Abstract
Pancreatic beta-cell loss through apoptosis represents a key factor in the pathogenesis of diabetes; however, no effective approaches to block this process and preserve endogenous beta-cell mass are currently available. To study the role of thioredoxin-interacting protein (TXNIP), a proapoptotic beta-cell factor we recently identified, we used HcB-19 (TXNIP nonsense mutation) and beta-cell-specific TXNIP knockout (bTKO) mice. Interestingly, HcB-19 mice demonstrate increased adiposity, but have lower blood glucose levels and increased pancreatic beta-cell mass (as assessed by morphometry). Moreover, HcB-19 mice are resistant to streptozotocin-induced diabetes. When intercrossed with obese, insulin-resistant, and diabetic mice, double-mutant BTBRlepob/obtxniphcb/hcb are even more obese, but are protected against diabetes and beta-cell apoptosis, resulting in a 3-fold increase in beta-cell mass. Beta-cell-specific TXNIP deletion also enhanced beta-cell mass (P<0.005) and protected against diabetes, and terminal deoxynucleotidyl transferase-mediated nick end labeling (TUNEL) revealed a ∼50-fold reduction in beta-cell apoptosis in streptozotocin-treated bTKO mice. We further discovered that TXNIP deficiency induces Akt/Bcl-xL signaling and inhibits mitochondrial beta-cell death, suggesting that these mechanisms may mediate the beta-cell protective effects of TXNIP deficiency. These results suggest that lowering beta-cell TXNIP expression could serve as a novel strategy for the treatment of type 1 and type 2 diabetes by promoting endogenous beta-cell survival.—Chen, J., Hui, S. T., Couto, F. M., Mungrue, I. N., Davis, D. B., Attie, A. D., Lusis, A. J., Davis, R. A., Shalev, A. Thioredoxin-interacting protein deficiency induces Akt/Bcl-xL signaling and pancreatic beta-cell mass and protects against diabetes.
Keywords: apoptosis, islets, obesity, insulin sensitivity, bTKO, TXNIP
Pancreatic beta-cell loss through apoptosis is a key factor in the pathogenesis of both type 1 and type 2 diabetes. Whereas in type 1 diabetes beta-cell destruction is caused by an autoimmune process, type 2 diabetes results from a combination of insulin resistance and impaired beta-cell function and survival (1,2,3,4,5). As long as pancreatic beta cells can produce enough insulin to overcome the insulin resistance, normal glucose homeostasis is maintained. However, once this functional beta-cell mass fails to compensate for the increased insulin requirements, impaired glucose tolerance, hyperglycemia and diabetes ensues. Diabetes has become a worldwide epidemic owing to the widespread increase in obesity, which is a major risk factor for insulin resistance. Despite major efforts to halt this epidemic and find a cure for diabetes, and even though the critical role of beta-cell apoptosis in the development and progression of diabetes has been recognized, current treatment strategies do not focus on the preservation of endogenous beta-cell mass. Novel approaches that could promote pancreatic beta-cell reserve, protect against apoptotic beta-cell loss, and help prevent diabetes are therefore urgently needed.
Thioredoxin-interacting protein (TXNIP) is a ubiquitously expressed 50 kD protein that binds to and inhibits thioredoxin and can thereby modulate the cellular redox state and induce oxidative stress (6,7,8,9,10). Structurally, TXNIP demonstrates some homology with arrestins, which have been implicated in obesity. However, TXNIP contains two unique cysteines not found in other arrestin domain-containing proteins (10). Interestingly, we observed that TXNIP expression is increased in human islets exposed to high glucose and in islets of insulin resistant and diabetic mice (11, 12). Moreover, we recently found that overexpression of TXNIP in INS-1 beta cells induces apoptosis (12), suggesting that this protein may be involved in the beta-cell apoptosis associated with diabetes.
The aim of the present study was, therefore, to determine whether reduction of TXNIP expression could be beta-cell protective and have antidiabetic properties in vivo. To this end we crossed mice with generalized TXNIP deficiency (HcB-19) (13), with severely obese, insulin resistant, and diabetic mice lacking leptin to generate beta-cell-specific TXNIP knockout (bTKO) mice. We then analyzed the effects of TXNIP deficiency under conditions of obesity- and streptozotocin (STZ) -induced diabetes. The results of these studies provide the first evidence for the critical in vivo role TXNIP plays in controlling pancreatic beta-cell survival and glucose homeostasis, and identify TXNIP reduction as a powerful strategy to preserve endogenous beta-cell mass in both type 1 and type 2 diabetes.
MATERIALS AND METHODS
Animal studies
All mouse studies were approved by the University of Wisconsin Animal Care and Use Committee, and the National Institutes of Health (NIH) principles of laboratory animal care were followed. Mice were maintained under standardized conditions on a fixed 12 h light-dark cycle and received a regular chow [Teklad Rodent diet (W) 8604 Harlan, Indianapolis, IN, USA] and water ad libitum.
The C3H congenic TXNIP-deficient HcB-19 (HcB) mice harboring a naturally occurring nonsense mutation in the TXNIP gene and the control C3H/DiSnA (C3H) strain have been described elsewhere (13,14,15). BHcB mice were generated by backcrossing HcB-19 mice for 10 generations into a C57BL/6 background. C57BL/6 (B6) control mice were obtained from Jackson Laboratory (Bar Harbor, ME, USA).
We generated the double-mutant congenic mouse BTBR lepob/obtxniphcb/hcb (BTBR.ob/hcb) by backcrossing HcB-19 mice for 7 generations into a BTBR background and intercrossing them with BTBRlepob/+ mice. Double-heterozygous BTBRlepob/+txniphcb/+ mice were used for maintaining the line and as controls. (Phenotypically and biochemically, these mice were indistinguishable from wild-type BTBRlep+/+ txnip+/+ mice.) Genotyping for the leptinob mutation was performed by Polymerase chain reaction (PCR) with the forward primer 5′-TGTCCAAGATGGACCAGACTC-3′ and the reverse primer 5′-ACTGGTCTGAGGCAGGGAGCA-3′ followed by restriction digest with DdeI, according to the Jackson protocol. The txnipHcB-19 allele was detected using the D3mit76 and D3mit101 markers, as described elsewhere (13).
The bTKO was generated using the Cre-loxP system. Briefly, exons 2–8 of the TXNIP gene (total of 8 exons) were flanked by loxP sites and a floxed TXNIP mouse was generated by introducing this targeting vector. Crossing with Rip-Cre mice (Jackson Laboratory) expressing the Cre recombinase under the control of the rat insulin promoter yielded the bTKO mouse. Mice were then backcrossed for three generations into a C57BL/6 background, and lox/lox littermates were used as controls. Genotyping for txniplox/lox was performed by PCR using the primers NeoF1: 5′-CGCTGACTCCTCAAGATGGGT-3′; R2: 5′-GGAAAGACAACGCCAGAAGG-3′; and GT1: 5′-ACGTGCTACTTCCATTTGTC-3′ For rip-cre+/− we used primers Cre1: 5′-GTTCGCAAGAACCTGATGGACA-3′ and Cre2: 5′-CTAGAGCCTGTTTTGCACGTTC-3′. Appropriate recombination and excision of the TXNIP gene was confirmed using primers F3: 5′-TGAGGTGGTCTTCAACGACC-3′; R2: 5′-GGAAAGACAACGCCAGAAGG-3′; and R3: 5′-CCTTGAGGAAGCTCGAAGCC-3′. Beta-cell-specific deletion of TXNIP was confirmed by reverse transcriptase-polymerase chain reaction (RT-PCR) using the primers Fc-mTXNIP: 5′-GGTGATGTTCAAGAAGATCAAG-3′ and Rc-mTXNIP: 5′-CCTCAGTGTAAGTGGGTGG-3′. GAPDH served as an internal control and was amplified using the primers F-GAPDH: 5′-GAAGGTGAAGGTCGGAGTC-3′ and R-GAPDH: GAAGATGGTGATGGGATTTC-3′.
For measurement of food intake, mice were housed 2–4 animals per cage and given ad libitum access to chow. An exact amount of food was weighed out and the amount eaten during 1 wk per mouse was measured. Special attention was paid to avoid or, if present, account for food crumbs fallen into the cage bedding. Measurements were performed on 4 consecutive weeks on age and sex matched mice and the means used for comparison between TXNIP-deficient and control mice.
After sacrifice, gonadal fat pads were isolated and weighed before being used for histology. Subcutaneous fat was collected from the abdominal wall and brown adipose tissue was dissected from the interscapular area.
Whole body fat mass was assessed by dual energy X-ray absorptiometry (DXA) using a PIXImus densitometer (GE Lunar, Madison, WI, USA).
Diabetes was induced in mice by multiple low-dose STZ injections, an established method known to lead to beta-cell apoptosis (16,17,18). In brief, 40 mg STZ/kg body weight, prepared freshly in 0.1 mM sodium citrate at pH 4.5, was injected daily i.p. for 5 consecutive days into BHcB and C57BL/6 mice. We used BHcB rather than HcB-19 mice because mice on the C3H background have been shown to carry a STZ resistance gene (19). The same regimen was used for the bTKO experiments, but mice received 4 STZ injections because this already led to overt diabetes in our control lox/lox mice. Because female mice do not respond to STZ, only male mice were used for all STZ experiments. Nonfasting blood glucose was checked immediately before the first STZ injections and at 4, 8, 11, 15, and 18 days after the initial injection.
Islet isolation
Mouse pancreatic islets were isolated by collagenase digestion, as described elsewhere (20,21,22).
RT-PCR
RNA from mouse islets was isolated using the RNAqueous–4PCR Kit (Ambion, Inc., Austin, TX, USA). RNA from all other tissues was isolated using TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. RNA was converted to cDNA with the First Strand cDNA synthesis kit (Roche Applied Science, Indianapolis, IN, USA). Quantitative real-time RT-PCR was performed on a Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) and all samples were analyzed in triplicates and corrected for the 18S ribosomal subunit (Applied Biosystems) run as an internal standard.
Hypothalamic TXNIP expression was measured by real-time RT-PCR using the primers mTXNIP forward: 5′-CGAGTCAAAGCCGTCAGGAT-3′ and mTXNIP reverse: 5′-TTCATAGCGCAAGTAGTCCAAAGT-3′ and FAM labeled probe: CTCAGCAGTGCAAACA. Akt mRNA expression was quantified using the forward primer 5′-TGCCCACACGCTTACTGAGA-3′ and the reverse primer 5′-CAAAGCAGAGGCGGTCGT-3′.
Immunoblotting
Protein extracts were prepared as described elsewhere (22) and expression of total or phosphorylated/activated Akt was assessed by Akt and specific (Ser-473 p-Akt antibodies (Cell Signaling, Danvers, MA, USA) (1:1000 dilution at 4°C overnight). Beta-actin (1:200) (Abcam, Cambridge, MA, USA) was used as a loading control. The secondary antibodies used were anti-rabbit immunoglobulin G (IgG) (Bio-Rad, Hercules, CA, USA) and anti-mouse IgG (1:5000) (Amersham, Piscataway, NJ, USA). Bands were visualized by Lumigen PS-3 detection reagent (Amersham) and quantified by ImageQuant Version 5.1 (GE Healthcare Life Sciences, Piscataway, NJ, USA).
Immunohistochemistry
Pancreata were cleared of fat, weighed, and fixed in 4% formaldehyde, and processed in an automated Shandon Citadel 100 (Thermo Scientific, Waltham, MA, USA) machine before paraffin embedding and preparation of 10 μm sections. For light microscopy, insulin was stained using the ready-to-use predilute guinea pig anti-insulin (Zymed, San Francisco, CA, USA), biotinylated anti-guinea pig IgG secondary antibody (1:200) and Vectastain ABC-AP with Vector Blue alkaline phosphatase substrate kit III (Vector, Burlingame, CA, USA). Counterstaining was performed using Nuclear Fast Red (Trevigen, Gaithersburg MD, USA).
For fluorescent imaging, beta cells were visualized by insulin staining using guinea pig anti-insulin antibody (Zymed) and Cy3-conjugated anti-guinea pig IgG (1:500, Jackson ImmunoResearch, West Grove, PA, USA). Glucagon and somatostatin were detected using the primary antibodies mouse anti-glucagon (1:100) (Sigma-Aldrich Corp., St. Louis, MO, USA) and goat anti-somatostatin (1:100) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and the secondary antibodies donkey anti-mouse FITC (Jackson ImmunoResearch) and donkey anti-goat Alexa Fluor 488 (Invitrogen), respectively. The Vectashield with 4′,6′-diamidino-2-phenylidole (DAPI) mounting solution (Vector) was used for visualization of nuclei. Primary antibodies for cleaved caspase-9 (1:50), Bcl-xL (1:25) and p-Akt (Ser-473 (1:50) were from Cell Signaling; the secondary biotinylated anti-rabbit IgG (1:200) and Streptoavidin Alexa-Fluor 488 were from Invitrogen. Sections were incubated overnight at 4°C with the primary antibody, followed by 1 h incubation with secondary antibody and 1 h with Alexa Fluor.
Assessment of beta-cell apoptosis and proliferation
Beta-cell apoptosis was assessed with the DeadEnd Fluorometric terminal deoxynucleotidyl transferase-mediated nick end labeling (TUNEL) System (Promega, Madison, WI, USA) according to the manufacturer’s instructions, but including a permeabilization step (10 min in a 1% Triton X-100 PBS solution). Proliferation was assessed by staining for Ki67 (1:200, Abcam). Beta cells were visualized by insulin staining using guinea pig anti-insulin antibody (Zymed) and Cy3-conjugated anti-guinea pig IgG (1:500) (Jackson ImmunoResearch). The Vectashield with DAPI mounting solution (Vector) was used for visualization of nuclei. At least 3 mice per group and 2 different sections per pancreas were used to calculate the average percentage of TUNEL- or Ki67-positive beta cells per islet.
Assessment of pancreatic beta-cell mass
For measurement of whole pancreas insulin content, mice were sacrificed after a 4 h fast and their whole pancreas insulin content (μg/pancreas) was assessed by acid-ethanol extraction and insulin ELISA.
For morphometric analysis of beta-cell mass, pancreata were cleared of fat and lymph nodes and weighed before fixation. Each pancreas was sectioned at 3–5 different positions 300 μm apart, and 10 sections were prepared per position (to ensure that each position contained distinct islets). The sections (1 for each position; i.e., 3–5 per pancreas) were then immunostained for insulin using Vector Blue, and the beta-cell mass was assessed by point-counting morphometry, as described elsewhere (23,24,25). Images were obtained at ×5 with a Spot Insight camera (Diagnostic Instruments, Sterling Heights, MI, USA) and analyzed using the MetaMorph Imaging Software 3.0 (Universal Imaging Corp., West Chester, PA, USA). The beta-cell mass was then determined by first obtaining the fraction of the cross-sectional area of pancreatic tissue positive for insulin staining and then multiplying this by the pancreas weight obtained before fixation. Analysis consisted of 3–4 mice (age and sex-matched) per group.
Measurements of biochemical parameters
All measurements were performed after a 4 h fast. Blood glucose was determined using an OneTouchUltra glucometer (LifeScan, Milpitas, CA, USA), serum insulin levels were detected by ELISA, and serum triglycerides with a commercial kit from Sigma. Serum ketone bodies were measured using the Catachem kit (Catachem, Bridgeport, CT, USA) and leptin, adiponectin, and glucagon levels were assessed using RIA (Linco, St. Charles, MO, USA).
For glucose tolerance tests or insulin tolerance tests, age- and sex-matched mice were deprived of food for 4 h and then given glucose (3 g/kg) or regular insulin (1 IU/kg) by i.p. injection. Blood glucose was measured at 0, 15, 30, 60, 90 and 120 min. Since lean TXNIP-deficient mice became severely hypoglycemic in response to 1 IU/kg of insulin, the dose was reduced for these experiments to 0.55 IU/kg (26).
Statistical analysis
Student’s t tests were used to calculate the significance of a difference between 2 means. For data sets of more than 2 groups and to analyze changes over time, we performed 1-way and 2-way ANOVA calculations.
RESULTS
TXNIP-deficient mice are obese but hypoglycemic
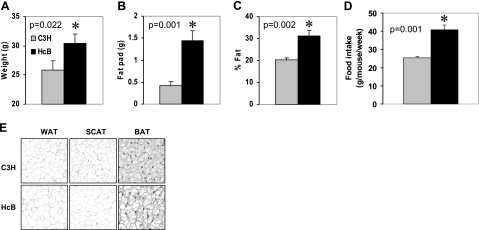
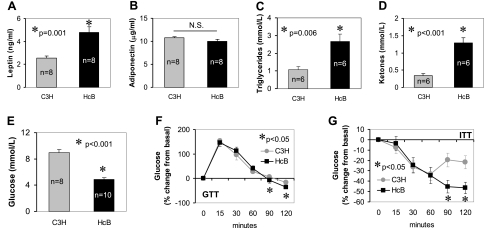
To study the in vivo effects of TXNIP, we used HcB-19 mice, a congenic C3H mouse strain that harbors a naturally occurring point mutation in the TXNIP gene, resulting in dramatically reduced TXNIP mRNA and protein levels (13) and compared them to control C3H mice. Surprisingly, we noticed that HcB-19 mice were more obese, as demonstrated by their significantly elevated whole body and gonadal fat pad weight and their increased percentage of whole body fat mass as assessed by dual energy X-ray absorptiometry (DXA) (Fig. 1A–C). (Although 9-month-old male mice were used for the data shown, findings in females as well as younger mice were comparable, as shown in Supplemental Table S1.) Even though there was no difference in body weight between HcB-19 and C3H mice at weaning, male HcB-19 mice were already significantly heavier at 4 wk of age, whereas in females the difference became significant only at 16 wk. TXNIP-deficient mice were also found to have increased food intake (Fig. 1D), which may at least in part explain their obesity. Histology of white, subcutaneous, and brown adipose tissue only revealed increased lipid accumulation, as would be expected with obesity, but no major structural change in the TXNIP-deficient mice (Fig. 1E). Also consistent with the increased fat mass, TXNIP-deficient mice had significantly elevated serum leptin levels, whereas adiponectin levels remained unchanged (Fig. 2A, B). In addition, TXNIP-deficient mice had elevated serum triglycerides and ketones (Fig. 2C, D) in accordance with previous findings (14, 15). Although both obesity and hyperlipidemia would suggest that these mice might be insulin resistant and potentially diabetic, TXNIP-deficient mice had significantly lower blood glucose levels compared to controls (Fig. 2E). (Whereas we show blood glucose levels of 4-month-old mice after a 4 h fast, very similar results were also obtained in 8-wk-old and 9-month-old mice and random blood glucose levels were also significantly lower in TXNIP-deficient mice; data not shown). In addition, glucose and insulin tolerance tests revealed that HcB-19 mice had no impairment in their glucose tolerance and were insulin sensitive. In fact, at the 90 and 120 min time points, their decrease in blood glucose was significantly more pronounced than in C3H mice.
Figure 1.
Adiposity in TXNIP-deficient mice. A–C) Assessment of body weight (A), gonadal fat pad weight (B), and percentage of whole body fat mass (C) by dual energy X-ray absorptiometry in 9-month-old male TXNIP-deficient HcB-19 and control C3H mice. D) Weekly food intake as measured over a 4-wk period in HcB-19 and C3H mice. Bars represent means ± se; n = 3–6 mice per group. E) Hematoxylin staining of white (WAT), subcutaneous (SCAT), and brown (BAT) adipose tissue sections (×5).
Figure 2.
Metabolic characteristics of TXNIP-deficient mice. Blood was collected after a 4 h fast from TXNIP-deficient Hcb-19 and control C3H mice and analyzed for leptin (A), adiponectin (B), triglycerides (C), ketones (D), and glucose (E). Bars represent means ± se; n = mice/group. Glucose tolerance tests (GTT) (F) or insulin tolerance tests (ITT) (G) were performed by injecting glucose (3 g/kg) or regular insulin (0.55 IU/kg) i.p. and measuring blood glucose at the designated time points (n=6 and 9 mice/group, respectively). Mean ± se percentage changes from basal are shown.
To address the issue of whether these findings may be strain-specific, we also backcrossed HcB-19 mice for 10 generations into a C57BL/6 (B6) background and generated a new congenic line (BHcB). BHcB mice also demonstrated increased adiposity compared to B6 controls (Supplemental Table S2). Biochemically, BHcB mice showed the same characteristics as HcB-19 mice and had elevated serum triglycerides as well as ketones. (In contrast to the HcB-19, however, only BHcB females had elevated ketones, whereas all other parameters showed no gender difference.) In addition, compared to their B6 controls, BHcB mice were also hypoglycemic (Supplemental Fig. S1), indicating that the lower blood glucose levels of TXNIP-deficient mice are not dependent on the genetic background. Despite these lower blood glucose concentrations, serum insulin levels were slightly higher in the TXNIP-deficient mice even though the difference did not reach statistical significance (Supplemental Table S2). At the same time, serum glucagon levels were appropriately elevated in response to the hypoglycemia in the TXNIP-deficient mice, demonstrating that there was no defect in glucagon secretion.
TXNIP-deficient mice have increased pancreatic beta-cell mass
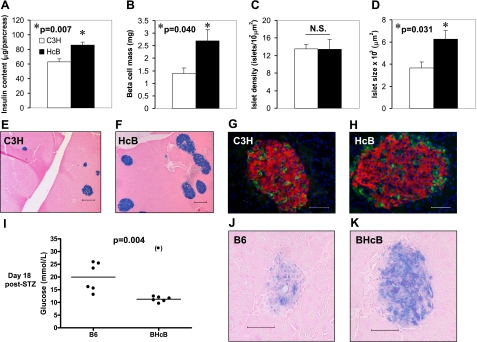
The unusual combination of decreased blood glucose levels and obesity, combined with our previous findings of TXNIP inducing beta-cell apoptosis, raised the possibility that TXNIP deficiency might affect functional beta-cell mass. As a measure of beta-cell mass, we therefore first assessed whole pancreas insulin content in HcB-19 and C3H mice. We found that whole pancreas insulin content was significantly higher in TXNIP-deficient HcB-19 mice as compared to age- and sex-matched control C3H mice (Fig. 3A). To further confirm these findings, we also analyzed beta-cell mass directly by morphometry and again observed a ∼2-fold increase in pancreatic beta-cell mass in the HcB-19 mice (Fig. 3B). Although there was no difference in islet number between HcB-19 and C3H mice (Fig. 3C), these studies further revealed that the islet size was significantly larger in the TXNIP-deficient HcB-19 mice (Fig. 3D–F). At the same time, islet morphology, architecture, and abundance of alpha and delta cells, as assessed by staining for glucagon and somatostatin, were not affected by TXNIP deficiency (Fig. 1G, H).
Figure 3.
Effects of TXNIP deficiency on beta-cell mass and diabetes susceptibility. A–D) Whole pancreas insulin content (A), beta-cell mass (B), islet density (C), and islet size (D) in 9-month-old HcB-19 and control C3H mice. Bars represent means ± se of at least 4 mice/group. E, F) Representative pancreas sections of C3H (E) and HcB-19 mice (F) stained for insulin (blue). G, H) Immunofluorescent images of a C3H (G) and HcB-19 islet (H): red = insulin; green = glucagon/somatostatin; blue = nuclei/DAPI. I–K) B6 and BHcB mice received multiple low-dose STZ injections. Blood glucose levels and representative islets on day 18 after the initial STZ injection are shown; blue = insulin. Scale bars = 200 μm (E, F); 50 μm (G, H, J, K).
TXNIP-deficient mice are protected against STZ-induced diabetes
Based on our earlier data showing that TXNIP induces beta-cell apoptosis (12, 27) and the higher beta-cell mass of TXNIP-deficient mice observed in the present studies, we hypothesized that TXNIP deficiency may protect against diabetes by promoting beta-cell survival and preserving beta-cell mass. To test this hypothesis, we used the STZ-induced diabetes model. (Due to an STZ resistance gene in the C3H mice (19), we used our B6/BHcB mice for these studies.) Of 7 B6 control mice receiving multiple low-dose STZ injections, 5 mice became overtly diabetic (blood glucose>15 mmol/L), 1 mouse showed borderline elevated blood glucose levels of >13 mmol/L, and 1 mouse died 1 day after the first STZ injection. In contrast, only 1 of 7 TXNIP-deficient BHcB mice exposed to the same regimen became diabetic, and the remaining 6 mice maintained normal blood glucose levels for 18 days after the initial STZ injection (Fig. 3I). In addition, histological analysis of pancreata from STZ-treated B6 mice showed severely disrupted or totally absent islets, whereas normal insulin-containing islets were seen in STZ-treated BHcB mice (Fig. 3J, K) suggesting that TXNIP deficiency promotes beta-cell survival even under conditions of STZ-induced diabetes.
TXNIP deficiency rescues BTBRlepob/ob mice from diabetes
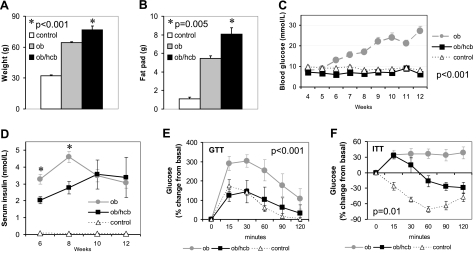
While the obesity of HcB-19 mice is rather mild, and multiple low-dose STZ injections represent a model for type 1 diabetes, we also wanted to assess the potential protective effects of TXNIP deficiency in the context of severe obesity, insulin resistance, and type 2 diabetes. One of the most severe models of obesity and diabetes is the BTBRlepob/ob mouse. On the BTBR genetic background, the leptin mutation leads not only to dramatic obesity and insulin resistance, but in contrast to the B6 background, also leads to severe diabetes starting at ∼6 wk of age and resulting in beta-cell loss and complete decompensation at 8–10 wk (28, 29). To study the effects of TXNIP deficiency in these BTBRlepob/ob mice (BTBR.ob), we therefore generated a double-mutant congenic mouse BTBRlepob/obtxniphcb/hcb (BTBR.ob/hcb) by backcrossing HcB-19 mice for 7 generations into a BTBR background and intercrossing them with BTBRlepob/+ mice. Interestingly, double-mutant mice were even more obese (Fig. 4A, B), but completely protected against diabetes even at 12 wk of age (Fig. 4C). In fact, even though not quite apparent in Fig. 4C, blood glucose levels of obese BTBR.ob/hcb mice were significantly lower than those of lean control double-heterozygous mice (P<0.001). (While again fasting blood glucose levels are shown, BTBR.ob/hcb mice also maintained normal random blood glucose levels, whereas those of BTBR.ob mice rose to ∼30 mmol/L.) Serum insulin levels of obese mice were in general ∼50-fold higher than those of lean controls (P<0.001), providing further indication of their insulin resistance. In addition, insulin levels of BTBR.ob mice were significantly higher at 6 and 8 wk compared to TXNIP-deficient BTBR.ob/hcb littermates (Fig. 4D). However, while insulin levels of BTBR.ob/hcb continued to rise and only leveled off at 10 wk, there was a significant decline in the insulin levels of BTBR.ob mice from 8 to 12 wk (P=0.024) consistent with their diabetes decompensation and worsening hyperglycemia.
Figure 4.
Adiposity and glucose homeostasis in double-mutant congenic BTBR.ob/hcb mice. A, B) Body weight (A) and gonadal fat pad weight (B) as measured in 12-wk-old BTBR leptinob/ob txniphcb/hcb (ob/hcb), BTBR leptinob/ob (ob), and BTBR leptinob/+ txniphcb/+ (control) littermates. Bars represent means ± se; P values refer to ob/hcb vs. ob. Ob or ob/hcb vs. control: P < 0.001. C) Fasting blood glucose levels (means±se). D) Serum insulin levels (means±se); *P = 0.01 vs. ob/hcb. E, F) For glucose tolerance tests (GTT) (E) or insulin tolerance tests (ITT) (F), mice received glucose (3 g/kg) or regular insulin (1 IU/kg) by i.p. injection; blood glucose was measured at the designated time points. Mean ± se percentage changes from basal are shown; P values refer to ob/hcb vs. ob. Ob/hcb vs. control: GTT, nonsignificant; ITT, P = 0.004 (n=3–7 mice/group).
TXNIP deficiency improves insulin sensitivity of BTBRlepob/ob mice
To assess the effects of TXNIP deficiency on glucose tolerance, we performed glucose tolerance tests at 5 wk of age prior to the development of overt diabetes. While BTBR.ob mice were already clearly glucose intolerant, the glucose tolerance of TXNIP-deficient BTBR.ob/hcb mice was not significantly different from lean controls (Fig. 4E). In insulin tolerance tests, BTBR.ob mice also failed to demonstrate a response to insulin, while it was still preserved in BTBR.ob/hcb mice (data not shown). At 11 wk, the insulin tolerance test revealed a paradoxical increase in blood glucose in the obese mice in response to the insulin injection. However, while the blood glucose of BTBR.ob mice remained high throughout the test, it decreased significantly in BTBR.ob/hcb mice (Fig. 4F). Even though the insulin sensitivity of BTBR.ob/hcb mice was still impaired compared to lean controls, it was clearly improved compared to BTBR.ob mice.
TXNIP deficiency promotes pancreatic beta-cell mass and survival in BTBRlepob/ob mice
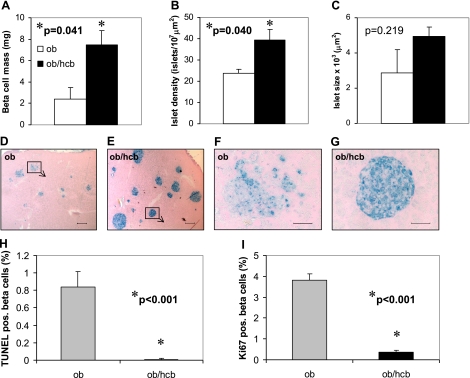
To determine how TXNIP deficiency affects pancreatic beta-cell mass in the face of severe obesity and insulin resistance, we performed morphometry on pancreata of BTBR.ob/hcb mice and their BTBR.ob littermates. Interestingly, we found that beta-cell mass was >3-fold higher in TXNIP-deficient mice (Fig. 5A). This was due to a significantly higher islet number (Fig. 5B) and a trend toward larger islets in the BTBR.ob/hcb mice (Fig. 5C). Immunohistochemistry revealed large, intact, and healthy looking islets in the TXNIP-deficient mice, whereas the majority (∼70%) of islets in BTBR.ob mice were disrupted (Fig. 5D, E). (In fact, this destruction is likely the cause for the large variability in islet size observed in the BTBR.ob mice.)
Figure 5.
Beta-cell mass, apoptosis, and proliferation in double-mutant congenic BTBR.ob/hcb mice. A–C) Pancreatic beta-cell mass (A), islet density (B), and islet size (C) assessed by morphometry in 12-wk-old BTBR.ob and TXNIP-deficient BTBR.ob/hcb mice. Bars represent means ± se; n = 3 mice/group. D–G) Representative pancreas sections of BTBR.ob and BTBR.ob/hcb mice stained for insulin (blue). Scale bars = 200 μm (D, E); 50 μm (F, G; focus on islets marked by boxes in D, E). H) Quantification of beta-cell apoptosis in BTBR.ob and BTBR.ob/hcb mice as assessed by TUNEL. Bars represent means ± se; ∼40 islets and >3000 beta cells analyzed per group. I) Quantification of beta-cell proliferation in BTBR.ob and BTBR.ob/hcb mice as measured by Ki67 staining. Bars represent means ± se; ∼50 islets and >5000 beta cells analyzed per group.
To further study the mechanisms leading to the observed increase in beta-cell mass, we quantified beta-cell apoptosis and proliferation in BTBR.ob/hcb and BTBR.ob pancreata. The results revealed that TXNIP deficiency led to a significant reduction in beta-cell apoptosis even in the context of severe obesity and insulin resistance (Fig. 5H), although no increase in proliferation was found (Fig. 5I). In fact, the number of Ki67 positive beta cells was higher in the BTBR.ob mice as opposed to BTBR.ob/hcb. This finding could be explained by the long-term hyperglycemia present in the BTBR.ob (but not BTBR.ob/hcb) mice that may have acted as a proliferation stimulus.
Beta-cell-specific TXNIP bTKO are hypoglycemic
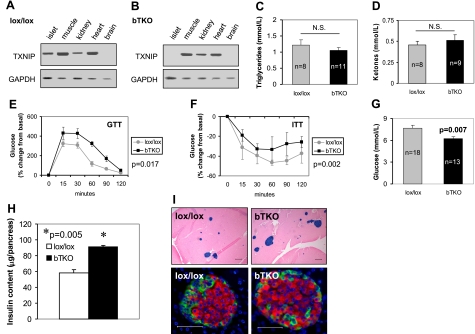
To further establish the role of TXNIP in beta-cell biology, we generated bTKO mice using the Cre-loxP system. We confirmed the txniplox/lox rip-cre+/− bTKO genotype by PCR and the beta-cell-specific deletion of TXNIP by RT-PCR (Fig. 6A, B). (Due to the very low TXNIP protein levels even in wild-type islets, we chose RT-PCR rather than immunoblotting to increase our sensitivity.) While a strong band was visible in islets of control lox/lox mice (Fig. 6A), TXNIP expression was absent in islets of bTKO littermates (Fig. 6B). In other tissues, TXNIP expression showed the same pattern in bTKO and control littermates.
Figure 6.
Characterization of bTKO mice. A, B) TXNIP expression in different tissues of lox/lox control (A) and bTKO mice (B) as measured by RT-PCR. C, D) Serum triglyceride (C) and ketone levels (D) in bTKO and control lox/lox mice after a 4 h fast. E, F) Glucose (E) and insulin tolerance tests (F) in bTKO and lox/lox littermates. After a 4 h fast, glucose (3 g/kg) or insulin (0.55 IU/kg) was administered i.p.; blood glucose was measured at the designated time points; n = 3–8 mice/group. G) Fasting blood glucose concentration in bTKO and lox/lox control mice; n = mice/group. H) Whole pancreas insulin content as a measure of beta-cell mass in 12-wk-old bTKO and control lox/lox mice; n = 4 mice/group. Means ± se are shown. I) Top panels: representative pancreas sections of lox/lox and bTKO mice stained for insulin (blue). Bottom panels: immunofluorescent images of a lox/lox and bTKO islet stained for insulin (red), glucagon/somatostatin (green), and nuclei (blue; DAPI). Scale bars = 200 μm (top); 50 μm (bottom).
Generation of beta-cell-specific knockout mice with RIP-Cre can, in certain cases, lead to simultaneous hypothalamic deletion of the gene of interest. As no TXNIP expression was seen by regular RT-PCR even in the brain of control mice, we also performed quantitative real-time RT-PCR on whole brain and isolated hypothalamus, which demonstrated that TXNIP is expressed in brain and hypothalamus and confirmed that the expression was not altered in bTKO mice (data not shown).
Initial characterization of these bTKO mice revealed that they were viable, fertile, and did not show any gross abnormalities. Furthermore, bTKO mice showed no increased adiposity and their body and gonadal fat pad weight was not significantly different from lox/lox controls (data not shown.) Also, in contrast to HcB-19, the bTKO mice had normal serum levels of triglycerides and ketones (Fig. 6C, D); glucose tolerance and insulin sensitivity were not improved but rather impaired in bTKO mice (Fig. 6E, F). This is consistent with the impairment of glucose tolerance described in other knockout mice generated by the RIP-Cre system (30). Interestingly, though, despite this impairment in insulin sensitivity, bTKO mice, like HcB-19 mice, had significantly reduced fasting blood glucose levels (Fig. 6G), suggesting an increase in functional beta-cell mass and underlining the importance of beta-cell TXNIP expression for glucose homeostasis.
Pancreatic beta-cell mass is increased in bTKO mice
To further assess the effects of beta-cell-specific TXNIP-deficiency on beta-cell mass, we again measured whole pancreas insulin content and found that it was significantly elevated in bTKO mice compared to lox/lox controls (Fig. 6H), consistent with our findings in HcB-19 mice. Again, islet architecture was not affected by lack of beta-cell TXNIP, but more islets of larger size were observed (Fig. 6I). These findings demonstrate that beta-cell-specific TXNIP deletion is sufficient for an induction of beta-cell mass similar to the one observed in HcB-19 mice with generalized TXNIP deficiency, indicating that beta-cell mass is regulated by beta-cell TXNIP expression.
bTKO mice are protected against STZ-induced diabetes
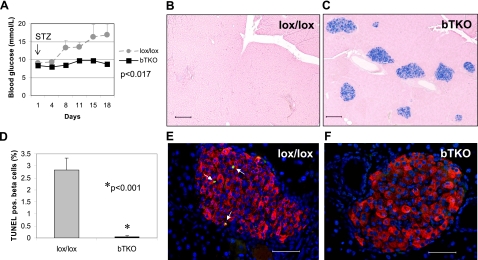
To assess whether bTKO mice might also be protected against diabetes, we again used the model of STZ-induced diabetes and administered multiple low-dose injections of STZ to bTKO mice and lox/lox controls and followed them for 18 days. As soon as 8 days after the initial STZ injection, there was a significant difference in blood glucose levels (P=0.011), and while control lox/lox mice did progress to overt diabetes, none of the bTKO mice became diabetic (Fig. 7A). This protection was due to preservation of functional beta-cell mass in the TXNIP-deficient mice, as analysis of the pancreata revealed that bTKO mice maintained normal islets with insulin producing beta cells, whereas islets in lox/lox mice were destroyed (Fig. 7B, C), consistent with our findings in BHcB and BTBR.ob/hcb mice (Figs. 3 and 5). We therefore sacrificed a subset of mice per group at 8 days (before beta-cell destruction was complete) and performed TUNEL analyses to determine whether this difference was again caused by altered levels of apoptosis. These studies revealed that beta-cell apoptosis was reduced over 50-fold in bTKO mice compared to their controls (Fig. 7D–F), whereas beta-cell proliferation as assessed by Ki67 staining remained unchanged (data not shown). Together, these data demonstrate that lack of beta-cell TXNIP is sufficient for protection against diabetes, and this effect is again conferred by a dramatic inhibition of beta-cell apoptosis.
Figure 7.
Protection against diabetes by beta-cell-specific deletion of TXNIP. A) Control lox/lox and bTKO mice (n=7 mice/group) received multiple low-dose STZ injections; blood glucose levels were assessed immediately prior to the first injection (arrow) and thereafter at the designated time points. At 8 days, 3 mice/group were killed for TUNEL analysis. B, C) Representative pancreas sections of lox/lox (B) and bTKO mice (C) 18 days after initial STZ injection, stained for insulin (blue). D–F) Beta-cell apoptosis in lox/lox and bTKO mice 8 days after initial STZ injection, as assessed by TUNEL. Bars represent means ± se; ∼25 islets and ∼2000 beta cells analyzed per group. White arrows point to TUNEL-positive apoptotic nuclei. Scale bars = 200 μm (B, C); 50 μm (E, F).
Lack of TXNIP induces Akt/Bcl-XL signaling and prevents mitochondrial beta-cell death
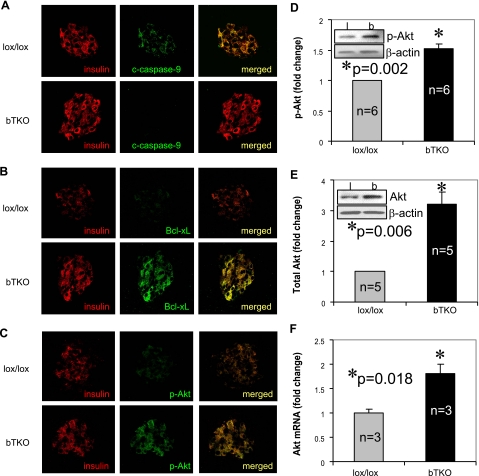
Recently, we discovered that TXNIP overexpression in INS-1 cells induces apoptosis through the intrinsic mitochondrial pathway (22), raising the possibility that the observed inhibition of beta-cell apoptosis in TXNIP deficient mice may be mediated by protection against mitochondrial cell death. To address this question, we analyzed pancreatic sections of STZ-treated bTKO and lox/lox control mice as well as congenic BTBR.ob/hcb and BTBR.ob mice for the expression of cleaved (activated) caspase-9. Caspase-9 is activated by cytochrome c released from the mitochondria, and serves as a classical marker of mitochondria-mediated apoptosis. Interestingly, although diabetic STZ-treated lox/lox and BTBR.ob mice showed clear expression of cleaved caspase-9 in their beta cells, no such expression was observed in the STZ-treated bTKO or BTBR.ob/hcb counterparts (Fig. 8A and Supplemental Fig. S2A). In contrast, antiapoptotic Bcl-xL was abundantly expressed in beta cells of TXNIP-deficient mice, but down-regulated in STZ-treated lox/lox and diabetic BTBR.ob mice (Fig. 8B and Fig. S2B). We also observed a ∼2-fold increase in Bcl-xL by immunoblotting, using isolated bTKO islets as compared to lox/lox controls (data not shown). Aside from interacting and inhibiting proapoptotic Bax, Bcl-xL also prevents apoptosis by directly protecting the outer mitochondrial membrane. It therefore represents another marker of mitochondrial signaling upstream of caspase-9.
Figure 8.
Induction of Akt/Bcl-xL signaling in bTKO mice. Control lox/lox and bTKO mice received multiple low-dose STZ injections; 8 days after the initial injection, pancreata were analyzed by immunohistochemistry for cleaved caspase-9 (A), Bcl-xL (B), and p-Akt (C). Using isolated islets of untreated lox/lox and bTKO mice, the induction of Akt was further quantified by immunoblotting for p-Akt (D) and total Akt (E) and by quantitative real-time RT-PCR for Akt mRNA (F). Bars represent means ± se; n = mice/group. Insert: representative immunoblot (D, E).
Mitochondrial cell death is regulated by multiple factors, but the Akt serine/threonine kinase signaling cascade is one of the major pathways involved and known to modulate Bcl-xL. Since Akt signaling has also been shown to play a critical role in pancreatic beta-cell survival (31,32,33), we investigated whether this pathway may be involved in the beta-cell effects observed in our TXNIP-deficient mice. In fact, the expression of Ser-473 phosphorylated/activated Akt (p-Akt) was clearly higher in STZ-treated bTKO mice as compared to lox/lox controls (Fig. 8C). To eliminate any contribution from differentially altered insulin levels or STZ-induced diabetes, we also analyzed p-Akt levels in isolated islets of untreated, nondiabetic bTKO and lox/lox mice. Immunoblotting revealed again a significant increase in p-Akt (P=0.002) (Fig. 8D) in the bTKO mice, confirming the key role TXNIP deficiency plays in this effect. Similar results were also obtained using isolated Hcb-19 and C3H islets (Fig. S2C). While Akt signaling is generally thought to be regulated primarily by Akt phosphorylation, we surprisingly found that lack of TXNIP also led to significantly increased total Akt protein levels as well as Akt mRNA expression (Fig. 8E, F) suggesting that in islets TXNIP deficiency may have effects on Akt phosphorylation and transcription. Taken together, these findings strongly suggest that TXNIP deficiency mediates its beta-cell protective effects at least in part through Akt/Bcl-xL signaling and prevention of mitochondrial beta-cell apoptosis.
DISCUSSION
The results of this study demonstrate for the first time that TXNIP deficiency, and especially beta-cell-specific deletion of TXNIP, protects against diabetes. Moreover, they reveal that lack of TXNIP induces Akt/Bcl-XL signaling, inhibits pancreatic beta-cell apoptosis, increases endogenous beta-cell mass and, in the case of generalized TXNIP deficiency, improves insulin sensitivity. Of note, TXNIP deficiency protected against both, STZ-induced diabetes as well as obesity-induced diabetes caused by the leptinob mutation.
Interestingly, the rescue from diabetes in the TXNIP-deficient double-mutant BTBR.ob/hcb mice was not associated with any improvement in obesity. In contrast, BTBR.ob/hcb mice were even more obese than their already severely obese BTBR.ob littermates. These findings represent a shift in paradigm, as in general obesity is associated with impaired glucose homeostasis. In contrast, our results establish that under certain circumstances obesity can go hand in hand with improved glucose control. Although counterintuitive, there is precedence for this phenomenon as increased fat mass was recently found to be associated with improved insulin sensitivity in transgenic ob/ob mice overexpressing adiponectin (34). In addition to elevated serum adiponectin levels, these transgenic mice also showed a larger number of small adipocytes in their fat pads and expansion of their subcutaneous but not abdominal adipose tissue. In contrast, in HcB-19 and BTBR.ob/hcb mice adiponectin levels and adipose tissue morphology remained unaffected by TXNIP deficiency (similar adipocyte size in BTBR.ob and BTBR.ob/hcb) and abdominal adiposity did increase, making any contribution from adiponectin or fat redistribution to the observed phenotype very unlikely. Given the dramatic effects of TXNIP deficiency on beta-cell apoptosis and beta-cell mass we observed in the present study, it seems more likely that the increased functional beta-cell mass together with the partially improved insulin sensitivity were able to overcome even severe obesity, promote normal glucose homeostasis, and prevent diabetes. This has major clinical implications, as fighting obesity and maintaining weight loss often remains unsuccessful, predisposing large segments of the population to developing diabetes. Therefore, targets that are effective in maintaining normal glucose control and preventing diabetes in the face of severe obesity are very promising.
The obese, insulin resistant, and diabetic BTBR.ob mice represent one of the most severe models of type 2 diabetes, and our results demonstrate that TXNIP deficiency was able to completely rescue these mice from diabetes. This protection was conferred by improvement of peripheral glucose homeostasis as well as beta-cell survival. The improvement in peripheral glucose homeostasis is consistent with findings from a recent study demonstrating that silencing of TXNIP expression enhanced glucose uptake in adipocytes and human skeletal muscle myocytes, while TXNIP overexpression inhibited glucose uptake (35). Interestingly, the same study showed that TXNIP expression is increased in skeletal muscle of patients with impaired glucose tolerance or diabetes. Together, these findings strongly support the importance of TXNIP in glucose homeostasis and diabetes in humans and raise the possibility that under conditions of impaired glucose tolerance or diabetes, increased TXNIP expression may decrease insulin sensitivity in specific organs such as skeletal muscle, thereby leading to a vicious cycle.
In addition, deletion of TXNIP has recently been shown to lead to impaired hepatic glucose production (36). Since in diabetes hepatic glucose production is often inadequately high, this effect may represent an additional mechanism by which lack of TXNIP improves peripheral glucose homeostasis.
However, even more striking than the observed improvement in insulin sensitivity was the effect of TXNIP deficiency on beta-cell survival. Under normal conditions, lack of TXNIP already led to significantly increased pancreatic beta-cell mass (Hcb-19 mice) and this effect became even more apparent when animals were stressed by multiple low-dose STZ administration or obesity. Our results further revealed that decreased apoptosis was the main mechanism leading to this increase in beta-cell mass. This is consistent with our recent in vitro findings of TXNIP deficiency protecting beta cells against glucose toxicity (22) and our previous observation that TXNIP overexpression induces beta-cell apoptosis (12). Moreover, we found that TXNIP expression is increased in islets of different diabetic and insulin resistant mice, including obese BTBR.ob and non-obese C57BL/6.azip (12, 22), suggesting that this protein is involved in the beta-cell death observed under these conditions.
Importantly, beta-cell-specific deletion of TXNIP in our bTKO mice also led to increased functional beta-cell mass and protection against beta-cell apoptosis and diabetes, further underlining the critical role beta-cell TXNIP plays for beta-cell survival. These protective effects were independent of any improvement in peripheral glucose homeostasis, as bTKO mice even displayed some insulin resistance compared to their lox/lox littermate controls. Furthermore, generalized and beta-cell-specific TXNIP deficiency protected against diabetes induced by multiple low-dose STZ injections, an established model of type 1 diabetes that leads to toxic and autoimmune destruction of the beta-cells. In contrast to the BTBR.ob/hcb mice, where in addition to beta-cell survival, the improved insulin sensitivity may have contributed to the protection against diabetes, these STZ findings strongly support a direct effect of TXNIP deficiency on beta-cell survival. Mice with beta-cell-specific overexpression of thioredoxin, a target inhibited by TXNIP, have also been found to be protected against STZ-induced diabetes (37), suggesting that the observed effects might be mediated by increased thioredoxin activity.
The results of the present study further revealed that TXNIP deficiency induced Akt signaling in islets of both bTKO and HcB-19 mice. This is consistent with recent data demonstrating Akt activation in skeletal muscle and heart in response to TXNIP deletion (38). Increased Akt expression and activity have previously been shown to inhibit apoptosis and promote beta-cell survival and proliferation (31,32,33), suggesting that Akt signaling may contribute to the elevated beta-cell mass observed in HcB-19 and bTKO mice and to their protection against beta-cell apoptosis and diabetes. If so, one would expect that beta-cell-specific overexpression of Akt may mimic these effects. In fact, the phenotype of transgenic mice with beta-cell-specific expression of constitutively active Akt is very similar to the one exhibited by our TXNIP-deficient mice, including mild fasting hypoglycemia, increased beta-cell mass, and resistance to STZ-induced beta-cell apoptosis and diabetes (32).
Moreover, we found that the downstream Akt target, Bcl-xL, was also upregulated, while the expression of cleaved caspase-9 was blunted in TXNIP-deficient mice. Since Bcl-xL is an antiapoptotic factor involved in maintaining mitochondrial membrane integrity, and cleaved caspase-9 is a marker of mitochondrial damage, these findings suggest that lack of TXNIP may inhibit mitochondrial beta-cell death, thereby protecting pancreatic beta-cell mass under conditions of STZ- and obesity-induced diabetes. Further support for this notion and for the role of TXNIP in these mechanisms comes from our recent observation that TXNIP overexpression in INS-1 beta-cells leads to cytochrome c release and increased apoptosis through the intrinsic mitochondrial pathway (22).
While beta-cell loss by apoptosis is a recognized feature of both type 1 and type 2 diabetes approaches to block this process are limited. Currently, the main stem of diabetes treatment is the maintenance of glucose homeostasis as closely to normal as possible in order to avoid the devastating complications of this disease. These treatments include oral hypoglycemics and insulin sensitizers, different insulin preparations administered daily by multiple injections or continuous insulin pumps and, in some patients with type 1 diabetes, whole pancreas or islet transplantation. However, none of these approaches targets the maintenance of endogenous beta-cell mass, even though it has been shown that even a small amount of preserved endogenous insulin secretion has great benefits in terms of clinical outcome (39). Therefore, finding a target that could be used to block beta-cell apoptosis and thereby preserve and enhance endogenous beta-cell mass would represent a major breakthrough. The results of the present study suggest that TXNIP may be such a candidate.
In addition, blocking TXNIP expression may also be beneficial in the setting of islet transplantation. A major impediment to islet transplantation is the large number of islets required in order to confer insulin independence, resulting in the need for several organ donors. This is in sharp contrast to the known small amount of beta-cell mass needed to maintain glucose homeostasis in vivo. It is therefore assumed that a large fraction of transplanted islets undergoes apoptosis and is lost. Reducing TXNIP expression may render islets less susceptible to stresses during the transplantation and in their ectopic location in the liver, where they are exposed to high glucose concentrations. This may prevent beta-cell loss, reduce the number of required islets and thereby improve the outcome of islet transplantation.
In summary, our findings demonstrate for the first time that lack of TXNIP protects against both STZ- and obesity-induced diabetes and thereby establish TXNIP as a powerful novel target for diabetes therapy. They further reveal that TXNIP mediates its effects through induction of Akt/Bcl-XL signaling, inhibition of mitochondrial cell death, and prevention of beta-cell apoptosis. Based on this discovery, future diabetes treatment approaches may attempt to reduce TXNIP expression in order to protect pancreatic beta cells against apoptosis, enhance endogenous beta-cell mass, and improve insulin sensitivity. Given our findings, such approaches are anticipated to be effective in type 1 as well as type 2 diabetes, even if complicated by severe obesity.
Supplementary Material
Acknowledgments
We thank Geetu Saxena, Hyunjoo Cha-Molstad, and Mary Rabaglia for their excellent technical assistance. This work was supported by grants from the American Diabetes Association (ADA; 7–07-CD-22), Juvenile Diabetes Research Foundation (1–2007-790) and National Institutes of Health (NIH; R01DK-078752) to A.S. and from ADA (1–05-IN-06) and NIH (R01HL-51648) to R.D.
References
- Mandrup-Poulsen T. Beta-cell apoptosis: stimuli and signaling. Diabetes. 2001;50:S58–S63. doi: 10.2337/diabetes.50.2007.s58. [DOI] [PubMed] [Google Scholar]
- Mandrup-Poulsen T. Apoptotic signal transduction pathways in diabetes. Biochem Pharmacol. 2003;66:1433–1440. doi: 10.1016/s0006-2952(03)00494-5. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S. Life and death of the pancreatic beta cells. Trends Endocrinol Metab. 2000;11:375–378. doi: 10.1016/s1043-2760(00)00305-2. [DOI] [PubMed] [Google Scholar]
- Pick A, Clark J, Kubstrup C, Levisetti M, Pugh W, Bonner-Weir S, Polonsky K S. Role of apoptosis in failure of beta-cell mass compensation for insulin resistance and beta-cell defects in the male Zucker diabetic fatty rat. Diabetes. 1998;47:358–364. doi: 10.2337/diabetes.47.3.358. [DOI] [PubMed] [Google Scholar]
- Mathis D, Vence L, Benoist C. Beta-cell death during progression to diabetes. Nature. 2001;414:792–798. doi: 10.1038/414792a. [DOI] [PubMed] [Google Scholar]
- Junn E, Han S H, Im J Y, Yang Y, Cho E W, Um H D, Kim D K, Lee K W, Han P L, Rhee S G, Choi I. Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J Immunol. 2000;164:6287–6295. doi: 10.4049/jimmunol.164.12.6287. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Masutani H, Nakamura H, Nishinaka Y, Yodoi J. Redox regulation by thioredoxin and thioredoxin-binding proteins. IUBMB Life. 2001;52:29–33. doi: 10.1080/15216540252774739. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Matsui M, Iwata S, Hirota K, Masutani H, Nakamura H, Takagi Y, Sono H, Gon Y, Yodoi J. Identification of thioredoxin-binding protein-2/vitamin D(3) up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J Biol Chem. 1999;274:21645–21650. doi: 10.1074/jbc.274.31.21645. [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Maehira F, Oshiro M, Asato T, Yanagawa Y, Takei H, Nakashima Y. A possible interaction of thioredoxin with VDUP1 in HeLa cells detected in a yeast two-hybrid system. Biochem Biophys Res Commun. 2000;271:796–800. doi: 10.1006/bbrc.2000.2699. [DOI] [PubMed] [Google Scholar]
- Patwari P, Higgins L J, Chutkow W A, Yoshioka J, Lee R T. The interaction of thioredoxin with TXNIP. Evidence for formation of a mixed disulfide by disulfide exchange. J Biol Chem. 2006;281:21884–21891. doi: 10.1074/jbc.M600427200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev A, Pise-Masison C A, Radonovich M, Hoffmann S C, Hirshberg B, Brady J N, Harlan D M. Oligonucleotide microarray analysis of intact human pancreatic islets: identification of glucose-responsive genes and a highly regulated TGFbeta signaling pathway. Endocrinology. 2002;143:3695–3698. doi: 10.1210/en.2002-220564. [DOI] [PubMed] [Google Scholar]
- Minn A H, Hafele C, Shalev A. Thioredoxin-interacting protein is stimulated by glucose through a carbohydrate response element and induces beta-cell apoptosis. Endocrinology. 2005;146:2397–2405. doi: 10.1210/en.2004-1378. [DOI] [PubMed] [Google Scholar]
- Bodnar J S, Chatterjee A, Castellani L W, Ross D A, Ohmen J, Cavalcoli J, Wu C, Dains K M, Catanese J, Chu M, Sheth S S, Charugundla K, Demant P, West D B, de Jong P, Lusis A J. Positional cloning of the combined hyperlipidemia gene Hyplip1. Nat Genet. 2002;30:110–116. doi: 10.1038/ng811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui T Y, Sheth S S, Diffley J M, Potter D W, Lusis A J, Attie A D, Davis R A. Mice lacking thioredoxin-interacting protein provide evidence linking cellular redox state to appropriate response to nutritional signals. J Biol Chem. 2004;279:24387–24393. doi: 10.1074/jbc.M401280200. [DOI] [PubMed] [Google Scholar]
- Sheth S S, Castellani L W, Chari S, Wagg C, Thipphavong C K, Bodnar J S, Tontonoz P, Attie A D, Lopaschuk G D, Lusis A J. Thioredoxin-interacting protein deficiency disrupts the fasting-feeding metabolic transition. J Lipid Res. 2005;46:123–134. doi: 10.1194/jlr.M400341-JLR200. [DOI] [PubMed] [Google Scholar]
- Cardinal J W, Margison G P, Mynett K J, Yates A P, Cameron D P, Elder R H. Increased susceptibility to streptozotocin-induced beta-cell apoptosis and delayed autoimmune diabetes in alkylpurine-DNA-N-glycosylase-deficient mice. Mol Cell Biol. 2001;21:5605–5613. doi: 10.1128/MCB.21.16.5605-5613.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A, Schott-Ohly P, Dohle C, Gleichmann H. Differential regulation of Th1-type and Th2-type cytokine profiles in pancreatic islets of C57BL/6 and BALB/c mice by multiple low doses of streptozotocin. Immunobiology. 2002;205:35–50. doi: 10.1078/0171-2985-00109. [DOI] [PubMed] [Google Scholar]
- Herold K C, Vezys V, Sun Q, Viktora D, Seung E, Reiner S, Brown D R. Regulation of cytokine production during development of autoimmune diabetes induced with multiple low doses of streptozotocin. J Immunol. 1996;156:3521–3527. [PubMed] [Google Scholar]
- Babaya N, Ikegami H, Fujisawa T, Nojima K, Itoi-Babaya M, Inoue K, Ohno T, Shibata M, Ogihara T. Susceptibility to streptozotocin-induced diabetes is mapped to mouse chromosome 11. Biochem Biophys Res Commun. 2005;328:158–164. doi: 10.1016/j.bbrc.2004.12.149. [DOI] [PubMed] [Google Scholar]
- Minn A H, Lan H, Rabaglia M E, Harlan D M, Peculis B A, Attie A D, Shalev A. Increased insulin translation from an insulin splice-variant overexpressed in diabetes, obesity, and insulin resistance. Mol Endocrinol. 2005;19:794–803. doi: 10.1210/me.2004-0119. [DOI] [PubMed] [Google Scholar]
- Lan H, Rabaglia M E, Stoehr J P, Nadler S T, Schueler K L, Zou F, Yandell B S, Attie A D. Gene expression profiles of nondiabetic and diabetic obese mice suggest a role of hepatic lipogenic capacity in diabetes susceptibility. Diabetes. 2003;52:688–700. doi: 10.2337/diabetes.52.3.688. [DOI] [PubMed] [Google Scholar]
- Chen J, Saxena G, Mungrue I N, Lusis A J, Shalev A. Thioredoxin-interacting protein: a critical link between glucose toxicity and beta cell apoptosis. Diabetes. 2008;57:938–944. doi: 10.2337/db07-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontoglio M, Sreenan S, Roe M, Pugh W, Ostrega D, Doyen A, Pick A J, Baldwin A, Velho G, Froguel P, Levisetti M, Bonner-Weir S, Bell G I, Yaniv M, Polonsky K S. Defective insulin secretion in hepatocyte nuclear factor 1alpha-deficient mice. J Clin Invest. 1998;101:2215–2222. doi: 10.1172/JCI2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner-Weir S. Regulation of pancreatic beta-cell mass in vivo. Recent Prog Horm Res. 1994;49:91–104. doi: 10.1016/b978-0-12-571149-4.50008-8. [DOI] [PubMed] [Google Scholar]
- Bock T, Pakkenberg B, Buschard K. Increased islet volume but unchanged islet number in ob/ob mice. Diabetes. 2003;52:1716–1722. doi: 10.2337/diabetes.52.7.1716. [DOI] [PubMed] [Google Scholar]
- Kwan E P, Xie L, Sheu L, Nolan C J, Prentki M, Betz A, Brose N, Gaisano H Y. Munc13–1 deficiency reduces insulin secretion and causes abnormal glucose tolerance. Diabetes. 2006;55:1421–1429. doi: 10.2337/db05-1263. [DOI] [PubMed] [Google Scholar]
- Minn A H, Pise-Masison C A, Radonovich M, Brady J N, Wang P, Kendziorski C, Shalev A. Gene expression profiling in INS-1 cells overexpressing thioredoxin-interacting protein. Biochem Biophys Res Commun. 2005;336:770–778. doi: 10.1016/j.bbrc.2005.08.161. [DOI] [PubMed] [Google Scholar]
- Stoehr J P, Nadler S T, Schueler K L, Rabaglia M E, Yandell B S, Metz S A, Attie A D. Genetic obesity unmasks nonlinear interactions between murine type 2 diabetes susceptibility loci. Diabetes. 2000;49:1946–1954. doi: 10.2337/diabetes.49.11.1946. [DOI] [PubMed] [Google Scholar]
- Clee S M, Nadler S T, Attie A D. Genetic and genomic studies of the BTBR ob/ob mouse model of type 2 diabetes. Am J Ther. 2005;12:491–498. doi: 10.1097/01.mjt.0000178781.89789.25. [DOI] [PubMed] [Google Scholar]
- Lee J Y, Ristow M, Lin X, White M F, Magnuson M A, Hennighausen L. RIP-Cre revisited, evidence for impairments of pancreatic beta-cell function. J Biol Chem. 2006;281:2649–2653. doi: 10.1074/jbc.M512373200. [DOI] [PubMed] [Google Scholar]
- Dickson L M, Rhodes C J. Pancreatic beta-cell growth and survival in the onset of type 2 diabetes: a role for protein kinase B in the Akt? Am J Physiol Endocrinol Metab. 2004;287:E192–E198. doi: 10.1152/ajpendo.00031.2004. [DOI] [PubMed] [Google Scholar]
- Tuttle R L, Gill N S, Pugh W, Lee J P, Koeberlein B, Furth E E, Polonsky K S, Naji A, Birnbaum M J. Regulation of pancreatic beta-cell growth and survival by the serine/threonine protein kinase Akt1/PKBalpha. Nat Med. 2001;7:1133–1137. doi: 10.1038/nm1001-1133. [DOI] [PubMed] [Google Scholar]
- Fatrai S, Elghazi L, Balcazar N, Cras-Meneur C, Krits I, Kiyokawa H, Bernal-Mizrachi E. Akt induces beta-cell proliferation by regulating cyclin D1, cyclin D2, and p21 levels and cyclin-dependent kinase-4 activity. Diabetes. 2006;55:318–325. doi: 10.2337/diabetes.55.02.06.db05-0757. [DOI] [PubMed] [Google Scholar]
- Kim J Y, van de Wall E, Laplante M, Azzara A, Trujillo M E, Hofmann S M, Schraw T, Durand J L, Li H, Li G, Jelicks L A, Mehler M F, Hui D Y, Deshaies Y, Shulman G I, Schwartz G J, Scherer P E. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh H, Carlsson E, Chutkow W A, Johansson L E, Storgaard H, Poulsen P, Saxena R, Ladd C, Schulze P C, Mazzini M J, Jensen C B, Krook A, Bjornholm M, Tornqvist H, Zierath J R, Ridderstrale M, Altshuler D, Lee R T, Vaag A, Groop L C, Mootha V K. TXNIP Regulates Peripheral Glucose Metabolism in Humans. PLoS Med. 2007;4:e158. doi: 10.1371/journal.pmed.0040158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chutkow W A, Patwari P, Yoshioka J, Lee R T. TXNIP is a critical regulator of hepatic glucose production. J Biol Chem. 2008;283:2397–2406. doi: 10.1074/jbc.M708169200. [DOI] [PubMed] [Google Scholar]
- Hotta M, Tashiro F, Ikegami H, Niwa H, Ogihara T, Yodoi J, Miyazaki J. Pancreatic beta cell-specific expression of thioredoxin, an antioxidative and antiapoptotic protein, prevents autoimmune and streptozotocin-induced diabetes. J Exp Med. 1998;188:1445–1451. doi: 10.1084/jem.188.8.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui S T, Andres A M, Miller A K, Spann N J, Potter D W, Post N M, Chen A Z, Sachithanantham S, Jung D Y, Kim J K, Davis R A. TXNIP balances metabolic and growth signaling via PTEN disulfide reduction. Proc Natl Acad Sci U S A. 2008;105:3921–3926. doi: 10.1073/pnas.0800293105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Diabetes Control and Complications Trial Research Group Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. Ann Intern Med. 1998;128:517–523. doi: 10.7326/0003-4819-128-7-199804010-00001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.