Abstract
Atherosclerosis is now recognized as an inflammatory disease involving the vascular wall. Recent results indicate that acute inflammation does not simply passively resolve as previously assumed but is actively terminated by a homeostatic process that is governed by specific lipid-derived mediators initiated by lipoxygenases. Experiments with animals and humans support a proinflammatory role for the 5-lipoxygenase system. In contrast, results from animal experiments show a range of responses with the 12/15-lipoxygenase pathways in atherosclerosis. To date, the only two clinical epidemiology human studies both support an antiatherogenic role for 12/15-lipoxygenase downstream actions. We tested the hypothesis that atherosclerosis results from a failure in the resolution of local inflammation by analyzing apolipoprotein E-deficient mice with 1) global leukocyte 12/15-lipoxygenase deficiency, 2) normal enzyme expression, or 3) macrophage-specific 12/15-lipoxygenase overexpression. Results from these indicate that 12/15-lipoxygenase expression protects mice against atherosclerosis via its role in the local biosynthesis of lipid mediators, including lipoxin A4, resolvin D1, and protectin D1. These mediators exert potent agonist actions on macrophages and vascular endothelial cells that can control the magnitude of the local inflammatory response. Taken together, these findings suggest that a failure of local endogenous resolution mechanisms may underlie the unremitting inflammation that fuels atherosclerosis.—Merched, A. J., Ko, K., Gotlinger, K. H., Serhan, C. N. Chan, L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators.
Keywords: lipoxygenase, innate immunity
Atherosclerosis is now widely appreciated as an inflammatory disease involving the vascular wall (1, 2). Advanced complex atheromata that set the stage for overt clinical events in atherosclerosis are preceded by less complex lesions. The earliest lesions are designated type I (an increase in intimal macrophages and presence of foam cells) and type II lesions (grossly visible fatty streaks), which are common in infancy and childhood (3, 4) that may progress to advanced atheromata or disappear. The factors that enable some lesions to progress while others regress remain unclear. It is clear, however, that lack of regression is associated with persistent inflammation in the vascular wall (reviewed in refs. 1, 2). In this context, it is noteworthy that inflammation does not simply “burn out” on its own as once thought, as specific tissue-level resolution programs are initiated for inflammation that actively govern the process via the biosynthesis of novel proresolving chemical mediators (5).
Resolution is programmed within the normal inflammatory response itself that enables the body to contain inflammation to minimize tissue and organ damage (6). It involves limiting cellular trafficking as well as nonphlogistic phagocytic removal of apoptotic cells, key parts of the integrated programs that are orchestrated by specialized lipid-derived mediators (6). The biosynthesis of these local acting mediators is regulated by availability of fatty acid precursors such as ω-3 polyunsaturated fatty acids (PUFAs) and the spatial and temporal control of specific lipoxygenase (LO) pathways (5).
12/15-LO (type 1 in humans and its ortholog in mice) and 5-LO (7) are key LO systems in leukocytes and other neighboring cells that can biosynthesize local products that can steer tissues toward chronic inflammation or complete resolution. One class of LO products, the lipoxins (LX, an acronym for lipoxygenase interaction products), was identified as “braking signals” in acute inflammation that can activate the resolution phase of an inflammatory response. Although neutrophil 5-LO initially generates the proinflammatory chemoattractants, such as leukotriene B4, from arachidonate (7), 12/15-LO products interact with 5-LO in a temporally distinct fashion to generate the anti-inflammatory LXs that are involved in resolution. Along with LXs, specialized lipid mediators known as resolvins and protectins were identified (5), which are generated from ω-3 essential PUFAs downstream of the 12/15-LO in human cells. They possess potent dual anti-inflammatory and proresolving actions that mediate resolution of inflammation.
Recent results (8, 9) indicated that leukotrienes and specifically the 5-LO system play critical roles in atheromas and cardiovascular diseases in humans. The possible proresolving actions of 12/15-LO downstream molecules in the inflammation associated with atherosclerosis in humans has been addressed in two recent reports. Wittwer et al. (10) first reported in a case-control study involving 498 Caucasians that heterozygotes for a −292C>T variant in the promoter of the 12/15-LO gene (which was associated with higher enzyme expression in vitro) showed a tendency toward protection against atherosclerosis. In another study, Assimes et al. (11) described a coding SNP (T560M) variant in the 12/15-LO gene that is associated with a 20-fold reduction in enzyme activity. Genotyping of atherosclerotic disease, vascular function, and genetic epidemiology (ADVANCE) and atherosclerosis risk in communities (ARIC) study cohorts (involving 3543 individuals) showed that heterozygote carriers of this near-null 560 M allele had an increased risk of clinical coronary artery disease (adjusted odds ratio, 1.62; P = 0.02). Thus, the only two available case-control studies to date support a protective role of 12/15-LO expression against coronary disease in humans.
Failure in mounting endogenous resolution mechanisms is increasingly being recognized as an important feature in diverse inflammatory disorders such as glomerulonephritis (12, 13), bronchial asthma (14), and inflammatory bowel disease (15). Hence, we hypothesized that atherosclerosis may result, in part, from local nonresolving forms of vascular inflammation. Here, we report that 12/15-LO is pivotal in protecting from atherosclerosis and that several of its products, namely, LXA4, resolvin D1 (RvD1), and protectin D1 (PD1), are potent local-acting proresolving mediators that exhibit robust proresolution actions regulating multiple proinflammatory cytokines produced by macrophages. These 12/15-LO-derived mediators also exert proresolution actions on vascular endothelial cells, which together suggest that a failure to effectively resolve local inflammatory insults initiated in the vessel wall may result in persistent inflammation and atherosclerosis progression.
MATERIALS AND METHODS
Mice
12/15-LO−/− and apoE−/− mice in C57BL/6J background were purchased from Jackson Laboratories (Bar Harbor, ME, USA). 12/15-LO−/− mice were backcrossed onto the C57BL/6J background for ≥11 generations. All mice were maintained under normal chow diet. We measured total cholesterol and triglyceride concentrations in plasma at the end of diet feeding using enzymatic procedures (Sigma, St. Louis, MO, USA). Fast protein liquid chromatography (FPLC) separation of lipoprotein particles was achieved as described previously (16). The cholesterol content of the FPLC fractions was measured by using an enzymatic kit (Sigma).
For the creation of the mouse transgenic line targeting gene expression to macrophages, we used a gene expression system containing a scavenger receptor promoter that had shown highly specific expression approach (17). We subcloned the human 15-LO cDNA into the EcoRV site of the scavenger receptor promoter expression cassette. Male pronuclei of fertilized ova from C57BL/6J mice were microinjected with this construct, and transgenic offspring and subsequent progeny were genotyped by polymerase chain reaction (PCR) of tail DNA. 12/15-LO specific mRNA expression in macrophages was characterized by RNase protection analyses performed on RNA by using the 510 bp fragment of 15-LO as a probe. For bone marrow transplantation, 15-wk-old female apoE−/−/12/15-LO−/− mice were subjected to 10 Gy total body irradiation to eliminate endogenous bone marrow stem cells and most of the bone marrow-derived cells. Bone marrow cells for repopulation were prepared from apoE−/−/12/15-LO−/− or apoE−/− mice, and transplantation was performed as described previously (18). After bone marrow transplantation, mice were kept on a regular chow diet for 15 wk. All procedures were approved by the animal protocol review committee of our institution.
Quantitative morphometry and immunohistochemistry
We performed cross-section analysis of the aortic sinus of mice at 22 wk. En face study of atherosclerotic lesion area was performed on all the other experiments, using complete aortas spanning from the root of the aorta to the iliac bifurcation. We prepared aortas for analysis as described previously (18). Cryostat sections of the aortic root and aorta were fixed in acetone for 10 min and air dried for at least 30 min. After blocking the endogenous peroxidase activity and washing in PBS (pH 7.4), we incubated the sections for 30 min with different monoclonal antibodies individually and exposed the sections to peroxidase or alkaline phosphatase-conjugated secondary antibody for 30 min. Primary antibodies used included rat anti-mouse macrophages Mac3 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), rat anti-mouse VCAM-1 (Santa Cruz), and rat anti-mouse CD18 (Pharmingen, San Diego, CA, USA). Collagen content of lesions was analyzed using trichrome staining.
Gene expression and macrophage functional studies
Macrophages were collected 3 days after activation with intraperitoneal injection of 1 ml of 3% aged Brewer’s thioglycolate. Cells were cultured and RNA was extracted as described in our previous study (19). The expression of specific genes was studied at the mRNA level by quantitative RT-PCR using a multiplex quantitative PCR (qPCR) system (Stratagene, La Jolla, CA, USA; ref. 19) with the following modifications. For normalization of gene-expression analysis, we used 8 mouse housekeeping genes as endogenous controls: cyclophilin A gene (PPIA), B2M (β-microglobulin) GAPDH, 5-aminolevulinate synthase (ALAS1), hydroxymethyl-bilane synthase (HMBS), 18S rRNA (RN18S), eukaryotic elongation factor 1g (EeE1g), and α-actin (ACTα). Moreover, using GeNorm (20), we selected PPIA and EeF1g as the two most stable housekeeping genes within our experimental conditions and used the geometric standardization as described previously (20). Macrophage uptake of apoptotic thymocytes was performed as described previously (19).
Human aortic endothelial cell (HAEC) experiment
HAECs were plated at 2.0 × 105 cells/well in 12-well tissue culture plates coated with 0.1% gelatin and grown in EGM-2 culture medium (Lonza, Walkersville, MD, USA) at 37°C in 5% CO2 for 20–24 h before the experiments were started. At the time of the experiment, the HAEC culture medium was removed and replaced by medium containing 5 U/ml tumor necrosis factor (TNF) -α, with either 100 nM of LXA4, PD1, RvD1, or ethanol (used as vehicle at 0.04%). The plate was incubated at 37°C in 5% CO2 for 2 h. Media were collected and frozen at 80°C until use for bioplex assay, as described below. For RNA extraction, the same cells were washed twice with PBS and lyzed for RNA preparation as indicated previously (19). Data are means ± sd obtained from three different cultures (n=3). We analyzed gene expression by real-time qPCR using the same approach as with macrophages. We identified the most stable housekeeping genes in our experiments e.g., β-actin and GAPDH, which we have used as internal controls for normalization to quantify the expression of different adhesion molecules and chemokines. Primers for human genes were designed as follows: β-actin: (5′-GCCATGTACGTTGCTATCCA-3′ and 5′-CCTCGTAGATGGGCACAGT-3′); GAPDH: (5′-TGGTATCGTGGAAGGACTCA-3′ and 5′-CCAGTAGAGGCAGGGATGAT-3′); ICAM-1: (5′-GGGAGAAGGAGCTGAAACG-3′ and 5′-CACGAGAAATTGGCTCCAT-3′), VCAM-1: (5′-TGTGAATCCATCCACAAAGC-3′ and 5′-GGTGAGAGTTGCATTTCCAG-3′); P-selectin: 5′-CTTCCTCAATGCCAGTCAGA-3′ and 5′- GCCGTTCAGTAGCAAGGAA-3′); MCP-1: (5′-GAATCACCAGCAGCAAGTGT-3′ and 5′-GTCTTCGGAGTTTGGGTTTG-3′).
Cytokine immunoassays
We used the Bioplex Protein Array system (Bio-Rad, Hercules, CA, USA) to measure a panel of 18 mouse and 27 human cytokines. The mouse panel includes CCL5 (regulated on activation, normal T cell expressed and secreted), colony stimulating factor (CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-gamma (IFNγ), interleukin (IL) -1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-10, IL-12P40, IL-12P70, IL-17, keratinocyte-derived chemokine (KC), macrophage inflammatory protein (MIP-1α), and TNF-α. The human panel includes CCL5, eotaxin, FGF basic, G-CSF, GM-CSF, IFNγ, IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL10, IL-12p70, IL-13, IL-15, IL-17, IP10, MCP-1, MIP-1α, MIP-1b, PDGF-bb, TNF-α, and VEGF. These are novel multiplexed, particle-based, flow cytometric assay that uses anti-cytokine monoclonal antibodies linked to microspheres incorporating distinct proportions of two fluorescent dyes. The minimum detectable dose was <10 pg/ml. Four- or 5-parameter logistic regression algorithms were used to quantify the standards. Serum samples were stored at −80οC before the assays to avoid protein degradation.
Mediator lipidomics: lipoxygenase pathway markers
Resident peritoneal macrophages were collected, and 12/15-LO-derived mediators were extracted before and after activation with 2 μM of the divalent cation ionophore A23187 for 20 min at 37°C. We determined plasma Lipoxin A4 and Leukotriene B4 in Sep-Pack-extracted samples (Waters, Milford, MA, USA) using specific enzyme-linked immunosorbent assay as described by the manufacturer (Neogen, Lexington, KY, USA). At least four animals were included in each group. To analyze other mediators by lipidomics, samples were extracted using solid-phased extraction C-18 SPE 500 mg columns (Alltech, Deerfield, IL, USA) as in Lu et al. (21). Criteria for identification of a specific mediator included a minimum of 4 to 6 diagnostic ions and matching retention time with compounds prepared by total organic synthesis (21, 22). Aorta and macrophage samples were analyzed using liquid chromatography-tandem mass spectrometry (LC/MS/MS), which was performed with a LCQ (ThermoFinnigan, San Jose, CA, USA) quadrupole ion trap spectrometer system equipped with an electrospray ionization probe. Samples were suspended in mobile phase immediately before injection into the HPLC, which consisted of a SpectraSYSTEMS P4000 (ThermoFinnigan) quaternary gradient pump, with a Thermo Electron BDS Hypersil C18 (100×2 mm, 5 μm) column (ThermoFisher Scientific, Waltham, MA, USA). The column was eluted at a flow rate of 0.2 ml/min with methanol/water/acetic acid (65:35:0.01, v/v/v) from 0 to 8 min and then a gradient increasing to 100% methanol from 8.01 to 30 min (21). Lipid mediators were quantified using the area beneath the peak obtained for synthetic standards and linear calibration as in Pouliot et al. (23) and Serhan (24).
For macrophage incubations with DHA, an Applied Biosystems (Foster City, CA, USA) 3200 Q-trap LC/MS/MS system equipped with a TurboV ionization source with a turbo ion spray probe was used. After extraction, samples were suspended in mobile phase and injected into the HPLC component, which consisted of an Agilent 1100 series binary gradient pump, with an Aglient Eclipse plus C18 (50×4.6 mm, 1.8 μm) column (Agilent Technologies, Santa Clara, CA, USA). The column was eluted at a flow rate of 0.4 ml/min with methanol/water/acetic acid (60:40:0.01, v/v/v) from 0 to 5 min and then a gradient increasing to 100% methanol from 5.01 to 13 min. Information-dependent acquisition (IDA) used multiple reaction monitoring (MRM) with a dwell time of 25 ms for each lipid mediator of interest, with source parameters set as follows: ion spray voltage, −4200 V; curtain gas, 20 U; ion source gas flow rates 1 and 2 at 50 U each; and temperature at 400°C. IDA criteria were as follows: the most abundant compound was chosen to fragment with no exclusion of former target and above the threshold of 200 counts per second (cps). For enhanced product ion (EPI) collision, energy was set at −25 V, with a spread of −5 V and +5 V, using dynamic fill-time. The mass range was 100–400 m/z, with a scan rate of 4000 atomic mass units (amu)/s and a complete cycle (MRM, IDA, and EPI) of ∼1 s. Lipid mediators prepared by total organic synthesis were used to obtain calibration curves for quantitation as in Hong et al. (22). Quantification and identification of products from macrophage incubations with 17HDHA was carried out postextraction with an Applied Biosystems Q-Star LC/MS/MS system equipped with a TurboIonSpray ionization source as in Schwab et al. (25).
Statistics
For two-group comparison, we used the t test routinely except when value distribution failed the normality test, in which case we used the Mann-Whitney rank sum test as specified (SigmaStat; Jandel Scientific, Corte Madera, CA, USA). One-way ANOVA was used to compare three groups. Values are expressed as means ± se. Values of P ≤ 0.05 were considered to be significant.
RESULTS
Transgenic overexpression of 12/15-LO is atheroprotective
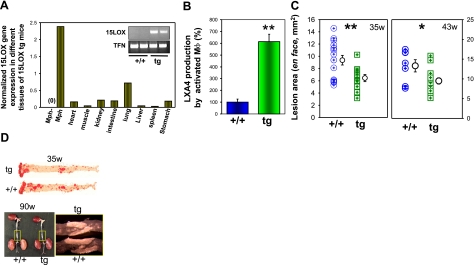
We created new macrophage-specific12/15-LO-overexpressing mice. Two transgenic lines, both characterized by high levels of human 12/15-LO mRNA and increased 15-HETE production by macrophages (data not shown), were bred in C57BL/6 background. The transgenic mice were viable, healthy, and fertile. We bred the 12/15LOtg+/0 mice into C57BL/6 apoE−/− background to establish apoE−/−/12/15-LOtg+/0 mice (designated tg in all figures) and apoE−/− littermates (designated +/+, referring to wild-type 12/15-LO expression, in all figures). Transgene expression was readily detected in peritoneal macrophages, and detectable mRNA levels were also found in tissues rich in macrophages, e.g., lung, but very low in other tissues (Fig. 1A). We then quantified the amount of LXA4 as a downstream product of 12/15-LO in activated peritoneal macrophages in the presence of 100 mM arachidonic acid (Fig. 1B) and observed a 6-fold increase in the amount of LXA4 produced by transgenic macrophages vs. wild-type macrophages (P<0.01). To determine the effect of 12/15-LO overexpression on atherosclerosis, we compared the extent of atherosclerosis in the two groups at 35 and 43 wk and found that apoE−/−/12/15-LOtg+/0 mice exhibited significantly smaller lesions at both time points by morphometric analysis of aortic lesion areas en face as compared with lesions of control apoE–/– mice (Fig. 1C, 6.45±1.95 vs. 9.37±3.03 mm2, P<0.01 at 35 wk and 9.48±0.81 vs. 13.31±1.55 mm2, P<0.05 at 43 wk). Thus, macrophage-specific 12/15-LO overexpression protects against the development of atherosclerosis in apoE−/− mice, an observation that corroborates earlier investigations that uncovered an antiatherogenic effect of macrophage-specific 12/15-LO overexpression in wild-type rabbits as well as in heterozygous Watanabe heritable hyperlipidaemic (WHHL) rabbits as compared with the corresponding nontransgenic littermates (26).
Figure 1.
Macrophage 12/15-LO expression delays atherosclerosis in apoE−/− mice. A) Characterization of the expression of 12/15-LO by real time qPCR in different tissues of transgenic mice showing high-level expression in macrophages and tissues rich in macrophages, e.g., lung. The primers were specific for the human 15-LO transgene transcripts and did not amplify mouse endogenous 12/15LO transcripts [see control macrophages (Mph-) and inset panels]. B) LXA4 production by activated macrophages. Adherent peritoneal macrophages were activated with ionophore A23187 and incubated with 100 mM of arachidonic acid. C) Aortic atherosclerotic lesion area in apoE−/−/15LOtg+/0 (tg) mice compared with apoE−/− littermate controls (+/+) by morphometric analysis of en face lesions performed at 35 and 43 wk on mice fed a regular chow. Bars represent means ± se (n=11, 15 and 7, 14, respectively; all females). D) Top: representative aortas displayed en face; atherosclerotic lesions are stained red by Oil Red O (35 w). Bottom: aortas taken from 90-wk-old tg and +/+ mice. The rectangular area from each aorta is viewed under higher magnification for a close-up view of the abdominal lesions. *P < 0.05; **P < 0.01.
Lack of 12/15-LO expression accelerates atherosclerosis
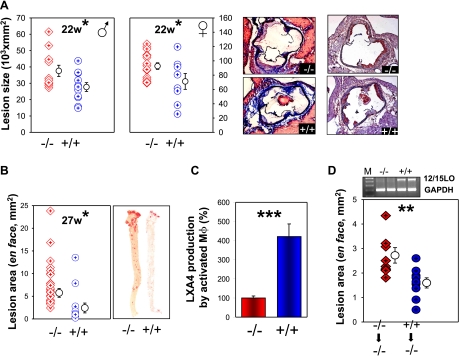
We next examined mice with 12/15-LO inactivation to obtain further evidence for whether the absence of 12/15-LO would produce an effect opposite to that of overexpression. There is considerable variability in the published data on the effect of lack of 12/15-LO in atherosclerosis (27,28,29,30). Thus, it was critical to examine the effect from a lack of leukocyte 12/15-LO in the same laboratory that examined 12/15-LO overexpressing mice at the same time, using essentially identical experimental conditions. As genetic heterogeneity could underlie phenotypic variability (31, 32), we obtained 12/15-LO−/− mice from Jackson Laboratories that had been bred into a homogeneous C57BL/6 background for ≥11 generations. We intercrossed them into C57BL/6 apoE−/− mice and compared atherosclerosis development in 12/15-LO−/−/apoE−/− mice (designated −/−, referring to 12/15-LO inactivation) and apoE−/− littermate controls (designated +/+ in all figures). At 22 wk of age, cross-sectional analysis of aorta roots showed that mice lacking 12/15-LO developed significantly larger atherosclerotic lesions as compared with mice with normal 12/15-LO expression (P<0.05; Fig. 2A). This was the case for both male (37,582±11,406 vs. 27,682±9018 μm2) and female mice (92,650±18,354 vs. 71,204±31,274 μm2). Histochemical staining revealed reduced amounts of collagen in the lesions in 12/15-LO−/− (−/−) mice as compared with those in +/+ mice that produced the enzyme (Fig. 2A, left histological sections). Macrophage immunostaining showed similar percentage compositions of lesions in both groups (Fig. 2A, right histological sections). The leukocyte content of lesions as determined by CD18 immunostaining revealed comparable proportions of positive areas between the two groups of mice (data not shown). We also observed no difference in the expression of the proinflammatory molecule VCAM-1 by immunostaining in the two genotypes (data not shown). To obtain corroborative evidence for an atheroprotective action of 12/15-LO expression, we characterized the extent of the lesion in the two groups of mice at one other time point using a different morphometric method. By en face analysis at 27 wk, we again found significantly larger lesion areas in 12/15-LO−/−/apoE−/− mice as compared with apoE−/− littermate controls (5.75±0.97 vs. 2.42±1.08 mm2; P<0.01; Fig. 2B). Quantification of the amount of LXA4, a downstream product of 12/15-LO, produced by activated macrophages showed an ∼4-fold increased LXA4 production by activated +/+ macrophages as compared with −/− macrophages (P<0.01; Fig. 2C).
Figure 2.
Leukocyte 12/15-LO deficiency diminishes the progression of atheroscleosis. A) Graphs show cross-sectional analysis of atherosclerosis lesion involvement of the aortic sinus in 12/15-LO−/−/apoE−/− (−/−) and apoE−/− (+/+) mice at 22 wk (males: n=11 −/−, 11 +/+; females: n=17 −/−, 8 +/+). Bars represent means ± se. Images show collagen staining (blue) by Masson’s trichrome (left panels) in −/− (top panel) vs. +/+ (bottom panel) and macrophage staining (red-brown) with Mac-3 antibody (right panels). B) En face lesion analysis at 27 wk. C) LXA4 production by ionophore-activated macrophages incubated with 100 mM of arachidonic acid.. D) En face aortic lesion shows −/− mice that received bone marrow transplantation from two groups of donors; 12/15LO−/− apoE−/− (−/−) and 12/15LO+/+ apoE−/− (+/+). 12/15-LO expression (top bands, lanes 3 and 4) in macrophages was confirmed by RT-PCR using GAPDH (bottom bands) as an internal normal control. *P < 0.05; **P < 0.01; ***P < 0.001.
Bone marrow-derived 12/15-LO mediates atheroprotection
In a corollary experiment, to determine whether 12/15-LO produced in bone marrow-derived cells mediated most of the protection, we reinstated leukocyte 12/15-LO expression in 12/15-LO−/−/apoE−/− mice by transplanting bone marrow cells from 12/15-LO−/−/apoE−/− and 12/15-LO+/+/apoE−/− donors to 12/15-LO−/−/apoE−/− mice (Fig. 2D). RT-PCR analysis confirmed that macrophages of recipient mice acquired 12/15-LO expression only after they had received bone marrow cells from 12/15-LO+/+/apoE−/− mice but not from 12/15-LO−/−/apoE−/− donors (Fig. 2D). Morphometric analysis of aortic atherosclerotic lesions in the recipients 15 wk after transplantation revealed that mice receiving 12/15-LO+/+/apoE−/− bone marrow displayed 41% smaller lesion areas (P<0.01) as compared with mice receiving 12/15-LO−/−/apoE−/− bone marrow (1.60±0.62 vs. 2.72±0.95 mm2; Fig. 2D). Thus, in support of conclusions from an overexpression model, use of a genetic knockout demonstrates a protective role of leukocyte 12/15-LO expression against atherosclerosis development in apoE−/− mice. Moreover, we found that most, if not all, of the atheroprotective effects of 12/15-LO expression were mediated by bone marrow-derived cells.
Anti-inflammatory actions of 12/15-LO
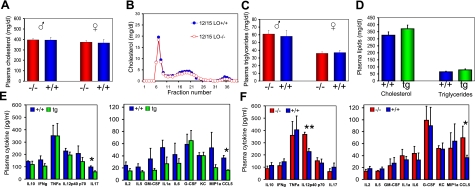
There were no significant differences in total plasma cholesterol or triglycerides in the three genotypes and no differences in the lipoprotein cholesterol profile of the apoE−/−/12/15-LO−/− (−/−) mice or apoE−/−/12/15-LO+/+ (+/+) mice (Fig. 3A–D). To determine the contribution of nonlipoprotein circulating factors on the overall inflammatory balance related to 12/15-LO expression, we measured circulating inflammatory cytokines from the transgenic (overexpression), wild-type, and knockout mice (Fig. 3E, F). Among the 18 cytokines tested (IL-1α, IL-1β, IL-3, IL-4, IL-5, IL-6, IL-8, IL-13, IL-12P70, IL-12P40, IL-17, TNF-α, IFN-γ, MIP-1, KC, G-CSF, GM-CSF, and CCL5), only 2 showed genotype-dependent changes (P<0.05) in the apoE−/−/12/15LOtg+/0 transgenic (tg) mice (Fig. 3E), and 2 in the apoE−/−/12/15-LO−/− (−/−) mice (Fig. 3F) as compared with their respective apoE−/− (+/+) littermate controls. IL-17 and CCL5 were down-regulated in transgenic mice (39 and 55% of control, respectively). IL-12p40 and again CCL5 were up-regulated in the knockout group (162 and 192% of controls, respectively). Interestingly, variation in CCL5 level was consistent in the three mouse genotypes, being inversely related to the gene dosage of 12/15-LO (see later). The level of two other proinflammatory cytokines (IL-12p40 and IL-17) decreased in the presence of higher 12/15-LO expression levels. Taken together, these results strongly suggest that 12/15-LO expression attenuates the inflammatory cytokine load in these animals.
Figure 3.
Plasma lipid and cytokine levels in 12/15-LO–/–/apoE−/− (−/−) ,apoE−/− (+/+) and apoE−/−/12/15-LOtg (tg) mice. A) Plasma cholesterol concentration in −/− and +/+ mice for both males and females. Values are means ± se (n=10 and 10 for males, and n=14 and 8 for females, respectively). B) Plasma lipoprotein profile by FPLC (males). C) Plasma triglycerides in male and female −/− and +/+ mice. D) Plasma cholesterol and triglycerides in +/+ and tg mice (all female). Values are means ± se (n=13 and 8). E) IL-17 and CCL5 were dowregulated in tg mice vs. +/+ controls. F) IL-12p40 and CCL5 were upregulated in −/− mice vs. +/+ controls. We measured 18 cytokines using Bioplex Protein Array system (Bio-Rad) as detailed in Material and Methods. Plasma from 4 to 6 mice was used for the determination of each cytokine. Values are means ± se; *P < 0.05, **P < 0.01.
12/15-LO modulates RvD1, PD1, and LXA4 production
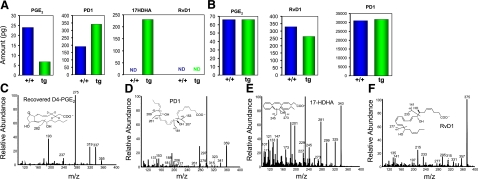
LXs have potent proresolving actions. More recently, the resolvins and protectins were identified as novel families of chemical mediators derived from eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (5, 33, 34). We studied the possible role of 12/15-LO-mediated protection via the metabolic conversion of DHA to downstream bioactive products in macrophages (Fig. 4). To this end, we isolated macrophages from apoE−/−/12/15LOtg+/0 mice and apoE−/− mice and incubated them in the presence of 0.2 μg/ml of DHA during the resolution phase of LPS exposure (Fig. 4A). 12/15-LO overexpression was associated with lower prostaglandin (PG)E2 and increased PD1 and 17HDHA levels. We identified and quantified PGE2, PD1, and 17HDHA by their structures (Fig. 4C–E), using authentic standards, and found a 75% increase in PD1. We did not identify RvD1 in these incubation conditions (Fig. 4A, F). To further assess the role of 17HDHA in the D-series resolvin/protectin pathways, we incubated macrophages from the two genotypes with 17HDHA and quantified PGE2, PD1, and RvD1 production (Fig. 4B). In these, all three mediators were identified and determined to be produced in similar amounts in the two genotypes. These results suggest that 17HpDHA may be rate limiting in the production of both PD1 and RvD1 by macrophages. Therefore, in the presence of DHA, 12/15-LO appears to modulate the production of PD1 and RvD1 through 17HDHA, which appears to be the rate-limiting product.
Figure 4.
Lipid mediators produced by LPS-activated macrophages from wild-type and 12/15-LOtg mice. A) Macrophages from three different mice were incubated with DHA (0.2 μg/ml) after exposure to 500 ng/ml of LPS. Samples were pooled and extracted for lipidomic study. Compared to those from wild-type (+/+) mice, macrophages isolated from 12/15LOtg mice produced markedly reduced PGE2, increased amounts of PD1 and 17HDHA. RvD1 was not identified in these conditions. B) Lipid mediators produced by macrophages incubated with 17HDHA (0.15 μg/ml) for 30 min. C–E) LC/MS/MS spectra of recovered D4-PGE2, PD1, and 17HDHA (respectively) identified in macrophages from 12/15-LOtg mice incubated with DHA. F) LC/MS/MS spectra of RvD1 produced by macrophages from wild-type mice incubated with 17HDHA.
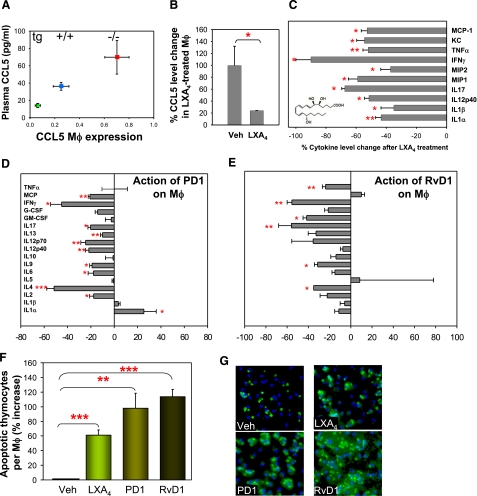
We next studied the actions of the three chemical mediators, LXA4, PD1, and RvD1, on the functions of macrophages and vascular endothelial cells, major cell types involved in atherosclerosis. We were intrigued by the negative correlation of CCL5 expression and the 12/15-LO gene dosage. By qPCR analysis, we found macrophage CCL5 expression (Fig. 5A) to be inversely related to the gene dosage of 12/15LO (0.70±0.22, 0.25±0.16, and 0.06±0.04 for 12/15-LO−/−/apoE−/− mice, 12/15-LO+/+/apoE−/−, and apoE−/−/15LOtg+/0, respectively, P<0.001 per ANOVA). The change in CCL5 transcript level in macrophages was strongly correlated with a similar negative association of CCL5 plasma concentration with 12/15-LO gene dosage in vivo (Fig. 5A). These observations indicate that 12/15-LO expression lowers plasma CCL5 levels partly or wholly by directly or indirectly down-regulating macrophage CCL5 transcription in vivo. To assess whether LXA4 mediates the action of the latter on CCL5 production, we incubated isolated peritoneal macrophages in the presence of synthetic LXA4 and found that LXA4 down-regulated CCL5 production by 76% (Fig. 5B), an observation that is consistent with LXA4 being a key mediator of 12/15-LO gene dosage-dependent down-regulation of CCL5 expression in macrophages.
Figure 5.
LXA4, RvD1, and PD1 action on macrophages. A) Correlation of CCL5 plasma levels with macrophage (Mφ) CCL5 transcript expression in relation to 12/15LO gene dosage. CCL5 transcript levels in peritoneal macrophages by real-time RT-PCR. Each measurement is on RNA isolated from macrophages from 3 to 6 mice from each genotype. Error bars = sem. B) CCL5 secretion by macrophages treated with 50 nM of LXA4 shows down-regulation of CCL5. C) Macrophage global cytokine response to LXA4 treatment shows significant reduction of 10 inflammatory cytokines. D, E) PD1 and RvD1 actions on macrophages. Macrophages were activated with LPS for 4 h, then incubated with PD1 (D) or RvD1 (E). Cytokines produced under these conditions were measured by bioplex technology as described in Materials and Methods. Negative values indicate that the cytokine produced was reduced in the presence of PD1 or RvD1 compared to the amount produced in their absence. PD1 and RvD1 down-regulate LPS-induced production of the vast majority of the cytokines tested. n = 3 to 4. F) Actions of LXA4, PD1, and RvD1 (100 nM) on phagocytic activity of macrophages toward apoptotic thymocytes. Data are from 4 separate incubations; >500 cells counted in each assay. Values are means ± se; *P < 0.05, **P < 0.01, ***P < 0.001 vs. vehicle. G) Representative views of macrophages (nuclei stained by DAPI, blue) that have taken up CellTracker-labeled apoptotic thymocytes (green).
Proresolving actions of LXA4, PD1, and RvD1 on macrophages
LXA4 stops neutrophil chemotaxis, TNF-α-induced superoxide, IL-1β, and MIP-2 production by neutrophils, as well as monokine and metalloprotease production by synovial fibroblasts, and stimulates nonphlogistic macrophage activation (35, 36). Thus, LXA4 exhibits proresolving actions that are not mediated by CCL5. To analyze the anti-inflammatory actions of LXA4 on cytokines other than CCL5, we examined the effect of this chemical mediator on the expression of 10 other proinflammatory cytokines in macrophages (IL-1α, IL-1β, IL-12p40, IL-17, MIP-1, MIP-2, IFN-γ, TNF-α, KC, and MCP-1). Indeed, LXA4 significantly lowered by ∼40–90% the production of all 10 inflammatory cytokines examined (Fig. 5C), many of which have been implicated in the pathogenesis of atherosclerosis (reviewed in refs. 1, 2).
To determine whether PD1 and RvD1, the other LO-downstream chemical mediators, have the capacity to act as bioactive local mediators, or autocoids, that have potent proresolving properties (5), we exposed macrophages to LPS in vitro for 4 h and added 17HDHA, PD1, or RvD1 to the incubation. Under these conditions, PD1 (Fig. 5D) and RvD1 (Fig. 5E) both had an almost uniformly reducing action on all cytokines screened (10/17 showing reduction and 1/17 stimulation for PD1, and 6/17 showing reduction, no stimulation, for RvD1), reflecting an overall inhibitory effect on global inflammatory responses. 17HDHA had a modest effect on only 4 of the 17 cytokines (data not shown). It was next of interest to determine whether the three lipid mediators identified in the present study modulate the phagocytic activity of macrophages toward apoptotic cells, because removal of apoptotic cells in atherosclerosis is an important proresolving function of macrophages (37). To this end, we exposed isolated peritoneal macrophages to LXA4, PD1, or RvD1 and found that they each increased macrophage uptake of apoptotic thymocytes by 60, 100, and 115%, respectively, compared to vehicle-treated cells (Fig. 5F, G). These findings corroborate and extend our recent demonstration that PD1 and another lipid mediator, RvE1, increase macrophage ingestion of apoptotic polymorphonuclear leukocytes in an acute inflammation model (25). Interestingly, 12/15-LO has been shown to modulate actin polymerization and enhance phagocytosis of apoptotic cells (38, 39). Herein we have identified specific downstream products that mediate this action of 12/15-LO. Thus, the protective effect of macrophage expression of 12/15-LO is mediated by facilitated production of PD1 and RvD1 (the latter through up-regulation of the rate-limiting 17HpDHA), which orchestrate proresolving actions via multiple mechanisms. These DHA-dependent lipid mediators complement the action of LXA4, which is also turned on by the transcellular action of 12/15-LO and 5-LO.
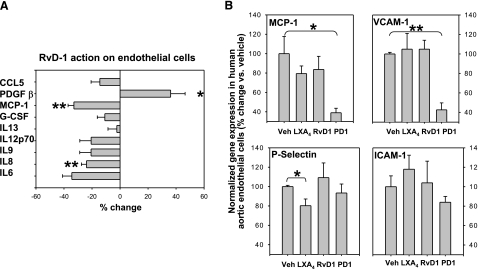
Proresolving actions of LXA4, PD1, and RvD1 on vascular endothelial cells
In addition to macrophages, vascular endothelial cells represent another key cell type involved in atherosclerosis. We incubated TNF-α-activated HAECs in the presence of LXA4, RvD1 or PD1 (100 nM, individually) for 2 h and assayed the culture media for the presence of different chemokines, cytokines, growth factors, and other proteins involved in inflammation. Of 27 such molecules screened, 9 were readily detectable in the media, and on these RvD1 showed the largest effect (Fig. 6A), significantly down-regulating the proinflammatory MCP-1 and IL8 (40,41,42) and up-regulating the anti-inflammatory PDGFβ (43). PD1 reduced MCP-1 production (data not shown), whereas LXA4 did not show any significant effect under these conditions (data not shown). Vascular endothelial cells modulate atherosclerosis development and progression also via the expression of adhesion molecules that regulate the recruitment of leukocytes to the lesion. We examined the expression of adhesion molecules in HAECs by real-time qPCR (Fig. 6B) and found that PD1 down-regulates the expression of VCAM-1 (and MCP-1), whereas LXA4 down-regulates that of P-selectin. Thus, the 12/15-LO downstream products, LXA4, PD1, and RvD1, exhibit potent proresolving actions on macrophages and vascular endothelial cells. Failure to stimulate the production of these lipid mediators would allow inflammation to linger on and atherosclerosis to progress, culminating in clinical adverse events. Natural or synthetic compounds that stimulate the production or mimic the action of these proresolution mediators may be an ideal means to promote resolution and protect against atherosclerosis progression.
Figure 6.
Actions of LXA4, PD1, and RvD1 on the expression of chemokines, cytokines, and adhesion molecules by HAECs. A) RvD1 action on HAECs shows significant inhibition of IL8 and MCP-1 and increase of PDGF β as measured by bioplex technology. B) Effect of incubation with LO products on proteins involved in adhesions of leukocytes, evaluated by real time qPCR. Data are from 3 different cultures. Values are means ± se; *P < 0.05, **P < 0.01 vs. vehicle.
DISCUSSION
Increasing evidence and awareness point to aberrant inflammation as a principal factor in the progression of atherosclerosis in humans (1, 2). The appreciation that the natural course of acute inflammation is resolution (5, 6), together with the present results showing that LXA4, RvD1, and PD1 mediate the antiatherosclerosis actions with macrophage-specific 12/15-LO overexpression, strongly supports the conclusion that atherosclerosis is a nonresolving form of vascular inflammation. The disruption of atherosclerotic plaques in humans via percutaneous transluminal coronary angioplasty leads to rapid appearance (within 10 s) of leukotrienes and lipoxins in the lumen of the vessel (44). The ratio of leukotrienes to lipoxins favors leukotriene formation in the lumen and lesion, which suggests that the absence and/or deficiency of intraluminal LXA4 generation in humans may lead to an inability to counterregulate local vascular inflammation and hence the progression of local insult from acute to chronic, resulting in the disease phenotype, i.e., atherosclerosis.
Recent results (45, 46) also showed that 5-LO and the 5-LO-activating protein (FLAP) is associated with increased incidence of cardiovascular disease. Leukotriene B4 production is upregulated during atherosclerosis in humans, and 5-LO is found associated with atherosclerotic lesions (8, 47, 48). The enhanced production of proinflammatory mediators, such as leukotrienes, underscores the balance toward a proinflammatory milieu around atherosclerotic lesions, as well as the genetic tendency toward acquiring this disease in select human populations. Thus, diminished capacity to generate local LXA4 at the sites of vascular inflammation or local vascular insults may contribute to the nonresolving vascular inflammation and its progression to atherosclerosis. Furthermore, as discussed in the introduction, only two case control studies (10, 11) on human genetic variants of 12/15-LO, a key enzyme involved in LXA4 biosynthesis, have been published, and both supported a protective role for 12/15-LO expression against coronary artery disease.
Indeed, we found that the addition of LXA4 to isolated macrophages leads to a down-regulation of macrophage genes, such as CCL5 (Fig. 5), that are involved in controlling the local balance of inflammatory mediators in vivo. Of interest, proresolving lipid mediators up-regulate CCR5 on the surface of human leukocytes that are directly involved with the binding of local chemokines and cytokines and their accelerated clearance during apoptosis via macrophages (49). In fact, CCL5 is but one of many proinflammatory cytokines normally produced by macrophages that are globally suppressed by exposure to LXA4, consistent with the lipid mediator producing a proresolution milieu locally, putting a brake on atherosclerosis development.
In addition to the lipoxins, resolvins, and protectins are two novel families of locally generated lipid mediators derived from ω-3 fatty acids (EPA and DHA) that display potent anti-inflammatory and proresolving actions in vivo. In murine systems, the 12/15-LO plays a critical role in the biosynthesis of protectins and resolvins of the D series (34, 50). Recent results (51) demonstrate that 12/15-LO-deficient mice have an impaired ability to generate D-series resolvins and protectins and thus are unable to efficiently repair wounds of the corneal epithelia. Alzheimer’s plaque lesions in humans and mouse models also appear to have defective 12/15-LO expression and are unable to generate the DHA-derived protectins (52). In this study, we found that 12/15-LO expression stimulates the production of protectin (PD)1 from 17-HDHA in macrophages during the resolution phase of inflammation. Furthermore, these cells also have the capacity to produce resolvin (Rv)D1. Both PD1 and RvD1 are potent proresolution lipid mediators derived from ω-3 fatty acids downstream of 12/15-LO. Individually, they suppress a large proportion of the proinflammatory cytokines produced by macrophages (Fig. 5D, E). Like LXA4, PD1 and RvD1 also stimulate the phagocytic activity of macrophages toward apoptotic cells (Fig. 5F, E), an anti-inflammatory and proresolution function that is thought to be important in both acute inflammation (25) and in atherosclerosis (37). Complementing their action on macrophages, the three lipid mediators downstream of 12/15-LO also exhibit concerted inhibitory actions on adhesion molecule and chemokine expression by vascular endothelial cells, putting a brake on the recruitment of inflammatory cells, to allow the resolution phase to set in and give the vascular wall a chance to return to normality. Hence, it appears that deficiencies in 12/15-LO, which plays a critical role in initiating the biosynthesis of anti-inflammatory and proresolving lipid mediators including lipoxins, resolvins, and protectins during the course of local acute inflammation, can lead to an inability to efficiently resolve recurring bouts of inflammation and hence chronic inflammatory states that can give rise to progressive atherosclerosis.
In summation (Fig. 7), the present results demonstrate that regulated expression of the 12/15-LO and some of its pathway products derived from arachidonic acid, specifically LXA4, and from ω-3 fatty acids, RvD1 and PD1, can each directly regulate macrophage functions and gene expression of interest in the progression of atherosclerosis. Adding back these specific proresolving lipid mediators down-regulates gene expression held to play critical roles in controlling local inflammation and the development of atherosclerosis. Hence, the present results suggest that appropriate targeted regulation of 12/15-LO and/or the use of stable mimetics of resolvins, protectins, and/or lipoxin may be a new approach in reducing the progression of atherosclerosis. These results also point to the possibility that global deficiencies in 12/15-LO, myeloid lineage and/or specific macrophages in humans can lead to aberrant local vascular inflammation and the atherosclerotic phenotype.
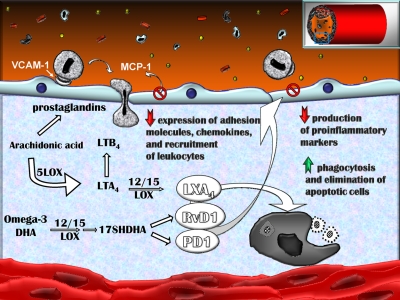
Figure 7.
Model of atherosclerosis as a nonresolving form of vascular inflammation. The essential ω-6 PUFA arachidonic acid (AA) is released from phospholipids in cells by the action of cytosolic phospholipase A2. After specific enzymatic steps, AA is converted into different families of mediators: prostaglandins (PGs) and leukotrienes (LTs), which are mostly proinflammatory molecules, and lipoxins such as LXA4, a stop-inflammation mediator. The essential ω-3 PUFA DHA is converted to two novel mediators, RvD1 and PD1, that promote resolution. We propose a model in which absence of macrophage 12/15LO leads to a deficiency in proresolving end products, RvD1 and PD1, as well as LXA4, locally at the site of the ongoing inflammation, crippling multiple proresolving functions, leaving the proinflammatory milieu unabated, and fueling atherosclerosis progression. LOX, lipoxygenase.
Acknowledgments
We thank Dr. Colin Funk (University of Pennsylvania, Philadelphia, PA, USA) for sending 12/15-LO−/− mice that we used for pilot experiments before purchasing them from Jackson Laboratory for the definitive experiments. This work was supported by American Heart Association grants to A.M. (0465093Y and 0730172N) and grants from the National Institutes of Health (P50 DE016191 to C.N.S., and HL-51586 to L.C.).
References
- Hansson G K. Mechanisms of disease-Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Stary H C, Chandler A B, Glagov S, Guyton J R, Insull W, Rosenfeld M E, Schaffer S A, Schwartz C J, Wagner W D, Wissler R W. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis—a report from the committee on vascular-lesions of the Council on Arteriosclerosis, American-Heart-Association. Circulation. 1994;89:2462–2478. doi: 10.1161/01.cir.89.5.2462. [DOI] [PubMed] [Google Scholar]
- Stary H C. Evolution and progression of atherosclerotic lesions in coronary-arteries of children and young-adults. Arteriosclerosis. 1989;9:I19–I32. [PubMed] [Google Scholar]
- Serhan C N. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Ann Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- Serhan C N, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983;220:568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- Qiu H, Gabrielsen A, Agardh H E, Wan M, Wetterholm A, Wong C H, Hedin U, Swedenborg J, Hansson G K, Samuelsson B, Paulsson-Berne G, Haeggstrom J Z. Expression of 5-lipoxygenase and leukotriene A4 hydrolase in human atherosclerotic lesions correlates with symptoms of plaque instability. Proc Natl Acad Sci U S A. 2006;103:8161–8166. doi: 10.1073/pnas.0602414103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanbroek R, Grabner R, Lotzer K, Hildner M, Urbach A, Ruhling K, Moos M P, Kaiser B, Cohnert T U, Wahlers T, Zieske A, Plenz G, Robenek H, Salbach P, Kuhn H, Radmark O, Samuelsson B, Habenicht A J. Expanding expression of the 5-lipoxygenase pathway within the arterial wall during human atherogenesis. Proc Natl Acad Sci U S A. 2003;100:1238–1243. doi: 10.1073/pnas.242716099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittwer J, Bayer M, Mosandl A, Muntwyler J, Hersberger M. The c.-292C>T promoter polymorphism increases reticulocyte-type 15-lipoxygenase-1 activity and could be atheroprotective. Clin Chem Lab Med. 2007;45:487–492. doi: 10.1515/CCLM.2007.103. [DOI] [PubMed] [Google Scholar]
- Assimes T L, Knowles J W, Priest J R, Basu A, Borchert A, Volcik K A, Grove M L, Tabor H K, Southwick A, Tabibiazar R, Sidney S, Boerwinkle E, Go A S, Iribarren C, Hlatky M A, Fortmann S P, Myers R M, Kuhn H, Risch N, Quertermous T. A near null variant of 12/15-LOX encoded by a novel SNP in ALOX15 and the risk of coronary artery disease. Atherosclerosis. 2007;198:136–144. doi: 10.1016/j.atherosclerosis.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield J S, Hong S, Vaidya V S, Lu Y, Fredman G, Serhan C N, Bonventre J V. Resolvin D Series and protectin D1 mitigate acute kidney injury. J Immunol. 2006;177:5902–5911. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- Munger K A, Montero A, Fukunaga M, Uda S, Yura T, Imai E, Kaneda Y, Valdivielso J M, Badr K F. Transfection of rat kidney with human 15-lipoxygenase suppresses inflammation and preserves function in experimental glomerulonephritis. Proc Natl Acad Sci U S A. 1999;96:13375–13380. doi: 10.1073/pnas.96.23.13375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B D, DeSanctis G T, Devchand P R, Kim E, Ackerman K, Schmidt B A, Szczeklik W, Drazen J M, Serhan C N. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A(4) Nat Med. 2002;8:1018–1023. doi: 10.1038/nm748. [DOI] [PubMed] [Google Scholar]
- Goh J, Godson C, Brady H R, MacMathuna P. Lipoxins: pro-resolution lipid mediators in intestinal inflammation. Gastroenterology. 2003;124:1043–1054. doi: 10.1053/gast.2003.50154. [DOI] [PubMed] [Google Scholar]
- Oka K, Pastore L, Kim I H, Merched A, Nomura S, Lee H J, Merched-Sauvage M, Arden-Riley C, Lee B, Finegold M, Beaudet A, Chan L. Long-term stable correction of low-density lipoprotein receptor-deficient mice with a helper-dependent adenoviral vector expressing the very low-density lipoprotein receptor. Circulation. 2001;103:1274–1281. doi: 10.1161/01.cir.103.9.1274. [DOI] [PubMed] [Google Scholar]
- Horvai A, Palinski W, Wu H, Moulton K S, Kalla K, Glass C K. Scavenger receptor A gene regulatory elements target gene expression to macrophages and to foam cells of atherosclerotic lesions. Proc Natl Acad Sci U S A. 1995;92:5391–595. doi: 10.1073/pnas.92.12.5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merched A J, Williams E, Chan L. Macrophage-specific p53 expression plays a crucial role in atherosclerosis development and plaque remodeling. Arterioscler Thromb Vasc Biol. 2003;23:1608–1614. doi: 10.1161/01.ATV.0000084825.88022.53. [DOI] [PubMed] [Google Scholar]
- Merched A J, Chan L. Absence of p21Waf1/Cip1/Sdi1 modulates macrophage differentiation and inflammatory response and protects against atherosclerosis. Circulation. 2004;110:3830–3841. doi: 10.1161/01.CIR.0000148681.01282.89. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:research 0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Hong S, Gotlinger K, Serhan C N. Lipid mediator informatics and proteomics in inflammation resolution. ScientificWorldJournal. 2006;6:589–614. doi: 10.1100/tsw.2006.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Lu Y, Yang R, Gotlinger K H, Petasis N A, Serhan C N. Resolvin D1, protectin D1, and related docosahexaenoic acid-derived products: Analysis via electrospray/low energy tandem mass spectrometry based on spectra and fragmentation mechanisms. J Am Soc Mass Spectrom. 2007;18:128–144. doi: 10.1016/j.jasms.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouliot M, Clish C B, Petasis N A, Van Dyke T E, Serhan C N. Lipoxin A(4) analogues inhibit leukocyte recruitment to Porphyromonas gingivalis: a role for cyclooxygenase-2 and lipoxins in periodontal disease. Biochemistry. 2000;39:4761–4768. doi: 10.1021/bi992551b. [DOI] [PubMed] [Google Scholar]
- Serhan C N. High-performance liquid chromatography separation and determination of lipoxins. Methods Enzymol. 1990;187:167–175. doi: 10.1016/0076-6879(90)87022-u. [DOI] [PubMed] [Google Scholar]
- Schwab J M, Chiang N, Arita M, Serhan C N. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Herderick E, Cornhill J F, Zsigmond E, Kim H S, Kuhn H, Guevara N V, Chan L. Macrophage-mediated 15-lipoxygenase expression protects against atherosclerosis development. J Clin Invest. 1996;98:2201–2208. doi: 10.1172/JCI119029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyrus T, Pratico D, Zhao L, Witztum J L, Rader D J, Rokach J, FitzGerald G A, Funk C D. Absence of 12/15-lipoxygenase expression decreases lipid peroxidation and atherogenesis in apolipoprotein e-deficient mice. Circulation. 2001;103:2277–2282. doi: 10.1161/01.cir.103.18.2277. [DOI] [PubMed] [Google Scholar]
- George J, Afek A, Shaish A, Levkovitz H, Bloom N, Cyrus T, Zhao L, Funk C D, Sigal E, Harats D. 12/15-Lipoxygenase gene disruption attenuates atherogenesis in LDL receptor-deficient mice. Circulation. 2001;104:1646–1650. doi: 10.1161/hc3901.095772. [DOI] [PubMed] [Google Scholar]
- Huo Y, Zhao L, Hyman M C, Shashkin P, Harry B L, Burcin T, Forlow S B, Stark M A, Smith D F, Clarke S, Srinivasan S, Hedrick C C, Pratico D, Witztum J L, Nadler J L, Funk C D, Ley K. Critical role of macrophage 12/15-lipoxygenase for atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;110:2024–2031. doi: 10.1161/01.CIR.0000143628.37680.F6. [DOI] [PubMed] [Google Scholar]
- Reilly K B, Srinivasan S, Hatley M E, Patricia M K, Lannigan J, Bolick D T, Vandenhoff G, Pei H, Natarajan R, Nadler J L, Hedrick C C. 12/15-Lipoxygenase activity mediates inflammatory monocyte/endothelial interactions and atherosclerosis in vivo. J Biol Chem. 2004;279:9440–9450. doi: 10.1074/jbc.M303857200. [DOI] [PubMed] [Google Scholar]
- Moore K J, Kunjathoor V V, Koehn S L, Manning J J, Tseng A A, Silver J M, McKee M, Freeman M W. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J Clin Invest. 2005;115:2192–2201. doi: 10.1172/JCI24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon C A, Blachowicz L, Lukens J, Nissenbaum M, Getz G S. Genetic background selectively influences innominate artery atherosclerosis: immune system deficiency as a probe. Arterioscler Thromb Vasc Biol. 2003;23:1449–1454. doi: 10.1161/01.ATV.0000079793.58054.2E. [DOI] [PubMed] [Google Scholar]
- Serhan C N, Clish C B, Brannon J, Colgan S P, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C N, Hong S, Gronert K, Colgan S P, Devchand P R, Mirick G, Moussignac R L. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachicha M, Pouliot M, Petasis N A, Serhan C N. Lipoxin (LX)A4 and aspirin-triggered 15-epi-LXA4 inhibit tumor necrosis factor 1alpha-initiated neutrophil responses and trafficking: regulators of a cytokine-chemokine axis. J Exp Med. 1999;189:1923–1930. doi: 10.1084/jem.189.12.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodin-Semrl S, Taddeo B, Tseng D, Varga J, Fiore S. Lipoxin A4 inhibits IL-1 beta-induced IL-6, IL-8, and matrix metalloproteinase-3 production in human synovial fibroblasts and enhances synthesis of tissue inhibitors of metalloproteinases. J Immunol. 2000;164:2660–2666. doi: 10.4049/jimmunol.164.5.2660. [DOI] [PubMed] [Google Scholar]
- Fadok V A, Chimini G. The phagocytosis of apoptotic cells. Semin Immunol. 2001;13:365–372. doi: 10.1006/smim.2001.0333. [DOI] [PubMed] [Google Scholar]
- Miller Y I, Chang M K, Funk C D, Feramisco J R, Witztum J L. 12/15-lipoxygenase translocation enhances site-specific actin polymerization in macrophages phagocytosing apoptotic cells. J Biol Chem. 2001;276:19431–19439. doi: 10.1074/jbc.M011276200. [DOI] [PubMed] [Google Scholar]
- Miller Y I, Worrall D S, Funk C D, Feramisco J R, Witztum J L. Actin polymerization in macrophages in response to oxidized LDL and apoptotic cells: role of 12/15-lipoxygenase and phosphoinositide 3-kinase. Mol Biol Cell. 2003;14:4196–4206. doi: 10.1091/mbc.E03-02-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisvert W A, Santiago R, Curtiss L K, Terkeltaub R A. A leukocyte homologue of the IL-8 receptor CXCR-2 mediates the accumulation of macrophages in atherosclerotic lesions of LDL receptor-deficient mice. J Clin Invest. 1998;101:353–363. doi: 10.1172/JCI1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boring L, Gosling J, Cleary M, Charo I F. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- Gosling J, Slaymaker S, Gu L, Tseng S, Zlot C H, Young S G, Rollins B J, Charo I F. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Invest. 1999;103:773–778. doi: 10.1172/JCI5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Kozaki K, Farr A G, Martin P J, Lindahl P, Betsholtz C, Raines E W. The absence of platelet-derived growth factor-B in circulating cells promotes immune and inflammatory responses in atherosclerosis-prone ApoE−/− mice. Am J Pathol. 2005;167:901–912. doi: 10.1016/S0002-9440(10)62061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezinski D A, Nesto R W, Serhan C N. Angioplasty triggers intracoronary leukotrienes and lipoxin A4. Impact of aspirin therapy. Circulation. 1992;86:56–63. doi: 10.1161/01.cir.86.1.56. [DOI] [PubMed] [Google Scholar]
- Helgadottir A, Manolescu A, Thorleifsson G, Gretarsdottir S, Jonsdottir H, Thorsteinsdottir U, Samani N J, Gudmundsson G, Grant S F, Thorgeirsson G, Sveinbjornsdottir S, Valdimarsson E M, Matthiasson S E, Johannsson H, Gudmundsdottir O, Gurney M E, Sainz J, Thorhallsdottir M, Andresdottir M, Frigge M L, Topol E J, Kong A, Gudnason V, Hakonarson H, Gulcher J R, Stefansson K. The gene encoding 5-lipoxygenase activating protein confers risk of myocardial infarction and stroke. Nat Genet. 2004;36:233–239. doi: 10.1038/ng1311. [DOI] [PubMed] [Google Scholar]
- Helgadottir A, Gretarsdottir S, St Clair D, Manolescu A, Cheung J, Thorleifsson G, Pasdar A, Grant S F, Whalley L J, Hakonarson H, Thorsteinsdottir U, Kong A, Gulcher J, Stefansson K, MacLeod M J. Association between the gene encoding 5-lipoxygenase-activating protein and stroke replicated in a Scottish population. Am J Hum Genet. 2005;76:505–509. doi: 10.1086/428066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer J H, Allayee H, Dwyer K M, Fan J, Wu H, Mar R, Lusis A J, Mehrabian M. Arachidonate 5-lipoxygenase promoter genotype, dietary arachidonic acid, and atherosclerosis. N Engl J Med. 2004;350:29–37. doi: 10.1056/NEJMoa025079. [DOI] [PubMed] [Google Scholar]
- Mehrabian M, Allayee H, Wong J, Shi W, Wang X P, Shaposhnik Z, Funk C D, Lusis A J. Identification of 5-lipoxygenase as a major gene contributing to atherosclerosis susceptibility in mice. Circ Res. 2002;91:120–126. doi: 10.1161/01.res.0000028008.99774.7f. [DOI] [PubMed] [Google Scholar]
- Ariel A, Fredman G, Sun Y P, Kantarci A, Van Dyke T E, Luster A D, Serhan C N. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nat Immunol. 2006;7:1209–1216. doi: 10.1038/ni1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C N, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, Yang R, Colgan S P, Petasis N A. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol. 2006;176:1848–1859. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- Gronert K, Maheshwari N, Khan N, Hassan I R, Dunn M, Laniado S M. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J Biol Chem. 2005;280:15267–15278. doi: 10.1074/jbc.M410638200. [DOI] [PubMed] [Google Scholar]
- Lukiw W J, Cui J G, Marcheselli V L, Bodker M, Botkjaer A, Gotlinger K, Serhan C N, Bazan N G. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]