc.M312123200. [DOI] [PubMed] [Google Scholar]
- Fushimi K, Troeberg L, Nakamura H, Lim N H, Nagase H. Functional differences of the catalytic and non-catalytic domains in human ADAMTS-4 and ADAMTS-5 in aggrecanolytic activity. J Biol Chem. 2007;283:6706–6716. doi: 10.1074/jbc.M708647200. [DOI] [PubMed] [Google Scholar]
- Edelman J, March L, Ghosh P. A double-blind placebo-controlled clinical study of a pleiotropic osteoarthritis drug (pentosan polysulphate, Cartrophen®) in 105 patients with osteoarthritis (OA) of the knee and hip joints. Osteoarthritis Cartilage. 1994;2:35. [Google Scholar]
- Ghosh P, Edelman J, March L, Smith M. Effects of pentosan polysulfate in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled pilot study. Curr Ther Res. 2005;66:552–571. doi: 10.1016/j.curtheres.2005.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P. The pathobiology of osteoarthritis and the rationale for the use of pentosan polysulfate for its treatment. Semin Arthritis Rheum. 1999;28:211–267. doi: 10.1016/s0049-0172(99)80021-3. [DOI] [PubMed] [Google Scholar]
- Munteanu S E, Ilic M Z, Handley C J. Calcium pentosan polysulfate inhibits the catabolism of aggrecan in articular cartilage explant cultures. Arthritis Rheum. 2000;43:2211–2218. doi: 10.1002/1529-0131(200010)43:10<2211::AID-ANR8>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Takizawa M, Ohuchi E, Yamanaka H, Nakamura H, Ikeda E, Ghosh P, Okada Y. Production of tissue inhibitor of metalloproteinases 3 is selectively enhanced by calcium pentosan polysulfate in human rheumatoid synovial fibroblasts. Arthritis Rheum. 2000;43:812–820. doi: 10.1002/1529-0131(200004)43:4<812::AID-ANR11>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Arris C E, Bevitt D J, Mohamed J, Li Z, Langton K P, Barker M D, Clarke M P, McKie N. Expression of mutant and wild-type TIMP3 in primary gingival fibroblasts from Sorsby’s fundus dystrophy patients. Biochim Biophys Acta. 2003;1638:20–28. doi: 10.1016/s0925-4439(03)00036-x. [DOI] [PubMed] [Google Scholar]
- Kashiwagi M, Tortorella M, Nagase H, Brew K. TIMP-3 is a potent inhibitor of aggrecanase 1 (ADAM-TS4) and aggrecanase 2 (ADAM-TS5) J Biol Chem. 2001;276:12501–12504. doi: 10.1074/jbc.C000848200. [DOI] [PubMed] [Google Scholar]
- Gendron C, Kashiwagi M, Hughes C, Caterson B, Nagase H. TIMP-3 inhibits aggrecanase-mediated glycosaminoglycan release from cartilage explants stimulated by catabolic factors. FEBS Lett. 2003;555:431–436. doi: 10.1016/s0014-5793(03)01295-x. [DOI] [PubMed] [Google Scholar]
- Hascall V C, Sajdera S W. Protein polysaccharide complex from bovine nasal cartilage: the function of glycoprotein in the formation of aggregates. J Biol Chem. 1969;244:2384–2396. [PubMed] [Google Scholar]
- Emonard H, Bellon G, Troeberg L, Berton A, Robinet A, Henriet P, Marbaix E, Kirkegaard K, Patthy L, Eeckhout Y, Nagase H, Hornebeck W, Courtoy P J. Low density lipoprotein receptor-related protein mediates endocytic clearance of pro-MMP-2: TIMP-2 complex through a thrombospondin-independent mechanism. J Biol Chem. 2004;279:54944–54951. doi: 10.1074/jbc.M406792200. [DOI] [PubMed] [Google Scholar]
- Caterson B, Christner J E, Baker J R, Couchman J R. Production and characterization of monoclonal antibodies directed against connective tissue proteoglycans. Fed Proc. 1985;44:386–393. [PubMed] [Google Scholar]
- Hughes C E, Caterson B, Fosang A J, Roughley P J, Mort J S. Monoclonal antibodies that specifically recognize neoepitope sequences generated by ‘aggrecanase’ and matrix metalloproteinase cleavage of aggrecan: application to catabolism in situ and in vitro. Biochem J. 1995;305:799–804. doi: 10.1042/bj3050799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterson B, Hughes C E, Roughley P, Mort J S. Anabolic and catabolic markers of proteoglycan metabolism in osteoarthritis. Acta Orthop Scand. 1995;266:121–124. [PubMed] [Google Scholar]
- Ogata Y, Itoh Y, Nagase H. Steps involved in activation of the pro-matrix metalloproteinase 9 (progelatinase B)-tissue inhibitor of metalloproteinases-1 complex by 4-aminophenylmercuric acetate and proteinases. J Biol Chem. 1995;270:18506–18511. doi: 10.1074/jbc.270.31.18506. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Ito A, Iwata K, Tanzawa K, Mori Y, Nagase H. Plasma membrane-bound tissue inhibitor of metalloproteinases (TIMP)-2 specifically inhibits matrix metalloproteinase 2 (gelatinase A) activated on the cell surface. J Biol Chem. 1998;273:24360–24367. doi: 10.1074/jbc.273.38.24360. [DOI] [PubMed] [Google Scholar]
- Farndale R W, Buttle D J, Barrett A J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- Knight C G, Willenbrock F, Murphy G. A novel coumarin-labelled peptide for sensitive continuous assays of the matrix metalloproteinases. FEBS Lett. 1992;296:263–266. doi: 10.1016/0014-5793(92)80300-6. [DOI] [PubMed] [Google Scholar]
- Bieth J G. Theoretical and practical aspects of proteinase inhibition kinetics. Methods Enzymol. 1995;248:59–84. doi: 10.1016/0076-6879(95)48007-2. [DOI] [PubMed] [Google Scholar]
- Leco K J, Waterhouse P, Sanchez O H, Gowing K L, Poole A R, Wakeham A, Mak T W, Khokha R. Spontaneous air space enlargement in the lungs of mice lacking tissue inhibitor of metalloproteinases-3 (TIMP-3) J Clin Invest. 2001;108:817–829. doi: 10.1172/JCI12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W H, Yu S, Meng Q, Brew K, Woessner J F. TIMP-3 binds to sulfated glycosaminoglycans of the extracellular matrix. J Biol Chem. 2000;275:31226–31232. doi: 10.1074/jbc.M000907200. [DOI] [PubMed] [Google Scholar]
- Herz J, Strickland D K. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest. 2001;108:779–784. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahebjam S, Khokha R, Mort J S. Increased collagen and aggrecan degradation with age in the joints of Timp3(-/-) mice. Arthritis Rheum. 2007;56:905–909. doi: 10.1002/art.22427. [DOI] [PubMed] [Google Scholar]
- Wayne G J, Deng S J, Amour A, Borman S, Matico R, Carter H L, Murphy G. TIMP-3 inhibition of ADAMTS-4 (Aggrecanase-1) is modulated by interactions between aggrecan and the C-terminal domain of ADAMTS-4. J Biol Chem. 2007;282:20991–20998. doi: 10.1074/jbc.M610721200. [DOI] [PubMed] [Google Scholar]
- Wang W M, Ge G, Lim N H, Nagase H, Greenspan D S. TIMP-3 inhibits the procollagen N-proteinase ADAMTS-2. Biochem J. 2006;398:515–519. doi: 10.1042/BJ20060630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler G S, Apte S S, Willenbrock F, Murphy G. Human tissue inhibitor of metalloproteinases 3 interacts with both the N- and C-terminal domains of gelatinases A and B. Regulation by polyanions. J Biol Chem. 1999;274:10846–10851. doi: 10.1074/jbc.274.16.10846. [DOI] [PubMed] [Google Scholar]
- Olson S T, Björk I, Sheffer R, Craig P A, Shore J D, Choay J. Role of the antithrombin-binding pentasaccharide in heparin acceleration of antithrombin-proteinase reactions: resolution of the antithrombin conformational change contribution to heparin rate enhancement. J Biol Chem. 1992;267:12528–12538. [PubMed] [Google Scholar]
- Whisstock J C, Pike R N, Jin L, Skinner R, Pei X Y, Carrell R W, Lesk A M. Conformational changes in serpins: II. The mechanism of activation of antithrombin by heparin dagger. J Mol Biol. 2000;301:1287–1305. doi: 10.1006/jmbi.2000.3982. [DOI] [PubMed] [Google Scholar]
- Gao G, Plaas A, Thompson V P, Jin S, Zuo F, Sandy J D. ADAMTS4 (aggrecanase-1) activation on the cell surface involves C-terminal cleavage by glycosylphosphatidyl inositol-anchored membrane type 4-matrix metalloproteinase and binding of the activated proteinase to chondroitin sulfate and heparan sulfate on syndecan-1. J Biol Chem. 2004;279:10042–10051. doi: 10.1074/jbc.M312100200. [DOI] [PubMed] [Google Scholar]
- Flannery C R, Zeng W, Corcoran C, Collins-Racie L A, Chockalingam P S, Hebert T, Mackie S A, McDonagh T, Crawford T K, Tomkinson K N, LaVallie E R, Morris E A. Autocatalytic cleavage of ADAMTS-4 (Aggrecanase-1) reveals multiple glycosaminoglycan-binding sites. J Biol Chem. 2002;277:42775–42780. doi: 10.1074/jbc.M205309200. [DOI] [PubMed] [Google Scholar]
- Gao G, Westling J, Thompson V P, Howell T D, Gottschall P E, Sandy J D. Activation of the proteolytic activity of ADAMTS4 (aggrecanase-1) by C-terminal truncation. J Biol Chem. 2002;277:11034–11041. doi: 10.1074/jbc.M107443200. [DOI] [PubMed] [Google Scholar]