Abstract
Limitation of infarct size is a major goal of therapy for acute coronary syndromes, and research has focused on achieving rapid patency of infarct-related vessels. However, new understandings of epigenetic modifications during ischemia suggest additional targeted approaches that have not been extensively explored. Here, we show that ischemia induces histone deacetylase (HDAC) activity in the heart with deacetylation of histones H3/4 in vitro and in vivo. We show, utilizing a standard murine model of ischemia-reperfusion, that chemical HDAC inhibitors significantly reduce infarct area, even when delivered 1 h after the ischemic insult. We demonstrate that HDAC inhibitors prevent ischemia-induced activation of gene programs that include hypoxia inducible factor-1α, cell death, and vascular permeability in vivo and in vitro, thus providing potential mechanisms to explain reduced vascular leak and myocardial injury. In vitro, siRNA knockdown experiments implicate HDAC4 as a mediator of the effects in ischemic cardiac myocytes. These results demonstrate that HDAC inhibitors alter the response to ischemic injury in the heart and reduce infarct size, suggesting novel therapeutic approaches for acute coronary syndromes.—Granger, A., Abdullah, I., Huebner, F., Stout, A., Wang, T., Huebner, T., Epstein, J. A., Gruber, P. J. Histone deacetylase inhibition reduces myocardial ischemia-reperfusion injury in mice.
Keywords: heart, hypoxia inducible factor 1, cardiomyocytes
Reduction of ischemic injury is a major goal of therapy for both adult and pediatric medicine. In the heart, ischemic episodes are encountered in acute coronary syndromes, congenital coronary abnormalities, congenital cardiomyopathies, cyanotic heart disease, and during cardiac surgery. Over 1 million Americans die from myocardial infarction each year, with 25% of these dying from congestive heart failure (1, 2). With optimal medical management, the 5-yr survival of patients diagnosed with chronic postinfarction heart failure is only 50%. Some interventions have focused on myocardial protection during ischemic episodes, including the use of glucose and insulin to provide necessary metabolic substrates to ischemic tissues. However, new understandings of ischemia-induced epigenetic changes suggest additional targeted approaches. For example, in the brain, ischemia promotes deacetylation of core histones and dimethylation of histone H3 at lysine-9 (H3-K9) over the mu opioid receptor-1 promoter, a signature of epigenetic gene silencing (3). In addition, in the isolated kidney, ischemia-reperfusion (I/R) results in a significant change in the ratio of methylated to unmethylated cytosines at the C3 promoter (4).
The regulation of gene expression by histone modifications is well established (5,6,7,8). Acetylation of core histones H3 and H4 plays a prominent role in the regulation of transcriptional activity. The dynamics of histone acetylation result from a balance of two biochemical activities: histone acetylation by histone acetyl transferases (HATs) and histone deacetylation by histone deacetylases (HDACs). These covalent modifications provide marks for interactions with coactivator and repressor proteins to regulate transcription (9,10,11). In addition to histones, growing evidence suggests that HDACs and HATs have additional targets, including transcription factors (12).
Specific HDAC family members play essential roles in cardiac physiology. Loss of Hdac2 prevents reexpression of fetal genes and attenuates cardiac hypertrophy in hearts exposed to hypertrophic stimuli (13). HDAC4 and HDAC5 inhibit myogenesis by directly binding and repressing myogenic transcription factors of the myocyte enhancer factor-2 (MEF-2) family (14, 15). Mice lacking either HDAC5 or HDAC9 display increased sensitivity to cardiac stress signals and cardiac hypertrophy, with double-inactivation leading to embryonic lethality or early postnatal death from multiple cardiac abnormalities (16, 17).
HDAC inhibition has recently been shown to reduce ischemic injury in the brain (18, 19). For example, in a middle cerebral artery occlusion model of brain ischemia, administration of the HDAC inhibitor (HDACI) trichostatin A (TSA) resulted in a 48% decrease in injury volume in treated animals compared to nontreated controls. In addition, HDACIs have recently been shown to elicit a preconditioning effect in an isolated perfused heart model associated with phosphorylation of p38, as well as with improved cardiac remodeling in a chronic LAD ligation rat model (20, 21). Given the documented role that HDACs play in cardiac physiology, we sought to determine the effects of HDAC inhibition on acute I/R injury in the heart, identify the HDAC isoforms involved, and uncover the transcriptional programs that mediate the effect.
MATERIALS AND METHODS
Cardiac I/R model
In brief, anesthesia was induced in adult, 8-wk-old CD-1 mice with ketamine (100 μg/g), xylazine (20 μg/g), and isoflurane (1%). The heart was exposed through a left thoracotomy, and a 7-0 silk suture was placed around the left anterior descending artery (LAD) as it emerged from under the left atrium. The LAD was occluded over a short piece of P-10 tubing for a period of 45 min prior to removal for reperfusion. Ischemia was confirmed by both ST elevation on surface electrocardiogram and visual blanching. The three treatment protocols (T1–T3) involved different time points: T1 (1 h prior to ligation), T2 (45 min after reperfusion), or T3 (12 h after reperfusion). Hearts were harvested 48 h postligation. In brief, fluorescent microspheres were injected into the aortic root of excised hearts with the LAD reoccluded to determine the ischemic area at risk. Subsequently, whole hearts were perfused and then immersed in triphenyltetrazolium chloride (TTC) with incubation to determine nonviable areas, which appear white. Scriptaid, Nullscript, and TSA were all administered IP at 1 μg/g in 100 μl dimethyl sulfoxide (DMSO). Scriptaid (Biomol, Plymouth Meeting, PA, USA) is a synthetic HDACI whose inactive control is Nullscript (Biomol). Differences between the means of unpaired samples were evaluated by the Student’s 2-sided t test. P < 0.05 was considered to be statistically significant. All experiments were performed with approval and under guidelines of the University of Pennsylvania Institutional Animal Care and Use Committee.
Reagents
The antibody to HIF1A was kindly provided by C. Simon (University of Pennsylvania, Philadelphia, PA, USA) The antibody to myosin light chain-2 ventricular (MLC-2v), was kindly provided by Steve Kubalak (Medical University of South Carolina, Charleston, SC, USA). Antibodies to acetylated histones H3 and H4 and β-tubulin were from Upstate (Charlottesville, VA, USA). Antibodies to histone H3, TFII-I, and cleaved caspase 3 were from Cell Signaling Technology (Danvers, MA, USA). Nullscript, Scriptaid, and TSA were from Biomol. HDAC null mice were a generous gift of Eric Olson (Department of Molecular Biology, University of Texas Southwestern, Dallas, TX, USA).
Isolation of mouse neonatal cardiac myocytes and cell culture conditions
Cardiomyocyte cultures were prepared from hearts of 1-day-old CD-1 mouse pups (Charles River, Wilmington, MA, USA). Ventricular tissues were predigested overnight in 2 × 10−5 mmol/L trypsin-Hank’s balanced salt solution without Ca2+ (HBSS)at 4°C under constant shaking. Cells were dissociated by 5 rounds of 10-min digestions with purified type II collagenase (Worthington, Lakewood, NJ, USA) in HBSS at 37°C. Fibroblasts were removed from the cell suspension by two rounds of 1 h differential plating at 37°C in Dulbecco modified Eagle medium (DMEM)/M199 (4:1 ratio) medium containing 10% horse serum, 5% fetal bovine serum, penicillin (100 U/ml), streptomycin (100 μg/ml), HEPES (25 mmol/L), and glutamine (2 mmol/L) in the presence of 5% CO2. Myocytes were cultured on fibronectin-coated tissue culture plates at an initial density of 105 cells/cm2. Medium was exchanged to a 1.5% reduced serum medium containing 1% horse serum and 0.5% fetal bovine serum after 36 h. Cells were pretreated or not for 1 h with TSA (100 nmol/L), Nullscript (6 nmol/L), or Scriptaid (6 nmol/L).
When indicated, hypoxic treatment was carried out in a calibrated incubator with 1% O2, 5% CO2, and 94% N2 for 5 h.
Cell viability assay
Murine neonatal cardiac myocytes were plated in Permanox Labtek chamber slides (Nunc, Rochester, NY, USA). After pretreatment with Nullscript or Scriptaid for 1 h under standard conditions, cells were incubated in normoxia or hypoxia for 5 h, and nonviable cell numbers were determined with ethidium homodimer-1 (EthD-1; 5 μmol/L) from the Live/Dead Viability/Cytotoxicity Kit (Molecular Probes/Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s protocol. EthD-1 enters cells with damaged membranes and is excluded by the intact plasma membrane of live cells. After 30 min, cover glass was applied with Vectashield mounting solution containing DAPI (Vector Laboratories, Burlingame, CA, USA).
Microarray analysis
Neonatal mouse cardiac myocytes were cultured in the presence of Nullscript or Scriptaid under normoxic and hypoxic conditions for 4 h. Total RNA was isolated from the cells by using TRIzol reagent (Gibco/Life Technologies, Inc., Gaithersburg, MD, USA). RNA from cells cultured with Scriptaid was compared with mRNA from cells cultured with Nullscript, under normoxia or hypoxia, using the mouse MOE430A v2.0 array (Affymetrix, Santa Clara, CA, USA). A 2-fold change was considered a threshold for differences in gene expression with values of P < 0.05. Three microarrays were used for each condition. Affymetrix GCOS 1.4 was used to quantify probe intensities; default values provided by Affymetrix were applied to all analysis parameters. Border pixels were removed, and the average intensity of pixels within the 75th percentile was computed for each probe. The average of the lowest 2% of probe intensities occurring in each of 16 microarray sectors was set as background and subtracted from all features in that sector. Probe pairs were scored positive or negative for detection of the targeted sequence by comparing signals from the perfect match and mismatch probe features. The number of probe pairs meeting the default discrimination threshold (tau=0.015) was used to assign a call of absent, present, or marginal for each assayed gene, and a P value was calculated to reflect confidence in the detection call. Probe intensities were then normalized and summarized to a signal value for each transcript using the GC-RMA algorithm as implemented in ArrayAssist Lite v3.4 (Stratagene, La Jolla, CA, USA). Spotfire DecisionSite (Spotfire, Somerville, MA, USA) was used for all microarray analyses.
Quantitative reverse trascriptase-polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from isolated cardiac myocytes or whole hearts with TRIzol (Invitrogen). Reverse transcription was carried out with 1 μg total RNA using the Superscript First-Strand Synthesis System for RT-PCR (Invitrogen), according to the manufacturer’s instructions. Ten nanograms of cDNA was subjected to real-time PCR using Taqman gene expression assays (Applied Biosystems, Foster City, CA, USA) to assess the expression level of Vegfa (Mm00437304_m1), Hdac2 (Mm01193631_m1), Hdac3 (Mm01258403_gH), Hdac4 (Mm01299566_m1), Hdac5 (Mm00515917_m1), Hdac6 (Mm00515945_m1), Hdac9 (Mm00458456_m1), Egln1 (Mm00459770_m1), Egln2 (Mm00519067_m1), and Egln3 (Mm00472200_m1). β2-microglobulin (Mm00437762_m1) was used as an internal control.
siRNA transfection
siRNA (Ambion, Foster City, CA, USA) directed against Hdac2 (ID: 158016), Hdac3 (ID: 158916), Hdac4 (ID: 166635), Hdac5 (ID: 158919), Hdac6 (ID: 158922), Hdac8 (custom), Hdac9 (ID: 174326), or scrambled siRNA (ID: AM4611) were transfected with Lipofectamine 2000 (Invitrogen), according to the manufacturer’s instructions. After 48 h, knockdown efficiency was assessed by quantitative RT-PCR analysis, using the primers listed in the preceding section.
Immunoblot
Primary neonatal murine cardiac myocytes from harvest at P1 were lysed for 15 min at 4°C in a lysis buffer containing 25 mmol/L Tris, pH 7.5; 300 mmol/L NaCl; 1% Triton X-100; and protease inhibitor Complete (Roche, Palo Alto, CA, USA). Cellular debris was removed by centrifugation. Cytoplasmic and nuclear extracts were prepared using the NE-PER kit for nuclear fractionation (Pierce, Rockford, IL, USA), according to the manufacturer’s instructions. Protein concentration was determined by bicinchoninic acid (BCA) methods, and an equal amount (20 μg) was loaded on SDS NuPAGE 4–12% Bis-Tris gels (Invitrogen) and electrotransferred to polyvinylidene fluoride membranes (Amersham Pharmacia Biosciences, Piscataway, NJ, USA). Membranes were blocked with 5% nonfat dry milk in TBS and incubated with the primary antibody overnight at 4°C. Antigen-antibody complexes were visualized by chemiluminescence (Pierce) after incubating the membrane for 1 h with the appropriate secondary antibody conjugated to horseradish peroxidase (Amersham).
Immunohistochemistry
Murine neonatal cardiac myocytes were maintained at normoxia (21% O2) or hypoxia (1% O2) for 5 h. One group was pretreated with Nullscript or Scriptaid for 1 h. Cells were then fixed with 4% paraformaldehyde in PBS for 15 min and permeabilized with 0.2% Triton X-100 in PBS for 10 min. Subsequently, cells were preincubated with PBS containing 2% bovine serum albumin and incubated at 4°C overnight in a humidified chamber with rabbit antibodies directed against either cardiac MYL2 (MLC-2v, 1:200) or cleaved caspase 3 (1:100). After 5 washes, cells were incubated with 1:2000 Alexa Fluor 488 conjugated goat anti-rabbit immunoglobulin G (IgG) (Molecular Probes) and Phalloidin Alexa 568 (1:200) to visualize actin filaments. After 3 washes, cover glasses were applied with Vectashield mounting solution containing DAPI (Vector Laboratories) or TOPRO (Invitrogen) for confocal analysis on a Leica TPS confocal system (Leica Microsystems, Wetzlar, Germany).
Vascular permeability assay
Vascular permeability assay was performed as described previously, with some modifications (22). CD-1 mice were anesthetized with ketamine (100 μg/g, i.p.) plus xylazine (10 μg/g, i.p.). Evans blue (20 mg/g) was injected through the jugular vein over 10 s. Ten minutes after Evans blue injection, mice were sacrificed, and hearts were perfused with 5 ml PBS via the aorta. The left ventricle was harvested and dried in a SpeedVac (Thermo Scientific, Milford, MA, USA) for 4 h. Tissue was homogenized in 1 ml of formamide (Sigma, St. Louis, MO, USA), and the Evans blue was extracted at 70°C for 24 h. After centrifugation (13,000 rpm, 30 min, 4°C), 200 μl of the supernatant were analyzed by measuring absorbance at 620 nm.
Enzymatic activity assays
Fluorometric HDAC activity assay (Upstate) and colorimetric HAT activity assay (Biovision, Mountain View, CA, USA) were performed according to the manufacturer’s instructions. Results are reported as fluorescent units (FU).
RESULTS
Single-dose i.p. administration of HDACIs reduces size of myocardial infarction in vivo
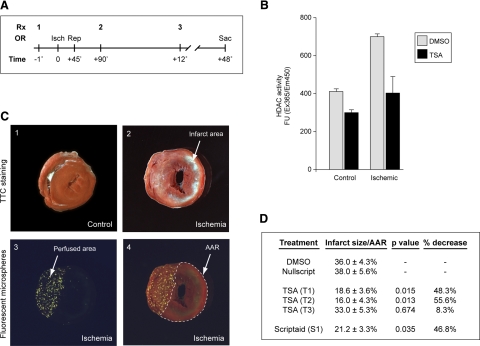
We utilized a standard in vivo model of cardiac I/R to examine the in vivo consequences of HDAC inhibition in the intact heart (Fig. 1) (23). After undergoing 45-minutes of LAD ischemia, followed by 48-hour reperfusion, control sham-operated or ischemic hearts were treated with either vector (DMSO) or the HDACI TSA (Fig. 1A). Hearts were harvested 48 h after reperfusion, left ventricular (LV) protein extracts were prepared, and HDAC activity was measured via a fluorometric assay. LV HDAC activity nearly doubled (411±14 to 700 ±9 FU; P=0.0002) in response to I/R injury. When treated with TSA, HDAC activity decreased to baseline (403±88 FU; P=0.03) (Fig. 1B). No significant changes in HAT activity were observed with I/R injury and HDACI treatment (Supplemental Fig. 1). Next, we asked whether myocardial infarct area could be reduced by treatment with HDACIs. We used an identical LAD I/R protocol: mice were sacrificed and hearts were excised and processed for TTC quantification of infarct area and fluorescent microsphere analysis of area at risk (Fig. 1C, D). Controls presented consistent infarct sizes with respect to area at risk (36.0±4.3%), whereas the single-dose pretreatment protocol (T1) with the HDACI TSA resulted in a dramatic, 48.3% reduction in infarct size (18.6±3.6%; P=0.015). Notably, even treatment 45 min after the injury via the T2 protocol resulted in significantly smaller infarct sizes (16.0±4.3%; P=0.013), a reduction of 55.6%. However, treatment 12 h after injury with the T3 protocol resulted in no significant decrease in infarct size (33.0±5.3%; P=0.674), suggesting that there is a treatment window after which I/R injury becomes resistant to epigenetic manipulations (Fig. 1D). In addition, treatment with the chemically distinct HDACI Scriptaid resulted in a nearly identical effect when compared to Nullscript, with a 46.8% reduction in infarct size (21.2±3.3%; P=0.035). These results strongly suggest that in murine models, HDACIs can reverse the induction of ischemia-induced HDAC activity in vivo and reduce myocardial infarct size by more than 50%. Therefore, we sought an in vitro system of ischemic cardiomyocyte injury to dissect the mechanisms that mediate the protective effect of HDACIs on myocardial ischemia.
Figure 1.
Single-dose i.p. administration of HDACIs reduces I/R-induced myocardial infarction in vivo. A) Mice were treated with either control (DMSO or Nullscript) or HDACI (TSA or Scriptaid) 1 h before ischemia (Isch) (1), 45 min after reperfusion (Rep) (2), or 12 h after reperfusion (3). After 48 h, mice were sacrificed (Sac) and quantified for infarct and area at risk (n=35). Rx, time of treatment protocol delivery; OR, operative maneuver. B) Cardiac extracts from the LV free wall of treated or control mice demonstrate an increase in HDAC activity with ischemia, returning to baseline levels with single-dose i.p. injections of HDACI (n=10). C) Quantification of the infarct size (area of infarct/area at risk). Comparison of area of infarct by TTC staining for control (1) and infarcted hearts (2). Visualization of the unaffected area perfused by fluorescent microspheres (3) and simultaneous measurements to calculate area at risk (AAR) (4). D) Control mice (DMSO or Nullscript) present consistent infarct areas, whereas mice treated either before ischemia (T1) or 45 min postreperfusion (T2) with HDACI show a marked reduction of infarct size (48.3%, P=0.015 to 55.6%, P=0.013). Treatment after 12 h (T3) is ineffective (8.3%, P=0.674).
Hypoxia induces HDAC activity in isolated cardiac myocytes
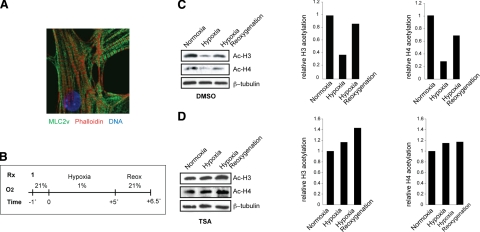
To determine whether hypoxia induced similar alterations of HDAC activity in vitro, neonatal mouse ventricular myocytes were harvested from postnatal day 1 (P1) CD-1 pups and cultured under normoxic (21% O2) conditions. Immunohistochemical staining with anti-MYL2 (MLC-2v) and phalloidin demonstrated normal sarcomeric structure and histological characteristics (Fig. 2A). Our in vitro hypoxic protocol consisted of a 5-h period of hypoxia (1% O2) followed by 90 min of reoxygenation (21% O2) (Fig. 2B). Cell lysates analyzed by Western blot with anti-acetylated histone H3 or H4 demonstrated a strong decrease in acetylated histones H3 and H4 with hypoxia that recovered on reoxygenation (Fig. 2C). We hypothesized that this event might be caused by either an increase in histone deacetylase activity, a decrease in histone acetyl transferase (HAT) activity, or a combination of the two. TSA completely blocked the decrease in both histone H3 and H4 acetylation with hypoxia (Fig. 2D). Treatment with other HDACIs (e.g., Scriptaid), but not controls (DMSO or Nullscript), had identical effects (data not shown). We also examined HDAC and HAT enzymatic activities of hypoxic myocytes identifying an increase in HDAC activity (Supplemental Fig. 2A), whereas there was no change in HAT activity compared to normoxic cells (Supplemental Fig. 2B). Thus, in our system, the primary mechanism of alterations in histone acetylation after ischemia in cardiac myocytes is due to an increase in HDAC activity.
Figure 2.
Inhibition of HDACs prevents deacetylation of histones H3 and H4 induced by hypoxia in isolated cardiac myocytes. A) Immunocytochemistry for MLC2v in isolated primary neonatal cardiac murine myocytes under normoxic conditions, demonstrating normal sarcomeric structure. B) Experimental protocol in vitro. Isolated cardiac myocytes were treated with either control (DMSO or Nullscript) or HDACI (TSA or Scriptaid) 1 h before hypoxia. Exposure to 5 h hypoxia was followed by reoxygenation for 90 min. C, D) Isolated cardiac myocytes were pretreated with DMSO (C) or TSA (100 nM) (D) for 1 h and exposed to 1% O2 for 5 h, followed by 90 min of reoxygenation. Cell lysates were subjected to Western blot analysis for acetylated histones H3 and H4, and relative acetylation level was estimated by densitometric quantification (n=3).
Inhibition of HDACs promotes cell survival during hypoxia in isolated cardiac myocytes
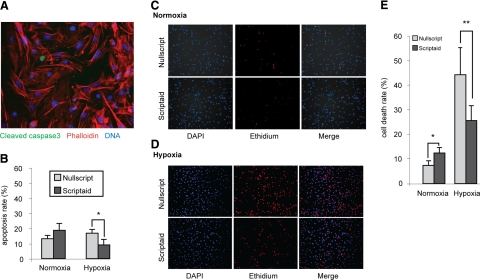
Given the pleiotropic effects seen with HDACIs in noncardiac systems, we examined mechanisms that might modulate this effect, first determining whether HDACIs affected hypoxia-induced apoptotic cell death (8, 11, 24). Under hypoxic or normoxic conditions, we examined cardiac myocytes by immunohistochemistry for cleaved caspase 3 (Fig. 3A). Consistent with other studies, we found cardiac myocytes resistant to hypoxia-induced apoptosis (25, 26). However, despite the low level of apoptosis, HDACIs reduced hypoxic-induced apoptosis in cardiac myocytes by 59% (P=0.01) (Fig. 3B).
Figure 3.
Inhibition of HDACs promotes cell survival during hypoxia in isolated cardiac myocytes. A) Immunocytochemistry for cleaved caspase 3 in isolated cardiac myocytes. B) Quantitative determination of nuclear cleaved caspase 3 staining. *P = 0.01; 500 cells counted in 5 fields for each condition; n = 3. C, D) Live/dead cell assay performed on isolated cardiac myocytes pretreated with Nullscript or Scriptaid for 1 h and cultured under normoxic (C) or hypoxic (D) conditions for 5 h. E) Quantitative evaluation. *P = 0.04; **P = 0.01; 500 cells counted in 5 fields for each condition; n = 3.
Because only a low level of apoptotic activity was observed in our in vitro cardiac myocyte I/R system, we tested whether HDACIs were protective of nonapoptotic cell death. Using identical experimental conditions, we determined overall cell death by ethidium staining (Figs. 3C–E). In contrast to minimal apoptosis activation, hypoxia induced a 5.5-fold increase in overall cell death in Nullscript-treated cells, whereas treatment with Scriptaid resulted in a hypoxia-induced increase of only 2.1-fold, a 62% reduction (P=0.01). It is worth noting that in normoxic conditions, HDAC inhibition produced an increase in cell death, consistent with a prior report in rat neonatal cardiac myocytes (1.5-fold, P=0.04) (27). These experiments demonstrate that environmental oxygen tension plays a critical role in the response of myocytes to HDACIs in that HDAC inhibition under normoxia induces cell death, whereas under hypoxia, they protect cardiac myocytes from cell death.
Inhibition of HDAC activity prevents hypoxia-induced increases in HIF1A and its target genes in isolated cardiac myocytes
Given the reduced damage of the heart in vivo and in vitro, we used microarray-based gene expression profiling to investigate the transcriptional consequences of HDAC inhibition in ischemic cardiac myocytes. Using our in vitro cardiac myocyte I/R protocol, we isolated and RNA purified cells, and gene expression was analyzed by Affymetrix Mouse Genome 430 2.0 Array, as described in detail in the Methods section (Fig. 4 and Supplemental Table 1). We performed a 2-way ANOVA yielding three false discovery rates and controlled P values, the first for each gene’s general responsiveness to hypoxia (comparing normoxia Nullscript [NN] to hypoxia Nullscript [HN]), the second for a gene’s general responsiveness to hypoxia with the HDACI Scriptaid treatment (comparing normoxia Scriptaid [NS] to hypoxia Scriptaid [HS]), and the third for a gene’s differential response to hypoxia depending on Scriptaid treatment (comparing hypoxia Nullscript [HN] to hypoxia Scriptaid [HS]). We identified genes that were significant for this third P value and further filtered them to yield a set that displayed a less than twofold change between NN and NS and a >2-fold change between NN and HN. Finally, we wanted to identify which genes were returned to their normoxic state and thus chose as a final criterion those probe sets with ≥25% return toward NN values under the HS condition.
Figure 4.
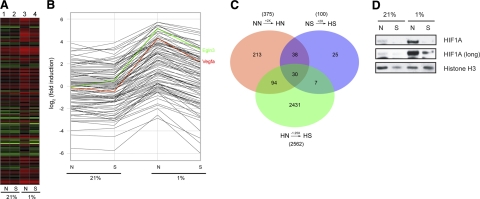
Expression analysis of in vitro ischemic cardiac myocytes reveals down-regulation of HIF1A target genes by HDACI under hypoxic conditions and coregulated gene sets. A) Neonatal cardiac myocytes were exposed to 5 h of hypoxia (1% O2), harvested, and processed for microarray analysis in biological triplicate. Lane 1: normoxia Nullscript (NN); lane 2: normoxia Scriptaid (NS); lane 3: hypoxia Nullscript (HN); lane 4: hypoxia Scriptaid (HS). Note the uniform reversal of gene expression changes by Scriptaid (lane 4) among hypoxia-induced genes (lane 3) toward their prehypoxic state (lanes 1 and 2). In addition, there were few significant changes among this group of coregulated genes with Scriptaid under normoxic conditions (lane 1 vs. 2). B) Gene profiles from the identical experiment in A made explicit, demonstrating the HIF1A-regulated genes Vegfa and Egln3. C) Venn diagram of sets of coregulated genes. The 124 genes that overlap between NN → HN and HN → HS make up the set of genes analyzed in A. D) Neonatal cardiac myocytes were pretreated with Nullscript (N) or Scriptaid (S) for 1 h and exposed to hypoxia for 5 h. Nuclear extracts were isolated and immunoblot analysis was performed with an anti-HIF1A antibody. Histone H3 was used as a nuclear loading control, and relative protein level was estimated by densitometric quantification.
This analysis revealed a series of coregulated genes that describe the ability of HDACIs to generally revert a subset of hypoxia-induced genes back toward their prehypoxic state. A heat map describing the expression profiles of 4 conditions (NN, HN, NS, HS) is illustrated in Fig. 4A. Here, 117 probe sets describe the set of commonly regulated genes that are not significantly changed by the administration of Scriptaid under normoxia (Fig. 4A, columns 1 and 2), are induced by hypoxia (column 3), and are repressed back toward their prehypoxic state by the administration of the HDACI Scriptaid (column 4). Predictably, we found numerous cellular functions were affected, many of which have been previously implicated in HIF1A signaling. Gene profiles are reported for each individual gene in Fig. 4B. Here, Vegfa (red) and Egln3 (green), known targets of HIF1A, were identified as two members of this commonly regulated gene set. Further analysis of this coregulated group of genes revealed that many were previously identified as regulated by HIF1A (Supplemental Table 1). However, there was also a set of coregulated genes lacking known association with HIF1A signaling, suggesting either a novel group of HIF1A regulated genes in cardiac myocytes or a group of genes regulated in a non-HIF1A-dependent manner. Visualization of the entire set of genes filtered for the response to hypoxia with and without Scriptaid treatment (Fig. 4C) demonstrates a relatively small number of genes significantly activated by hypoxia and differentially regulated by HDACIs. Indeed, far more genes were differentially regulated by HDACIs alone (2562) than by hypoxia (375), demonstrating the complexity that underlies this response. Western blot analysis of hypoxic cardiac myocytes revealed a significant HIF1A protein accumulation in the nucleus of cardiac myocytes under hypoxic conditions that was prevented by Scriptaid (Fig. 4D). Interestingly, pretreatment of hypoxic myocytes with the HDACI Scriptaid repressed this induction to baseline levels with no significant change at the mRNA levels (data not shown), extending observations in other cell types (28, 29).
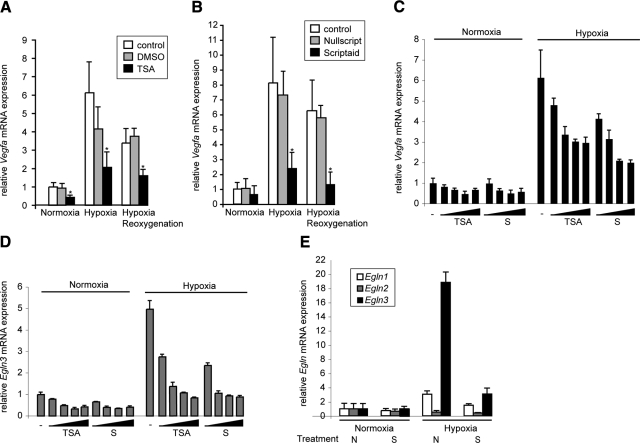
Microarray analysis revealed HDACI modification of both death pathways and HIF1A-dependent pathways. In addition, we were surprised to note that HDACI-mediated suppression of Vegfa, a well-characterized HIF1A target, correlated with cell survival. Using quantitative RT-PCR, we confirmed the microarray data for several genes, including Vegfa, Egln3, and Egln2 (Fig. 5), and Glut1 (data not shown). We measured Vegfa expression in isolated cardiac myocytes in an in vitro I/R assay and found that Vegfa was indeed strongly induced with hypoxia, with maintenance of mRNA levels during the reoxygenation (Fig. 5A, B). No significant changes were observed with a pretreatment with DMSO or Nullscript. As previously identified in our microarray experiments, this increase was prevented by the administration of either TSA (Fig. 5A) or Scriptaid (Fig. 5B). Progressive HDAC inhibition resulted in a dose-dependent reduction of Vegfa expression, an effect that was more pronounced under hypoxic conditions (Fig. 5C). The same pattern was observed for Egln3, another HIF1A target gene responsible for HIF1A degradation (Fig. 5D). To exclude the possibility of a general repressive effect of HDACIs on gene expression, we measured the expression of other members of the EglN family, Egln1 and Egln2 (Fig. 5E). A similar repressive trend was observed for Egln1, consistent with previous studies identifying Egln1 as an HIF1A target. As expected, Scriptaid also prevented the hypoxic induction of Egln1. In contrast, Egln2 expression was unaffected by the HDACI treatment and/or the oxygen level. Together, these data suggest that HDAC activity is specifically required for positive transcriptional regulation under hypoxia, and HDACIs prevent this response in a number of genes by down-regulation of HIF1A.
Figure 5.
Inhibition of HDACs prevents hypoxia-induced increases in Vegfa expression in isolated cardiac myocytes. A, B) Cells were pretreated with DMSO/TSA (A) or Nullscript/Scriptaid (B) for 1 h and exposed to 1% O2 for 5 h. Vegfa expression levels were determined by quantitative RT-PCR. β2-microglobulin was used as an internal control. *P < 0.05 vs. control; n = 5. C, D) Cells were pretreated with increasing amounts of TSA or Scriptaid (S) for 1 h and exposed to 1% O2 for 5 h. Vegfa (C) and Egln3 (D) expression levels were determined by quantitative RT-PCR analysis to detect gene expression using specific primers. β2-microglobulin was used as an internal control. E) Cells were pretreated with Nullscript or Scriptaid for 1 h and were exposed to 1% O2 for 5 h. Egln1, Egln2, and Egln3 expression levels were determined by quantitative RT-PCR. β2-microglobulin was used as an internal control.
Hdac4 is an important mediator of the hypoxic response in isolated cardiac myocytes
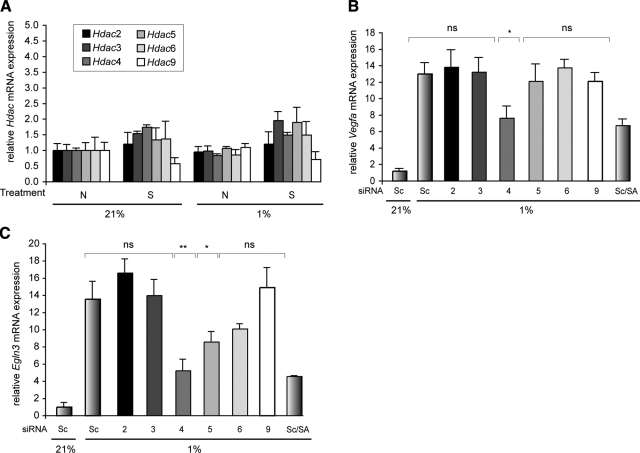
Because multiple Hdac isoforms play important roles in cardiac physiology, we examined the potential role of individual Hdac isoforms for their contribution to the hypoxic response (13, 16, 17). Of the Hdac isoforms expressed in the heart (Hdac2–6 and 9), we did not detect any significant changes in mRNA expression during hypoxia, either in the presence or absence of HDACI (Fig. 6A). Given the strong role that Hdac9 plays in cardiac physiology, we examined the hypoxic induction of Vegfa and Egln3 mRNAs in cardiac myocytes isolated from Hdac9-deficient pups (P1). Hdac9 deficiency in null mice did not prevent hypoxia-induced expression of Vegfa and Egln3, suggesting that other Hdac isoforms mediate the response (data not shown).
Figure 6.
HDAC4 plays a primary role in mediating the hypoxic response of Vegfa and Egln3 in isolated cardiac myocytes. A) Cells were pretreated with Nullscript (N) or Scriptaid (S) for 1 h and exposed to 1% O2 for 5 h. Quantitative RT-PCR analysis was performed to measure gene expression levels of Hdac2, -3, -4, -5, -6, and -9. Throughout these experiments, β2-microglobulin was used as an internal control (n=5). B, C) Cells were transfected with scrambled siRNA (Sc, negative control) or specific siRNA directed against Hdac2, -3, -4, -5, -6, or -9. After 48 h, cells were pretreated with Nullscript or Scriptaid (SA) for 1 h and exposed to 1% O2 for 5 h. Expression levels of the HIF1A target genes Vegfa (B) and Egln3 (C) were determined by quantitative RT-PCR analysis. ns, nonsignificant. *P < 0.05; **P < 0.005; n = 4.
RNA interference was used to deplete endogenous HDACs in neonatal cardiac myocytes, and knockdown efficiency was determined by quantitative RT-PCR with primers for individual Hdacs. Scrambled siRNA controls did not perturb the hypoxic response after 48 h of transfection. Individual, Hdac isoform-specific knockdown resulted in no changes in the expression of other HDAC isozymes (data not shown). Only individualknockdown of Hdac4 under hypoxic conditions fully recapitulated the effect of Scriptaid on the expression of Vegfa (Fig. 6B) and Egln3 (Fig. 6C). We also noticed a small, but significant, decrease of Egln3 expression with Hdac5 knockdown, whereas Vegfa expression remained unchanged, suggesting a potential role for HDAC5 in the hypoxic response. Taken together, these data suggest that in the context of our neonatal murine cardiomyocyte hypoxic assay system for the HIF1A targets Vegfa and Egln3, HDAC4 is the primary mediator of the HDACI-modulated repression of ischemic activation.
Single-dose i.p. administration of HDACI reduces I/R-induced Vegfa induction and vascular permeability in vivo
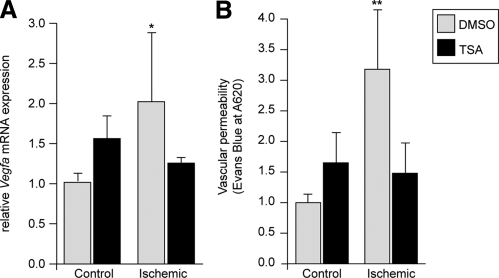
Although multiple mechanisms likely contribute to ischemic protection by HDACI in the intact heart, we asked whether there was a functional role for VEGFA in the process. Although VEGFA is an angiogenic factor that has been proposed as a treatment for myocardial infarction, its potent ability to stimulate vascular leak may have profound negative consequences (30). Thus, we hypothesized that a reduction in Vegfa activation may result in a decrease in vascular permeability, potentially reducing myocardial injury. We utilized our standard in vivo model of cardiac I/R to examine whether HDAC inhibition would be able to inhibit I/R-inducible Vegfa expression in the intact heart. Consistent with our in vitro experiments, Vegfa expression was significantly increased with I/R in vivo. Mirroring in vitro results, HDACIs completely blocked the ischemia-induced increase in Vegfa (Fig. 7A). Hearts from mice undergoing the I/R protocol treated with either vehicle (DMSO) or HDACI (TSA) were infused with Evans Blue and analyzed for vascular permeability (31). Ischemia resulted in a 3-fold increase in vascular permeability, subsequently halved by treatment with HDACI (Fig. 7B). These results strongly suggest that in murine models, HDACIs can 1) reverse the induction of ischemia-induced HDAC activity in vivo, 2) return ischemia-induced Vegfa induction to nonischemic levels, 3) prevent ischemia-induced vascular permeability, and 4) reduce myocardial infarct size by over 50%.
Figure 7.
Single-dose i.p. administration of HDACIs reduces I/R-induced Vegfa induction and vascular permeability in vivo. A) Vegfa mRNA levels from the LV free wall of treated or control mice increase with ischemia, returning to baseline levels with single-dose i.p. injections of HDACI. *P < 0.05; n = 5. B) Cardiac extracts from mice undergoing the I/R protocol treated with either vehicle (DMSO) or HDACI (TSA) were infused with Evans blue and analyzed for vascular permeability, which demonstrated a tripling of permeability with ischemia. This was subsequently halved by treatment with HDACI. **P = 0.003; n = 20.
DISCUSSION
These experiments demonstrate a profound, reproducible, and protective effect of HDACIs both in vitro and in vivo when cardiac myocytes are exposed to hypoxia. We demonstrate that HDAC inhibition can reduce the size of myocardial infarction due to I/R between 48.3 and 55.6%, depending on treatment protocols. Pretreatment resulted in a strong, significant, and reproducible reduction of infarct size; successful treatment is possible even postinjury, though benefit becomes negligible after 12 h. These observations are consistent with previous studies showing that HDACIs protect against brain ischemia, even when administrated 3 h after the onset of ischemia (32).
HDACs play pleiotropic roles in transcriptional regulation and cellular physiology (8). Although HDACIs have been shown to be proapoptotic in a number of cell types, their effects are highly context dependent. A striking example of this functional duality comes from studies of erythropoiesis, in which HDAC inhibition simultaneously negatively regulates IL-3-mediated growth of one class of erythroid precursors, while playing an important role in EPO-mediated differentiation and survival of other erythroid precursors (24). Indeed, in rat neonatal cardiac myocytes, under normoxic culture conditions, HDACIs are mildly proapoptotic (27). Yet, their role in I/R injury in the intact heart was previously unexplored.
One contributing mechanism for the profound ischemic protection with HDACIs is a reduction in ischemia-induced myocardial cell death. Importantly, environmental oxygen tension was critical to the response of the cell to HDACIs, such that HDACIs were protective of myocyte cell death only under hypoxic conditions. Our results support a significant reduction in nonapoptotic cell death by HDACIs, and to a lesser extent in apoptotic cell death. Despite the fact that cardiac myocytes are largely resistant to apoptosis, gene profiling demonstrated an HDACI-mediated reduction in Bnip3 and Bnip3l activation, suggesting a potential mechanism for a small reduction of apoptotic cell death. However, the majority of HDACI-mediated protection was due to a nonapoptotic mechanism, the mechanism of which remains a target for future study.
Both hypoxia and HDAC inhibition affect a vast array of pathways. Indeed, our comprehensive profiling of gene sets derived from normoxic and hypoxic cardiac myocytes treated with and without HDACI revealed important findings. First, under our selection criteria, far more genes were activated or suppressed in response to HDAC inhibition (2562) than by hypoxia alone (375). Second, a striking number of hypoxia-inducible genes were differentially regulated by HDACIs (124). Not surprisingly, a number of these differentially regulated genes were HIF1A targets. Consistent with this observation, our results demonstrate that HDAC inhibition prevents hypoxia-inducible stabilization of HIF1A protein in isolated murine cardiac myocytes. In addition, a number of downstream HIF1A hypoxia-inducible targets are similarly down-regulated, including Vegfa, Egln1, and Egln3. However, we also identified a number of HDACI-regulated, hypoxia-inducible genes with no known association to HIF1A, suggesting that these genes either are novel HIF1A targets or are regulated in a non-HIF1A-dependent yet hypoxia-inducible fashion.
HIF1A plays a critical role in the cellular response to hypoxia and tissue ischemia and is regulated by associated proteins and covalent modifications. In response to hypoxia, HIF1A accumulates and directly induces the transcriptional activity of a number of genes, including genes that control angiogenesis (such as Vegfa) and energy metabolism [for example, the glucose transporter 1 (GLUT1/Scl2a1), 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3/Pfkfb3)] (33). In the presence of oxygen, HIF proteins are hydroxylated on conserved prolyl residues by members of the egg-laying-defective nine (EglN) prolyl hydroxylases. The enzymatic activity of HDACs results in protein deacetylation, usually repressing the transcription of associated loci. In a direct transcriptional model, the application of HDACIs would, in general, release this HDAC-mediated repression, leading to an increase in histone acetylation and subsequent gene activation. Therefore, the ability of HDACIs to repress the ischemia-mediated induction of HIF1A and its target genes was, at first, counterintuitive. However, recent studies show that class II HDACs associate with HIF1A and modulate its stability (34, 35). For example, HDAC7 is able to increase transcriptional activity of HIF1A through the formation of a complex with HIF1A, HDAC7, and p300 (34). In addition, HDACIs repress HIF1A independently of von Hippel-Lindau tumor suppressor, suggesting that acetylation may regulate HIF function by targeting HIF1A:cofactor complexes, rather than by direct acetylation of HIF1A (36). However, the molecular link between HDACs and HIF1A remains controversial.
Because HDACI protected cardiac myocytes from ischemic injury both in vitro and in vivo and regulated the response of a coordinate set of genes in vitro, we asked whether an individual HDAC might be responsible for this response. We identified HDAC4 as the critical HDAC isoform likely mediating the protective response. Hdac4 null mice die perinatally with abnormal chondrocyte hypertrophy but lacking any identifiable cardiac defects (37). Nevertheless, recent studies suggest that HDAC4 may play a role in cardiac physiology. Cytosolic accumulation of HDAC4 is required for cardiomyocyte hypertrophy in response to calcium/calmodulin-dependent kinase II (CamKII) signaling (38). Phosphorylation of HDAC4 by CaMKII induces its nuclear export and leads to derepression of HDAC target genes. Our report, to our knowledge, is the first to show that HDAC4 is also involved in cellular response to cardiac ischemia, consistent with a recent study reporting that HDAC4 plays a role in mediating hypoxic response in chondrocytes (39). Knockdown of Hdac4 with siRNA increases HIF1A acetylation, and its subsequent degradation via the proteasome pathway in VHL-deficient cell line (35). In a series of preliminary experiments using a Gal4-Trap, we have demonstrated a functional interaction between HIF1A and HDAC4 in HEK293T cells, resulting in inhibition of transcription, suggesting the associated increases in hypoxia induced HDAC activity and transcriptional activation in cardiac myocytes might be due to either recruitment of a coactivator, as has been previously shown for HIF1A, HDAC7, and p300 (34) or HDAC4-mediated stabilization of HIF1A protein level as previously shown in VHL-deficient cell line (35). Although we did not examine additional post-translational modifications of HIF1A induced by class II HDACs, others, such as sumoylation, might also be considered. Furthermore, other HDAC family members might be important in the mediation of non-HIF1A-protective mechanisms.
The association of reduced Vegfa expression, reduced vascular permeability, and reduced infarct size is provocative. VEGFA is an important, multifunctional molecule and is directly regulated by HIF1A. With acute exposure, VEGFA is a potent stimulator of vascular permeability, whereas with prolonged exposure, it induces angiogenesis. Endothelial cells exposed to VEGFA develop fenestrations, caveolae, and vesiculo-vacuolar organelles (40, 41). Type 2 VEGF receptor (VEGFR2) associates with VE-cadherin that rapidly dissociates in response to VEGFA (42). VEGFA also inhibits gap junction communication via connexin 43 phosphorylation and Src activation (43, 44). Finally, VEGFA disrupts tight junctions by altering the phosphorylation of zonula occludin 1 and occludin (45). Consistent with our in vitro studies, Vegfa expression was significantly increased with I/R injury. Mirroring the in vitro results, HDACIs completely blocked the acute, ischemia-induced increase in Vegfa. Although VEGFA is an angiogenic factor that has been proposed as chronic treatment for myocardial infarction, its potent ability to stimulate vascular leak in the acute setting may have profound negative consequences (30). Although initially counterintuitive, we hypothesized that the reduction in Vegfa activation may result in a decrease in vascular permeability providing myocardial protection. The association of HDACI-mediated reduction in ischemic injury and HDACI-mediated reductions in Vegfa expression/vascular permeability is compelling and suggests further studies that may directly prove the association of acute inhibition of Vegfa expression with cardiac protection, while preserving the chronic, potential beneficial effects of VEGFA-mediated angiogenesis. Although the complete set of mechanisms that mediate the protective effect of HDACIs remains to be completely delineated, we clearly demonstrate that HDAC inhibition can reduce the size of myocardial infarction due to I/R injury by half. Importantly, treatment is possible even postinjury. Our results have important implications for the potential application of these compounds in the context of acute coronary syndromes such that the administration of HDACIs would only be predicted to have a beneficial effect in the context of active ischemia.
Supplementary Material
Acknowledgments
We thank Sherrie Vassallo for administrative and secretarial support and Thomas L. Spray for departmental support. This study was supported by the McCabe Foundation, University Research Foundation, and the Division of Pediatric Cardiothoracic Surgery, Children’s Hospital of Philadelphia (P.J.G.), and National Institutes of Health grant R01 HL071546 (J.A.E.). P.J.G., J.A.E., and I.A. have filed U.S. Patent Application R-3701: Inhibition of histone deactylase activity as treatment and preventive therapy for ischemic injury/inhibition of DNA methyl transferase activity as treatments and preventive therapy for ischemic injury.
References
- Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C J, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Dallas, TX, USA: American Heart Association; Heart Disease and Stroke Statistics–2007 Update. 2006 doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- Thom T, Haase N, Rosamond W, Howard V J, Rumsfeld J, Manolio T, Zheng Z J, Flegal K, O'Donnell C, Kittner S, Lloyd-Jones D, Goff D C, Jr, Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P. Heart disease and stroke statistics–2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- Formisano L, Noh K M, Miyawaki T, Mashiko T, Bennett M V, Zukin R S. Ischemic insults promote epigenetic reprogramming of mu opioid receptor expression in hippocampal neurons. Proc Natl Acad Sci U S A. 2007;104:4170–4175. doi: 10.1073/pnas.0611704104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt J R, Parker M D, Affleck L J, Corps C, Hostert L, Michalak E, Lodge J P. Ischemic epigenetics and the transplanted kidney. Transplant Proc. 2006;38:3344–3346. doi: 10.1016/j.transproceed.2006.10.112. [DOI] [PubMed] [Google Scholar]
- Kouzarides T. Acetylation: a regulatory modification to rival phosphorylation? EMBO J. 2000;19:1176–1179. doi: 10.1093/emboj/19.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T. Histone methylation in transcriptional control. Curr Opin Genet Dev. 2002;12:198–209. doi: 10.1016/s0959-437x(02)00287-3. [DOI] [PubMed] [Google Scholar]
- Gregory P D, Wagner K, Horz W. Histone acetylation and chromatin remodeling. Exp Cell Res. 2001;265:195–202. doi: 10.1006/excr.2001.5187. [DOI] [PubMed] [Google Scholar]
- Wade P A. Transcriptional control at regulatory checkpoints by histone deacetylases: molecular connections between cancer and chromatin. Hum Mol Genet. 2001;10:693–698. doi: 10.1093/hmg/10.7.693. [DOI] [PubMed] [Google Scholar]
- Fischle W, Wang Y, Allis C D. Histone and chromatin cross-talk. Curr Opin Cell Biol. 2003;15:172–183. doi: 10.1016/s0955-0674(03)00013-9. [DOI] [PubMed] [Google Scholar]
- Strahl B D, Allis C D. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wysocka J, Perlin J R, Leonelli L, Allis C D, Coonrod S A. Linking covalent histone modifications to epigenetics: the rigidity and plasticity of the marks. Cold Spring Harb Symp Quant Biol. 2004;69:161–169. doi: 10.1101/sqb.2004.69.161. [DOI] [PubMed] [Google Scholar]
- Minucci S, Pelicci P G. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer Nat. Rev Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- Trivedi C M, Luo Y, Yin Z, Zhang M, Zhu W, Wang T, Floss T, Goettlicher M, Noppinger P R, Wurst W, Ferrari V A, Abrams C S, Gruber P J, Epstein J A. Hdac2 regulates the cardiac hypertrophic response by modulating Gsk3beta activity. Nat Med. 2007;13:324–331. doi: 10.1038/nm1552. [DOI] [PubMed] [Google Scholar]
- Lemercier C, Verdel A, Galloo B, Curtet S, Brocard M P, Khochbin S. mHDA1/HDAC5 histone deacetylase interacts with and represses MEF2A transcriptional activity. J Biol Chem. 2000;275:15594–15599. doi: 10.1074/jbc.M908437199. [DOI] [PubMed] [Google Scholar]
- McKinsey T A, Zhang C L, Olson E N. Activation of the myocyte enhancer factor-2 transcription factor by calcium/calmodulin-dependent protein kinase-stimulated binding of 14–3-3 to histone deacetylase 5. Proc Natl Acad Sci U S A. 2000;97:14400–14405. doi: 10.1073/pnas.260501497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, McKinsey T A, Zhang C L, Richardson J A, Hill J A, Olson E N. Histone deacetylases 5 and 9 govern responsiveness of the heart to a subset of stress signals and play redundant roles in heart development. Mol Cell Biol. 2004;24:8467–8476. doi: 10.1128/MCB.24.19.8467-8476.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C L, McKinsey T A, Chang S, Antos C L, Hill J A, Olson E N. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell. 2002;110:479–488. doi: 10.1016/s0092-8674(02)00861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres M, Meisel A, Biniszkiewicz D, Namura S, Prass K, Ruscher K, Lipski A, Jaenisch R, Moskowitz M A, Dirnagl U. DNA methyltransferase contributes to delayed ischemic brain injury. J Neurosci. 2000;20:3175–3181. doi: 10.1523/JNEUROSCI.20-09-03175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres M, Fan G, Meisel A, Dirnagl U, Jaenisch R. Effects of cerebral ischemia in mice lacking DNA methyltransferase 1 in post-mitotic neurons. Neuroreport. 2001;12:3763–3766. doi: 10.1097/00001756-200112040-00032. [DOI] [PubMed] [Google Scholar]
- Lee T M, Lin M S, Chang N C. Inhibition of histone deacetylase on ventricular remodeling in infarcted rats. Am J Physiol Heart Circ Physiol. 2007;293:H968–H977. doi: 10.1152/ajpheart.00891.2006. [DOI] [PubMed] [Google Scholar]
- Zhao T C, Cheng G, Zhang L X, Tseng Y T, Padbury J F. Inhibition of histone deacetylases triggers pharmacologic preconditioning effects against myocardial ischemic injury. Cardiovasc Res. 2007;76:473–481. doi: 10.1016/j.cardiores.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Simard B, Gabra B H, Sirois P. Inhibitory effect of a novel bradykinin B1 receptor antagonist, R-954, on enhanced vascular permeability in type 1 diabetic mice. Can J Physiol Pharmacol. 2002;80:1203–1207. doi: 10.1139/y02-153. [DOI] [PubMed] [Google Scholar]
- Abdullah I, Lepore J J, Epstein J A, Parmacek M S, Gruber P J. MRL mice fail to heal the heart in response to ischemia-reperfusion injury. Wound Repair Regen. 2005;13:205–208. doi: 10.1111/j.1067-1927.2005.130212.x. [DOI] [PubMed] [Google Scholar]
- Yamamura K, Ohishi K, Katayama N, Yu Z, Kato K, Masuya M, Fujieda A, Sugimoto Y, Miyata E, Shibasaki T, Heike Y, Takaue Y, Shiku H. Pleiotropic role of histone deacetylases in the regulation of human adult erythropoiesis. Brit J Haematol. 2006;135:242–253. doi: 10.1111/j.1365-2141.2006.06275.x. [DOI] [PubMed] [Google Scholar]
- Sanchis D, Mayorga M, Ballester M, Comella J X. Lack of Apaf-1 expression confers resistance to cytochrome c-driven apoptosis in cardiomyocytes. Cell Death Differ. 2003;10:977–986. doi: 10.1038/sj.cdd.4401267. [DOI] [PubMed] [Google Scholar]
- Webster K A, Discher D J, Kaiser S, Hernandez O, Sato B, Bishopric N H. Hypoxia-activated apoptosis of cardiac myocytes requires reoxygenation or a pH shift and is independent of p53. J Clin Invest. 1999;104:239–252. doi: 10.1172/JCI5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcendor R R, Kirshenbaum L A, Imai S, Vatner S F, Sadoshima J. Silent information regulator 2alpha, a longevity factor and class III histone deacetylase, is an essential endogenous apoptosis inhibitor in cardiac myocytes. Circ Res. 2004;95:971–980. doi: 10.1161/01.RES.0000147557.75257.ff. [DOI] [PubMed] [Google Scholar]
- Deroanne C F, Bonjean K, Servotte S, Devy L, Colige A, Clausse N, Blacher S, Verdin E, Foidart J M, Nusgens B V, Castronovo V. Histone deacetylases inhibitors as anti-angiogenic agents altering vascular endothelial growth factor signaling. Oncogene. 2002;21:427–436. doi: 10.1038/sj.onc.1205108. [DOI] [PubMed] [Google Scholar]
- Kong X, Lin Z, Liang D, Fath D, Sang N, Caro J. Histone deacetylase inhibitors induce VHL and ubiquitin-independent proteasomal degradation of hypoxia-inducible factor 1alpha. Mol Cell Biol. 2006;26:2019–2028. doi: 10.1128/MCB.26.6.2019-2028.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis S M, Cheresh D A. Pathophysiological consequences of VEGF-induced vascular permeability. Nature. 2005;437:497–504. doi: 10.1038/nature03987. [DOI] [PubMed] [Google Scholar]
- Guimaraes C L, Trentin P G, Rae G A. Endothelin ET(B) receptor-mediated mechanisms involved in oleic acid-induced acute lung injury in mice. Clin Sci (Lond) 2002;103:340S–344S. doi: 10.1042/CS103S340S. [DOI] [PubMed] [Google Scholar]
- Kim H J, Rowe M, Ren M, Hong J S, Chen P S, Chuang D M. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. J Pharmacol Exp Therapeut. 2007;321:892–901. doi: 10.1124/jpet.107.120188. [DOI] [PubMed] [Google Scholar]
- Semenza G L. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- Kato H, Tamamizu-Kato S, Shibasaki F. Histone deacetylase 7 associates with hypoxia-inducible factor 1α and increases transcriptional activity. J Biol Chem. 2004;279:41966–41974. doi: 10.1074/jbc.M406320200. [DOI] [PubMed] [Google Scholar]
- Qian D Z, Kachhap S K, Collis S J, Verheul H M, Carducci M A, Atadja P, Pili R. Class II histone deacetylases are associated with VHL-independent regulation of hypoxia-inducible factor 1α. Cancer Res. 2006;66:8814–8821. doi: 10.1158/0008-5472.CAN-05-4598. [DOI] [PubMed] [Google Scholar]
- Fath D M, Kong X, Liang D, Lin Z, Chou A, Jiang Y, Fang J, Caro J, Sang N. Histone deacetylase inhibitors repress the transactivation potential of hypoxia-inducible factors independently of direct acetylation of HIF-α. J Biol Chem. 2006;281:13612–13619. doi: 10.1074/jbc.M600456200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega R B, Matsuda K, Oh J, Barbosa A C, Yang X, Meadows E, McAnally J, Pomajzl C, Shelton J M, Richardson J A, Karsenty G, Olson E N. Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell. 2004;119:555–566. doi: 10.1016/j.cell.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Backs J, Song K, Bezprozvannaya S, Chang S, Olson E N. CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J Clin Invest. 2006;116:1853–1864. doi: 10.1172/JCI27438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao M, Tamai N, Tsumaki N, Yoshikawa H, Myoui A. Oxygen tension regulates chondrocyte differentiation and function during endochondral ossification. J Biol Chem. 2006;281:31079–31092. doi: 10.1074/jbc.M602296200. [DOI] [PubMed] [Google Scholar]
- Feng D, Nagy J A, Hipp J, Dvorak H F, Dvorak A M. Vesiculo-vacuolar organelles and the regulation of venule permeability to macromolecules by vascular permeability factor, histamine, and serotonin. J Exp Med. 1996;183:1981–1986. doi: 10.1084/jem.183.5.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasile E, Qu H, Dvorak H F, Dvorak A M. Caveolae and vesiculo-vacuolar organelles in bovine capillary endothelial cells cultured with VPF/VEGF on floating Matrigel-collagen gels. J Histochem Cytochem. 1999;47:159–167. doi: 10.1177/002215549904700205. [DOI] [PubMed] [Google Scholar]
- Kevil C G, Payne D K, Mire E, Alexander J S. Vascular permeability factor/vascular endothelial cell growth factor-mediated permeability occurs through disorganization of endothelial junctional proteins. J Biol Chem. 1998;273:15099–15103. doi: 10.1074/jbc.273.24.15099. [DOI] [PubMed] [Google Scholar]
- Weis S, Cui J, Barnes L, Cheresh D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J Cell Biol. 2004;167:223–229. doi: 10.1083/jcb.200408130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis S, Shintani S, Weber A, Kirchmair R, Wood M, Cravens A, McSharry H, Iwakura A, Yoon Y S, Himes N, Burstein D, Doukas J, Soll R, Losordo D, Cheresh D. Src blockade stabilizes a Flk/cadherin complex, reducing edema and tissue injury following myocardial infarction. J Clin Invest. 2004;113:885–894. doi: 10.1172/JCI20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonetti D A, Barber A J, Hollinger L A, Wolpert E B, Gardner T W. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem. 1999;274:23463–23467. doi: 10.1074/jbc.274.33.23463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.