Abstract
Despite extensive literature on vascular endothelial growth factor (VEGF) expression and regulation by steroid hormones, the lack of clear understanding of the mechanisms of angiogenesis in the endometrium is a major limitation for use of antiangiogenic therapy targeting endometrial vessels. In the current work, we used the rhesus macaque as a primate model and the decidualized mouse uterus as a murine model to examine angiogenesis during endometrial breakdown and regeneration. We found that blockade of VEGF action with VEGF Trap, a potent VEGF blocker, completely inhibited neovascularization during endometrial regeneration in both models but had no marked effect on preexisting or newly formed vessels, suggesting that VEGF is essential for neoangiogenesis but not survival of mature vessels in this vascular bed. Blockade of VEGF also blocked reepithelialization in both the postmenstrual endometrium and the mouse uterus after decidual breakdown, evidence that VEGF has pleiotropic effects in the endometrium. In vitro studies with a scratch wound assay showed that the migration of luminal epithelial cells during repair involved signaling through VEGF receptor 2–neuropilin 1 (VEGFR2-NP1) receptors on endometrial stromal cells. The leading front of tissue growth during endometrial repair was strongly hypoxic, and this hypoxia was the local stimulus for VEGF expression and angiogenesis in this tissue. In summary, we provide novel experimental data indicating that VEGF is essential for endometrial neoangiogenesis during postmenstrual/postpartum repair.—Fan, X., Krieg, S., Kuo, C. J., Wiegand, S. J., Rabinovitch, M., Druzin, M. L., Brenner, R. M., Giudice, L. C., Nayak, N. R. VEGF blockade inhibits angiogenesis and reepithelialization of endometrium.
Keywords: VEGF Trap, luminal epithelium, rhesus macaque, mouse, hypoxia
Adult primate endometrium is a dynamic tissue undergoing regular cycles of menstruation, menstrual repair, proliferation, and secretory differentiation, all orchestrated by ovarian steroid hormones (1, 2). After menstruation, the endometrium regenerates from the remaining deeper zones and reepithelialization of the denuded surface occurs by outgrowth and spreading of the epithelium from the mouths of the residual glands (3). Angiogenesis, which occurs during the cycle, is presumed to be crucial to the repair and regeneration of the endometrium, but experimental evidence for this role is lacking.
During the past 2 decades, enormous progress has been made in understanding the mechanism of angiogenesis in different pathophysiological conditions, and antiangiogenic therapy is emerging as a major treatment modality of various cancers (4,5,6). Vascular endothelial growth factor (VEGF) is known to be required for normal as well as pathological angiogenesis in many tissues (5, 6). The actions of all 5 splice variants of VEGF are primarily mediated by 2 transmembrane tyrosine kinase receptors, VEGF receptor 1 (VEGFR1 or Flt1) and VEGF receptor 2 (VEGFR2 or Flk1) (4, 6). Although VEGFR1 binds to VEGF with high affinity, most of the biological effects of VEGF are mediated by VEGFR2. In addition, there are isoform-specific VEGF receptors expressed in both endothelial and nonendothelial cells, called neuropilins (NP1 and NP2) (7, 8). Several gene targeting studies in the mouse implicate neuropilins as important modulators of VEGF functions (9). For example, NP1 enhances the binding of VEGF165 to VEGFR2 and facilitates VEGF165-mediated functions (8). All VEGF isoforms and their receptors, including neuropilins, are expressed in the endometrium, and VEGF165 is the most abundant isoform (10, 11).
We recently found, in the rhesus macaque, heightened expression of VEGF in the newly formed surface epithelium and in the regenerating stroma during the postmenstrual repair and early proliferative phases (12). These increases correlated strongly with an increase in vascular proliferation in the upper endometrial zones. In women, up-regulation of VEGF and increases in vascular proliferation occur in the endometrium during the early to midproliferative phases (13,14,15). However, other angiogenic factors are also expressed in the endometrium and have been equally implicated in endometrial angiogenesis (2, 14, 16). Therefore, the relative contribution of each angiogenic factor in the endometrial cycle remains to be determined. In this work, we focused on the role of VEGF.
The development of highly potent VEGF inhibitors provides an opportunity to clarify these issues. VEGF-blocking antibodies, VEGF-receptor antibodies, soluble decoy receptors, and small-molecule inhibitors of VEGF-receptor tyrosine kinase activity are available to inhibit VEGF action in vivo (4, 6). Recent clinical trials with a humanized VEGF monoclonal antibody have shown promising results in human patients with various types of cancers and other angiogenesis-related pathological conditions (6). Soluble VEGF decoy receptors are also effective inhibitors of VEGF signaling but have short in vivo half lives. VEGF Trap is a receptor-based fusion protein that exhibits greater bioavailability and improved pharmcokinetic properties relative to soluble VEGF receptors, as well as greater affinity for VEGF than native VEGF receptors or antibodies (17). This high-affinity fusion protein has been shown to suppress angiogenesis in many types of tumors in experimental animals, resulting in marked reduction of tumor growth. In the studies reported below, we used VEGF Trap to inhibit binding of VEGF to its receptors. These studies were conducted in various biological/physiological systems, including the macaque endometrium, the decidualized mouse uterus, and cultured epithelial, stromal, and endothelial cells.
MATERIALS AND METHODS
VEGF Trap
VEGF Trap was supplied by Regeneron Pharmaceuticals (Tarrytown, NY, USA) (17). It is a soluble inhibitor of VEGF consisting of the second immunoglobulin (Ig) domain of human VEGFR1 and the third Ig domain of human VEGFR2 fused to the Fc fragment of human IgG1. The Fc fragment of human IgG1 was used in the control experiments and was also supplied by Regeneron Pharmaceuticals.
Hypoxyprobe™-1
Hypoxyprobe compound was procured from NPI, Inc. (Burlington, MA, USA) and used as hypoxia marker in this study (18). It is a hydrochloride salt of pimonidazole that is activated and forms protein adducts in hypoxic mammalian cells at oxygen partial pressures less than 10 mm Hg. The adduct formation (hypoxyprobe) in hypoxic cells can be detected using specific antibodies against pimonidazole adducts supplied by NPI, Inc. Hypoxyprobe is highly water soluble, chemically stable, and is taken up very efficiently by tissues in vivo.
Macaque experiments
All macaque experiments were conducted at the Oregon National Primate Research Center under approved protocols by the Primate Center Institutional Animal Care and Use Committee. Ovariectomized macaques were artificially cycled by sequential treatment of 17β-estradiol (E2; Sigma-Aldrich Corp., St. Louis, MO, USA) and progesterone (P; Sigma) as described previously (11, 12). Briefly, all macaques received subcutaneous (s.c.) implants of 3 cm Silastic capsules of E2. After 14 days, a 6 cm Silastic capsule of P was implanted s.c., and both implants remained in place for 14 days. Then at the end of the artificial cycle, the P implants were removed to induce menstruation and the E2 implants were left in place.
Experimental design
We used 6 macaques (age 6–10 yr), 3 controls and 3 VEGF Trap-treated, to study the effects of VEGF blockade on endometrial vascular development during the postmenstrual period. VEGF Trap was administered at a dose of 12.5 mg/kg i. v. on days 2, 4, and 6 after P withdrawal. The endometrium was collected on day 8. The control animals were treated similarly with a vehicle that contained only the Fc fragment of the VEGF Trap compound. Endometrial tissue samples were collected by hysterectomy and full-thickness uterine cross sections were prepared from each quarter for histology, immunohistochemistry (IHC), and in situ hybridization (ISH) studies.
Mouse experiments
Murine model of endometrial breakdown and regeneration
All mouse experiments were performed in the Research Animal Facility at Stanford University under approved protocols from the Administrative Panel on Laboratory Animal Care. Briefly, female mice (CD-1; Charles River, Wilmington, MA, USA) were mated with vasectomized males of the same strain to induce pseudopregnancy, then 20 μl sesame oil (Sigma) was injected into each uterine lumen on day 4 of pseudoprenancy (day 1: vaginal plug) to induce decidualization (Supplemental Fig. 1). On day 6 (2 days after oil injection), bilateral ovariectomy was performed to induce endometrial breakdown after visual confirmation of decidualization of both the uterine horns. All animals received daily low-dose s.c. injections of 10 ng E2 to prevent atrophy of the uterus following ovariectomy.
Experiment A
At the time of ovariectomy (day 0), one set of animals was injected (i.v.) with the vehicle (control) and another with a single injection of VEGF Trap (12.5 mg/kg) (Supplemental Fig. 1, Experiment A). Uteri from both the control and VEGF Trap treated animals were collected at 0 (n=3), 1 (n=3), 2 (n=6), 3 (n=3), 4 (n=3), and 5 (n=6) days after ovariectomy. One hour before tissue collection, a single dose of Hypoxyprobe-1 compound (60 mg/kg; NPI, Inc.) was injected i.p. into 3 mice from each treatment group (except day 0 samples) to detect hypoxic tissue regions (18). All tissues were processed for histology, IHC, and ISH studies.
Experiment B
Nine animals were decidualized and ovariectomized as above and were divided into 3 groups (Supplemental Fig. 1, Experiment B). Groups I (control) and II animals (n=3) were treated with the vehicle or VEGF Trap (12.5 mg/kg) on day 0, respectively; animals in group III (n=3) were treated with VEGF Trap on day 5. All 3 groups received daily i.p. injections of 3,5-bromodeoxyuridine (BrdU, 40 mg/kg; Sigma) from days 1–4 to label proliferating vessels, and uteri from all animals were collected on day 9 after ovariectomy. Specimens from groups I and II were also treated with a single i.p. injection of hypoxyprobe 1 h before tissue collection, as in Experiment A, for detection of hypoxic tissue regions.
Cell culture experiments
Isolation and culture of murine luminal epithelial (LE) and stromal cells
Uteri from 10–15 mice were pooled together to obtain enough LE cells for one complete set of experiments. LE and stromal cells were isolated following the same protocols as described previously (19, 20) with minor modifications. Briefly, uteri were opened lengthwise, cut into 3–5 mm pieces, spread on a 35 mm tissue culture plate with the luminal side up, and incubated with 0.625% trypsin (Difco, Detroit, MI, USA) in PBS without calcium and magnesium (Life Technologies, Inc., Grand Island, NY, USA) for 2 h at 4°C and 30 min at room temperature. The digested uterine tissues were transferred to a 15 ml Falcon tube (Becton Dickinson Labware, Franklin Lakes, NJ, USA) with 10 ml serum-containing medium (SCM) consisting of a 1:1 mixture of Dulbecco modified Eagle medium (DMEM) and Ham’s F-12 nutrient (Life Technologies, Inc.), 10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA), and 1% antibiotics (Life Technologies, Inc.). The tubes were vortexed gently to release the sheets of epithelial cells, and the supernatant containing LE sheets was collected. This procedure was repeated 3 times, and the supernatants were pooled, centrifuged at 1000 revolutions per minute (rpm) for 10 min, resuspended in SCM (1000 μl/uterus), seeded at 500 μl/well in a 24-well cell culture plate (Corning Inc., Corning, NY, USA), and incubated at 37°C with 5% CO2.
To isolate stromal cells, the remaining pooled uteri after LE cell isolation were subjected to collagenase/hyaluronidase (Sigma) digestion for 40 min at 37°C with continuous agitation, as described previously, for isolation of human endometrial stromal cells (20). The dispersed stromal fibroblasts were separated from clumps of gland cells and flakes of uterine tissues by filtering through a 40 μm cell strainer (BD Biosciences, San Jose, CA, USA). The cell suspension was centrifuged at 1800 rpm for 5 min and was resuspended (500 μl/uterus) in SCM. The cells were seeded (1×104 cells/insert) in the Transwell cell inserts (0.4 μm pore size; Corning), and the inserts were placed in 24-well cell culture plates (Corning) containing 800 μl of medium and incubated at 37°C with 5% CO2. The medium was aspirated from the inserts and then washed and replaced with fresh medium after 24 h to remove the nonadherent cells. Purity of the stromal and epithelial cells was assessed by vimentin and cytokeratin-7 (CK7) immunostaining (Supplemental Fig. 2C), respectively, and cell preparations greater than 95% purity were used in this study.
Endothelial cells
We used the bEnd3 mouse endothelial cell line (American Type Culture Collection, Manassas, VA, USA) for coculture experiments. The cells were seeded in the Transwell cell inserts (1×104 cells/insert) following the same protocol described above for stromal cells and were grown in DMEM supplemented with 15% FBS, 1% nonessential amino acids (Life Technologies, Inc.), 2 mM glutamine (Life Technologies, Inc.), 1 mM sodium pyruvate (Life Technologies, Inc.), and 1% antibiotics (Life Technologies, Inc.).
Scratch wound healing assay: effects of coculture
A scratch wound was made with a pipette tip in serum-starved confluent LE cells, and cell inserts containing either stromal or endothelial cells were transferred to the wells containing LE cells. In addition, the effects of various ligands, antagonists, and antibodies on postwound migration in these cocultures were assessed by incubating with either VEGF-164 alone (100 ng/ml), VEGF-164 plus VEGF trap (1.5 μg/ml), or VEGF-164 plus neutralizing antibodies against VEGFR1 (5 μg/ml; R&D Systems Inc., Minneapolis, MN, USA), VEGFR2 (1 μg/ml; R&D), or NP1 (10 μg/ml; R&D) for 12 h in serum-free medium. Serum-free medium alone served as a control. The wound images were taken at 0 and 12 h after wounding for calculation of the rate (μm/h) of wound healing (Supplemental Fig. 2A, B). Because the absolute rate of migration varied among different sets of cell preparations, each set of experiments (all treatment groups) was performed from the same cell preparations, and the results represent the means (percentage maximum) of at least 3 experiments.
RNA extraction and real-time polymerase chain reaction (PCR)
Total RNA extraction and real-time PCR experiments were performed following the manufacturer’s instructions and our previously published methods (20, 21). RNA from confluent epithelial and stromal cells grown in 24-well culture plates were extracted using Trizol (Invitrogen, Carlsbad, CA, USA) and purified by RNeasy Spin Columns (Qiagen, Valencia, CA, USA). About 2 μg of purified RNA was used for cDNA synthesis. Real-time PCR was performed with the Smart Cycler® System (Cepheid, Sunnyvale, CA, USA) using FastStart Universal SYBR Green Master mix (ROX; Roche, Indianapolis, IN, USA) and respective primers for each gene. GAPDH was used as the internal control. The thermal cycling conditions were an initial activation cycle at 95°C for 15 min, followed by 45 cycles of denaturation (94°C for 15 s), annealing, and amplification (60°C for 60 s). Final products were verified by agarose gel electrophoresis for each sample and negative controls and were quantified by external calibration curves obtained from serial dilutions of a known copy number of molecules (102–109 plasmids containing GAPDH, VEGFR1, VEGFR2, and NP1). All data were normalized with GAPDH expression. The following forward (F) and reverse (R) primers were used, respectively: VEGFR1: TTGAAAGAGTCACAGAGGAGGATGAG (F) and TCCGGCAGGTGGGTGATTTCT (R); VEGFR2: GTGATCCCAGATGACAGCCA (F) and GGTGAGCGCAGTGTGGTCC (R); NP1: CGATTTGGAGGACAGAGACTGC (F) and ATAGCGGATGGAAAACCCTGC (R); and GAPDH TCACTGCCACCCAGAAGACTGT (F) and CGTTCAGCTCTGGGATGACCTT (R).
ISH
ISH for VEGF expression in the monkey endometrium was performed following the same procedural details and probes as described previously (12). ISH in mouse sections were performed similar to monkey sections using a mouse homologous cDNA (415 to 791 bp, GenBank Accession No. NM_001025250). Frozen sections (10 μm) were fixed in cold 4% paraformaldehyde in PBS. The sections were hybridized with appropriate concentration of the 35S-labeled sense and antisense probes (∼5×106 cpm/ml). RNaseA-resistant hybrids were detected by autoradiography, and the sections were poststained with hematoxylin. Silver grains were counted over different cell types, and the abundance of silver grains was expressed as the number of grains per cell. These counts were then expressed as a percentage of the maximum signal in all the sections analyzed (12).
IHC
IHC of all tissue sections were performed in 10 μm frozen tissue sections fixed with 4% paraformaldehyde in PBS for 10 min. Immunostaining of cells in culture was performed identically to tissue sections, except the cells were fixed for 3 min. The details of IHC have been described previously (12, 21). The following primary antibodies were used for immunostaining: CD31 (1:200, rat monoclonal; BD Pharmingen, San Diego, CA, USA), vonWillebrand Factor (vWF; 1:4000; Dako Corporation, Carpinteria, CA, USA), Ki-67 (1:300; mouse monoclonal; BioGenex, San Ramon, CA, USA), NG2 (1:500; rabbit polyclonal; Chemicon, Temecula, CA, USA), CK7 (1:500, mouse monoclonal, Chemicon) and vimentin (1:400; Sigma). A BrdU staining kit from Zymed (Carlsbad, CA, USA) was used for detection of proliferating cells, and a hypoxyprobe kit with Cy3-conjugated antihypoxyprobe IgG (NPI, Inc.) was used for detection of hypoxic cells, following the manufacturer’s instructions. All specimens were visualized by immunofluorescence imaging using respective biotinylated second antibodies with the Vectastain kit and fluorescein- or Texas red-labeled avidin (Vector Laboratories, Burlingame, CA, USA). Sections for double immunostaining were performed after staining with the first antibody and avidin-biotin blocking (Vector Laboratories). Stained sections were mounted using Vectashield mounting medium with 4′,6′-diamidino-2-phenylidole (DAPI; Vector Laboratories) and examined using a Zeiss Axioskop 2 microscope equipped with a Zeiss AxioCam camera for fluorescence or bright-field imaging (Carl Zeiss, Oberkochen, Germany).
Fluorescence intensity measurement
The intensity of immunofluorescence was measured as an estimate of degree of hypoxia in the hypoxyprobe-stained tissue sections (22). The fluorescence intensity in luminal epithelium and subepithelial stroma was measured on digital images using ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA). A threshold intensity of 15 (range, 0–255) was used for distinguishing pixels from the background. The mean intensity value was calculated from at least 3 immunostained sections from each animal and expressed as a percentage of the maximum (% max) signal in all the sections analyzed.
Vascular density and proliferation measurement
CD31 (mouse) and vWF (macaque) fluorescence immunostaining were measured for calculation of an index of area density in different zones of endometrium (12, 22). Digital images were captured from 4 to 5 different regions of upper and lower zone endometrium. The distribution of intensity values in the regions of interest was visualized using the interactive 3D Surface Plot function of ImageJ software (22) (Fig. 1J, K). CD31/vWF-stained vessels were distinguished from background by a threshold value empirically determined for each set of IHC. The area density of vessels was calculated as the pixel values equal to or greater than the corresponding threshold. The mean value for upper and lower zones was calculated for each specimen and expressed as a percentage of the maximum signal. For vascular proliferation, the number of BrdU (mouse) and Ki-67 (macaque) positive nuclei (proliferating endothelial cells) in the CD31/vWF stained vessels was counted in the upper and lower zones of each endometrial section (12). Then, the number of proliferating endothelial cells per unit pixel area of vessels was calculated as quantitative index of vascular proliferation.
Figure 1.
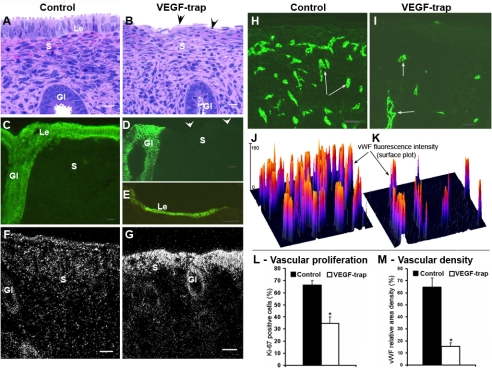
Effect of VEGF Trap in the rhesus macaque endometrium. A–E) Sections stained with hematoxylin and eosin (A, B) or cytokeratin 7 antibody (CK7; green fluorescence) (C–E). The luminal epithelium in most VEGF Trap-treated sections is absent (arrowheads; compare B with A; D with C), except a few that show patches of a thin layer of cells beginning to spread and cover the surface (E, Le). F, G) Darkfield VEGF-ISH images. VEGF mRNA signal is much higher in the uppermost zones of VEGF Trap-treated endometria (G). H, I) vWF immunostained vessels (green, arrows). J, K) Surface plots of vWF immunofluorescence corresponding to H, I. The peak height reflects fluorescence intensity, and the number of peaks reflects vascular density. The number of vessels in the upper endometrium is markedly reduced after VEGF Trap treatment (I) and is reflected by fewer peaks in the surface plot (K). L, M) Analyses of vascular proliferation and vascular density. Proliferating endothelial cells were detected by a double IHC procedure using Ki-67 and vWF antibodies. Bar graphs represent means ± se. *P < 0.001. Scale bars = 20 μm (A–D); 50 μm (E–I). Gl, gland; S, stroma. Arrows indicate blood vessels.
Detection of apoptotic cells
An in situ cell death detection kit (TUNEL technology) from Roche Diagnostics was used for detection of apoptotic cells in mouse endometrial sections following the manufacturer’s instructions. Paraffin sections (5 μm) were dewaxed and rehydrated using a standard protocol, and then subjected to proteinase K (20 μg/ml, Calbiochem, San Diego, CA, USA) digestion for 20 min at room temperature (25°C) and washed twice with PBS. The sections were then incubated with 50 μl of TUNEL reaction mixtures per section in a humidified chamber for 60 min at 37°C, washed with PBS, and mounted with Vectashield mounting medium with DAPI (Vector Laboratories).
Statistical analysis
Three to 6 samples in each experimental group were used, and all data were expressed as means ± se. The significance of differences between means was analyzed using 1-way ANOVA, followed by Fisher’s least significant difference (LSD) tests using the SPSS software (SPSS Inc, Chicago, IL, USA) (11, 12). Values of P < 0.05 were considered significant. Correlations between different attributes and coefficients of simple determination (R2) were calculated using the SPSS software (SPSS Inc.).
RESULTS
Macaque studies: VEGF Trap blocks postmenstrual angiogenesis and reepithelialization in the rhesus macaque endometrium
When macaques were withdrawn from P on day 0, treated with VEGF Trap or vehicle on days 2–4, and sampled on day 8, there were no gross changes in the endometrium except slight compaction of upper zone stroma in the VEGF Trap-treated animals. However, the luminal epithelium was either absent or greatly reduced in most endometrial sections from VEGF Trap-treated animals (compare Fig. 1A, B; arrowheads), although the glandular epithelium was unaffected. Cytokeratin immunostaining confirmed absence of luminal epithelium in most VEGF Trap-treated sections (Fig. 1D, arrowheads), except in a few areas where a very thin layer of epithelial cells was evident (Fig. 1E). VEGF mRNA expression in VEGF Trap-treated animals was substantially elevated in the superficial zone stroma compared with controls (Fig. 1F, G), but no marked differences in VEGFR1 or VEGFR2 mRNA expression were evident between the various treatment groups (data not shown). The upper third of endometrium was completely avascular after VEGF Trap treatment (Fig. 1I), although the number of vessels in the lower zone was largely unchanged (data not shown). Also, VEGF blockade significantly reduced endothelial cell proliferation and vascular density in the upper zones compared with controls (Fig. 1H–M). Overall, VEGF Trap treatment did not induce any marked changes in the duration of menstrual bleeding, as evidenced by examination of daily vaginal swabs, and bleeding stopped in all macaques by day 5 after P withdrawal, indicating that reepithelialization of the luminal surface in macaques is not a requirement for timely cessation of menstrual bleeding.
Murine in vivo studies
VEGF blockade inhibits angiogenesis and delays reepithelialization in a murine model of endometrial repair
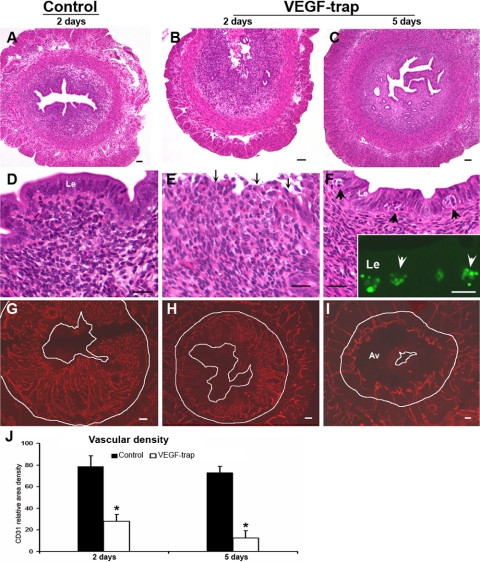
In mice, bilateral ovariectomy (P withdrawal) was performed to induce endometrial breakdown after decidualization. When a single dose of either VEGF Trap or the control vehicle was administered just before ovariectomy (Supplemental Fig. 1, Experiment A), the entire decidualized area of endometrium was shed by 24 h, indicating that VEGF blockade did not prevent endometrial tissue breakdown. In the controls, the upper endometrial stroma had completely regenerated and reepithelialization was complete within 2 days (Fig. 2A, D); however, in the VEGF Trap-treated animals, reepithelialization was not fully complete until 5 days after P withdrawal (Fig. 2C, F) and extensive apoptosis was evident within the newly formed luminal epithelium (Fig. 2F, arrowheads) as confirmed by TUNEL staining (Fig. 2F inset, arrowheads). On day 5, apoptosis was not evident in the luminal or glandular epithelium of controls or the glandular epithelium of VEGF Trap-treated animals. VEGF Trap significantly inhibited vascularization of the upper regenerated stroma, as evidenced by CD31 immunostaining (Fig. 2G–I) and measurements of vascular density (Fig. 2J) but did not alter the number of preexisting vessels in the basal zones or the myometrium (data not shown).
Figure 2.
Effect of VEGF Trap during endometrial repair and regeneration in the mice (for study design, see supplemental Fig. 1, Experiment A). Left panels represent samples from the control animals after 2 days of P withdrawal (A, D, G), and middle and right panels represent samples from VEGF Trap-treated animals after 2 (B, E, H) and 5 (C, F, I) days of P withdrawal, respectively. A–F) Histological preparations. The endometrium has fully regenerated and repithelialized 2 days after P withdrawal in controls (A, D), but the VEGF Trap-treated animals have a ragged surface on day 2 (B, E; arrows) and are not reepithelialized until day 5 (C, F). Apoptotic cells are evident in the epithelium at that time (F, arrowheads). Inset: apoptotic cells (green, arrowheads) in the luminal epithelium by TUNEL staining (F). G–I) CD31-immunostained vessels (red). Here and in Fig. 3, the outer white line demarcates separation of the endometrium from the myometrium, and the inner line demarcates the uterine lumen. The innermost endometrial layer is completely avascular (Av) after 5 days of VEGF Trap treatment (I). J) Analysis of vascular density at days 2 and 5. Bar graphs represent means ± se; *P < 0.001. Scale bars = 50 μm (A–C, G–I); 20 μm (D–F).
VEGF is not essential for maintenance of preexisting or newly formed mature endometrial vessels
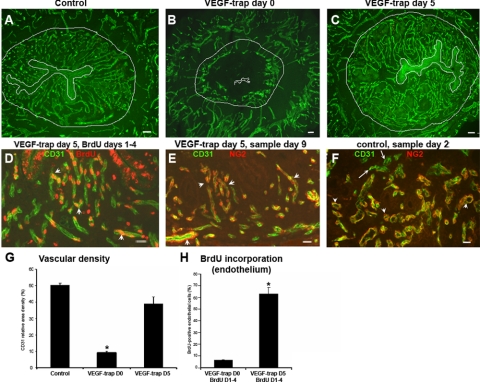
When VEGF Trap (or vehicle) was administered on either day 0 or day 5 after ovariectomy (Supplemental Fig. 1, Experiment B) and the uteri were sampled on day 9 after P withdrawal, the controls showed an extensive network of CD31-stained vessels on day 9 (Fig. 3A). In those treated with VEGF Trap on day 0 and sampled on day 9, vascular development was completely blocked in the innermost zones (Fig. 3B); but in those treated with VEGF Trap on day 5, vascular development had proceeded normally through day 9 (Fig. 3C). Vessels in the basal zone and the myometrium were unaffected by VEGF Trap treatment (Fig. 3A–C). Evidently, when VEGF Trap was administered after the innermost zones had fully vascularized, blockade of VEGF had no effect on either the neovessels in the innermost zones or the preexisting vessels in the basal and myometrial zones. Quantitative assessment of vessel density on day 9 confirmed these findings (Fig. 3G). Also, in animals treated with VEGF Trap on day 5 (Supplemental Fig. 1, Experiment B, group III), the nuclei of most endothelial cells in the innermost zones were labeled with BrdU on day 9 (Fig. 3D, arrowheads; H), confirming that after these vessels had proliferated, they were resistant to VEGF blockade. Samples from the same animals (VEGF Trap treatment on day 5, sampled on day 9), immunostained for NG2 (a pericyte marker) and CD31, revealed pericyte coverage in all vessels present in the innermost layer (Fig. 3E, arrowheads), suggesting that the newly formed pericyte-covered vessels in this zone are resistant to VEGF Trap treatment. In addition, colocalization of NG2 and CD31 in the control animals sampled at different time points (Experiment A) suggest that endometrial vessels probably acquire pericyte coverage at very early stages of angiogenesis because most vessels showed pericyte coverage in samples after day 2 of P withdrawal (Fig. 3F, arrowheads).
Figure 3.
Effect of VEGF Trap on preexisting and newly formed vessels in the mouse endometrium (see Supplemental Fig. 1, Experiment B). A–C) CD31 immunostaining of endometrial vessels (green) from the control (A) and after VEGF Trap treatments on day 0 (B) or day 5 (C), sampled on day 9. D) CD31 (green) and BrdU (red) colocalization. BrdU was administered on days 1–4, VEGF Trap on day 5, and sampled on day 9. Most vessels in the upper endometrium have BrdU incorporation (arrowheads, D), suggesting that these vessels are newly formed and not affected by VEGF trap treatment on day 5. E) Samples from same animals as in D, immunostained with CD31 (green) and NG2 (red, pericyte marker), showing pericyte coverage (arrowheads) of all vessels. F) CD31 (green) and NG2 (red) colocalization of control specimens sampled on day 2; except few (arrows), most vessels have acquired pericyte coverage (arrowheads). G, H) Analysis of vascular density (G) and BrdU incorporation (H) after VEGF Trap treatment at various time points. Bar graphs represent means ± se; *P < 0.001. Scale bars = 50 μm (A–C); 20 μm (D–F).
Blockade of endometrial angiogenesis leads to intense hypoxia and increased VEGF expression in the upper avascular zone
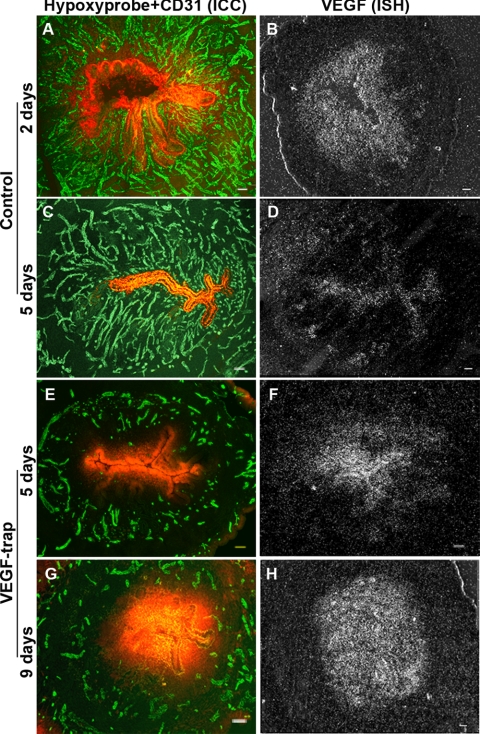
Hypoxyprobe injections revealed large differences in degree of hypoxia between the controls and the VEGF Trap-treated animals. In the controls (through day 3), only cells at the leading front of tissue growth, (i.e., the stromal cells closest to the lumen and the luminal epithelial cells) were intensely hypoxic (day 2, Fig. 4A, Supplemental Fig. 3A). VEGF was also strongly expressed in the same layer of hypoxic cells (day 2, Fig. 4B, Supplemental Fig. 3B). After the endometrium had completely regenerated and become fully vascularized (through day 5), hypoxia and VEGF levels decreased to a nadir in the luminal epithelium and were nondetectable in the stroma (Fig. 4C, D; Supplemental Fig. 3A, B).
Figure 4.
Hypoxia and VEGF expression following VEGF Trap treatment in the mouse endometrium. A–D represent samples from the control animals 2 days (A, B) and 5 days (C, D) after ovariectomy (P withdrawal), and E–H represent samples from animals treated with VEGF Trap on day 0 and sampled on day 5 (E, F) and day 9 (G, H) after ovariectomy (P withdrawal), respectively. Left panels show CD31 (green) and hypoxyprobe (red) colocalization by IHC (A, C, E, G); right panels show VEGF ISH (B, D, F, H). Note the dramatic increase in hypoxia in the CD31-negative avascular area (E, G) and increase in VEGF expression in the same zones of endometrium (F, H) after VEGF Trap treatment. Scale bars = 50 μm.
However, in VEGF Trap-treated animals, there was progressive and dramatic increase in hypoxia in the upper avascular stroma and luminal epithelium that was evident through day 9, with the highest degree of hypoxia evident in the luminal epithelium (Fig. 4E, G; Supplemental Fig. 3A). VEGF expression followed exactly the same pattern, gradient, and time course as hypoxia (Fig. 4F, H; Supplemental Fig. 3B), with the most intense expression in day 9 samples (Fig. 4H, Supplemental Fig. 3B). The hypoxyprobe staining intensity was significantly correlated with VEGF expression (grain counts) in the stroma (R2=0.902, P<0.001) and the luminal epithelium (R2=0.938, P<0.001) in both controls and VEGF Trap-treated animals. These results together suggest that ischemic hypoxia in the uppermost zones is the local stimulus for VEGF expression and angiogenesis.
Murine in vitro studies: VEGF stimulates luminal epithelial cell migration via its receptors on the stromal cells
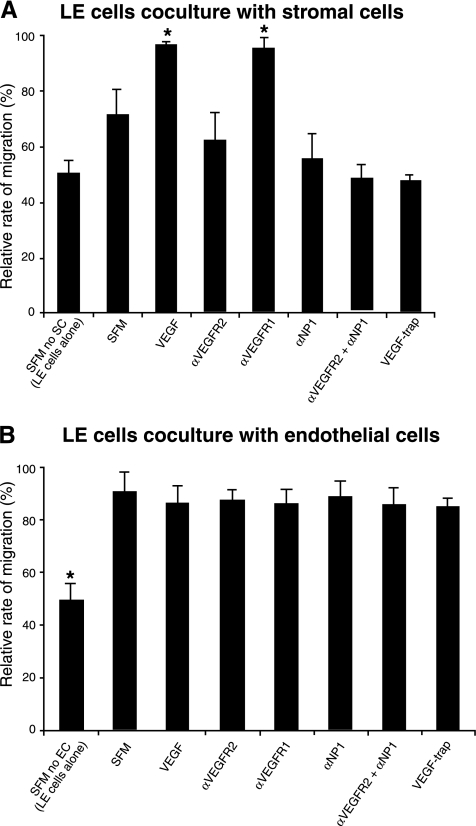
To determine whether VEGF can directly stimulate luminal epithelial cell migration or acts indirectly through stromal and/or endothelial cells, we examined the effect of VEGF-164 (the most abundant isoform in the endometrium) on luminal epithelial cell migration rate with an in vitro scratch wound assay. When the luminal epithelial cells were exposed to serum-free medium for the wound assay, many cells died and peeled off from the surface, and VEGF-164 treatment did not inhibit the cell death and had no effect on migration velocity of the cells (data not shown). But when these cells were cocultured with stromal or endothelial cells, cell death was markedly reduced and migration velocity was significantly increased (Fig. 5A, B), suggesting that soluble factors from both stromal and endothelial cells stimulate luminal epithelial cell migration and survival. The migration velocity of luminal epithelial cells was further significantly increased after VEGF-164 treatment when cocultured with stromal cells. This effect of VEGF-164 was completely inhibited by pretreatment with VEGF trap and neutralizing antibodies against VEGFR2, NP1 or a combination of both VEGFR2 and NP1, but not with the VEGFR1 antibody (Fig. 5A). The combined effect of VEGFR2 and NP1 antibodies was more dramatic than with either alone and was comparable to VEGF Trap treatment.
Figure 5.
Effect of VEGF-164 on mouse luminal epithelial (LE) cell migration in an in vitro scratch wound assay. LE cells were either cocultured with stromal or bEnd3 (endothelial) cells in transwell inserts. LE cell migration was determined in scratch wounds in serum-free medium (SFM) alone or SFM plus VEGF-164 (VEGF) with or without VEGF Trap or neutralizing antibodies against VEGFR1 (αVEGFR1), VEGFR2 (αVEGFR2), NP1 (αNP1), or αVEGFR2+ αNP1. A, B) Analysis of LE cell migration in scratch wounds cocultured with stromal (A) or endothelial (B) cells in the presence of VEGF or VEGF plus different VEGF inhibitors. The first bars in A (SFM no SC) and B (SFM no EC) show LE cell migration without coculture. Bar graphs represent means ± se; *P < 0.05.
Addition of VEGF-164 and/or VEGFR1, VEGFR2, or NP1 antibodies or VEGF Trap to the coculture of endothelial and LE cells, however, had no significant effect on migration of LE cells (Fig. 5B), suggesting that the effects of endothelial cells on migration of LE cells are not mediated by VEGF. Examination of mRNAs for VEGF receptors in isolated LE and stromal cells revealed that VEGFR2 transcripts were expressed only in the stromal cells at low levels, NP1 transcripts were expressed in both the cell types with much higher abundance in stromal cells, and VEGFR1 transcripts were nondetectable in both stromal and luminal epithelial cells (Supplemental Fig. 2D). These data suggest that VEGF affects the migration of luminal epithelial cells indirectly through VEGFR2 and NP1 receptors on stromal cells.
DISCUSSION
In the present study, we explored the role of VEGF in the early angiogenic processes associated with the postmenstrual regeneration of endometrium. First, VEGF is essential for the rapid burst of angiogenesis that occurs during the postmenstrual repair and early proliferative phases in the primate endometrium. Second, and more surprising, it plays a role in reepithelialization of endometrium during the postmenstrual repair phase. Our in vitro studies in the mouse endometrial cells suggest that this effect of VEGF on luminal epithelial cell migration is mediated by VEGFR2 and NP1 receptors on stromal cells. Third, VEGF action on endometrial endothelium is primarily limited to proliferating endothelial cells during neoangiogenesis as blockade of VEGF action had no effect on preexisting or newly formed mature vessels. Finally, the strong correlation between intense hypoxia and VEGF expression in the upper zones is consistent with hypoxia as the prime local regulator of VEGF expression in the endometrium.
VEGF regulation of angiogenesis
This study is the first to experimentally assess the effects of VEGF blockade on vascular development in the primate endometrium. Using the VEGF Trap, a potent inhibitor of all isoforms of VEGF-A, our results clearly show that VEGF plays a critical role in the early angiogenic processes during postmenstrual endometrial healing and regeneration in both mice and macaques. This result is consistent with our earlier findings in the macaques and several other studies in the human and nonhuman primates showing up-regulation of VEGF and increase in vascular proliferation in the postmenstrual/early-proliferative phase endometrium (12,13,14,15). Previous reports of VEGF inhibition in mouse endometrium show inconsistent effects on E2-induced vascular proliferation (23, 24). In contrast to a report by Heryanto et al. (23), Hastings et al. (24) did not observe significant effect of blockade of VEGF action on vascular proliferation in E2-treated mouse endometrium. Those authors used different VEGF inhibitors and completely different experimental models, discussed further below.
Like most adult organs and tissues, other than endocrine organs (22), the endometrial vascular bed does not seem to depend on VEGF for survival. Once endometrial neovessels developed, VEGF blockade had no significant effect on them. As evidenced by colocalization of NG2 and CD31 immunostaining (Fig. 3F), our results further suggest that endometrial vessels recruit pericytes and are stabilized at very early stages immediately following neoangiogenesis during endometrial regeneration. Thus, it is likely that on stabilization, these vessels become independent of VEGF action. Other reports indicate that blood vessels devoid of pericytes are more dependent on VEGF as an endothelial survival factor compared with vessels with pericytes (25). This result also explains the conflicting findings in the aforesaid studies on adult mouse endometrium (23, 24). The overall endothelial cell proliferation was very low in the model used by Hastings et al. (24), and small changes may be challenging to detect. Consequently, they observed very little effect of VEGF inhibition on endometrial vascularity.
A recent report (24) shows that the E2-induced vascular permeability in the mouse uterus is partly mediated through VEGF. Although our results show slight compaction of the upper endometrium in VEGF Trap-treated animals, it is unlikely the result of reduction in vascular permeability because the upper endometrium was completely avascular. Both our mouse and macaque experimental designs are not suitable to specifically address this question. Moreover, after inhibition of VEGF action, there was no significant effect on glands or stromal growth, suggesting that passive diffusion from the surrounding zones can provide required nutrients to support their growth.
Effects on reepithelialization
In this study, we observed a dramatic inhibition of reepithelialization after VEGF blockade during endometrial repair. Although LE cells highly express VEGF, a direct autocrine or paracrine action of VEGF on these cells is unlikely as LE cells lack the major VEGF signaling receptors (12). However, our in vitro coculture experiments in the mouse suggest that the action of VEGF on LE cells migration is likely mediated by signaling through VEGFR2 and NP1 receptors on stromal cells, not by an autocrine mechanism in LE cells or through endothelial cells, and suggest a crosstalk between luminal epithelium and stroma during tissue repair. Cooke et al. (19) showed that E2-induced proliferation of epithelial cells occurred only when the estrogen receptor was present in stromal cells, which implicated stromal cell-derived paracrine factors in E2-induced endometrial epithelial cell proliferation. Analogously, stromal cell-derived paracrine factors may mediate the effects of VEGF on LE cell migration. Numerous stromal derived factors are known to enhance adhesion and migration of keratinocytes during cutaneous wound healing (26), and future studies are needed to identify specific factors that are regulated by VEGF signaling.
Coculture of endothelial and LE cells significantly increased LE cell migration after wounding, but addition of VEGF or blocking VEGF action in the culture had no effect on the LE cells. Paracrine factors other than VEGF released by the vascular endothelium may facilitate endometrial reepithelialization by enhancing migration of LE cells. Numerous such endothelial-derived paracrine factors have been identified, including basic fibroblast growth factor, platelet-derived growth factor, insulin-like growth factor-1, heparin-binding epithelial growth factor and hepatocyte growth factor (26, 27). Thus, VEGF released from the epithelial and stromal cells in vivo may regulate LE cell functions in 2 ways, by acting though VEGFR2 and NP1 receptors on stromal cells and indirectly by stimulating the vascular endothelium to secrete paracrine factors that affect the nearby luminal epithelium.
Endometrial growth, ischemic hypoxia, and angiogenesis
Tissue hypoxia, primarily mediated by hypoxia-inducible factor 1 (HIF-1) (28), is a key regulator of VEGF expression and angiogenesis in a wide variety of tumors and other tissues. HIF-1α is primarily expressed during the secretory and menstrual phases in the human endometrium (29), and hypoxia also stimulates VEGF production in human endometrial stromal and epithelial cells in culture (30, 31). In contrast, a recent study in the human endometrium could not confirm substantial up-regulation of HIF proteins during the perimenstrual period (32), a time when the endometrial tissues are believed to be highly hypoxic and stimulate VEGF production, raising concern about the extent of hypoxia and hypoxia-regulated genes during this period. Also, another study (33) in the mouse showed disparate regulation of HIF in different hormonal conditions of the endometrium, demonstrating no correlation between HIF and VEGF expression during the preimplantation period. To our knowledge, ours is the first study to demonstrate direct evidence of hypoxia in vivo in the endometrium and a dynamic relation among endometrial hypoxia, VEGF expression, and angiogenesis.
The hallmark report by Judah Folkman in 1971 (34) proposed that growing masses of tumor cells can sense their increasing distance from existing local vasculature and release angiogenic factors, which in turn stimulate tumor angiogenesis. Numerous studies in subsequent years have confirmed this concept and established that tumor hypoxia and VEGF are the primary mediators of this tissue response. However, whether organ size or normal tissue mass is regulated by vascular endothelium has remained an open question, and this relation appears to be different in different organs. For example, it has been shown that testosterone stimulation in castrated rats causes a rapid burst of endothelial cell proliferation that precedes glandular regrowth and enlargement of prostate mass, suggesting that prostate is under tight control of the vascular endothelium (35). However, the endometrium can regenerate in absence of concomitant vascular growth (after VEGF blockade) in both nonhuman primates and rodents, indicating that endometrial tissue mass probably regulates neovessel development in this organ. As a result of tissue growth, the upper avascular zone creates a hypoxia gradient and a parallel VEGF expression gradient, highly consistent with the original model proposed for tumor angiogenesis.
Although vessels in different disease conditions may have different sensitivity to VEGF blockade, our findings have broad implications and provide new insights for developing therapeutic strategies for angiogenesis-dependent endometrial disorders. First, angiogenesis in the endometrium appears to be secondary to hormone-dependent tissue growth and hypoxia. After VEGF blockade, both endometrial glandular and stromal cells grew in a highly hypoxic environment and stimulated VEGF expression. Second, endometrial vessels recruit mural cells and stabilize at very early stages of neoangiogenesis and are insensitive to VEGF inhibitors. Thus, the strategies for long-term anti-VEGF therapy targeting endometrial vessels should be carefully examined. Because VEGF stimulates LE cell migration acting through its receptors on stromal cells, its potential pleiotropic role in endometrial cancer and hyperplasia warrants further investigation. Nevertheless, our study suggests that inhibiting endogenous VEGF may be a promising therapeutic approach to block neovessel formation in this tissue.
Supplementary Material
Acknowledgments
This work was supported by grants from the Andrew Mellon Foundation, the U.S. National Institutes of Health (NIH) Building Interdisciplinary Research Careers in Women’s Health, the Children Health Initiative at Stanford to N.R.N., and NIH Specialized Cooperative Centers Program in Reproduction (SCCPR) 1U54HD055764. We thank Sabita Dhal (Stanford University, Stanford, CA, USA), Kunie Mah (Oregon National Primate Research Center, Beaverton, OR, USA), and Kim Chi Vo (University of California, San Francisco, CA, USA) for technical assistance. S.J.W. is an employee of Regeneron Pharmaceuticals, holds an equity position in the company, and is an inventor on several patents related to the VEGF Trap.
References
- Jabbour H N, Kelly R W, Fraser H M, Critchley H O. Endocrine regulation of menstruation. Endocr Rev. 2006;27:17–46. doi: 10.1210/er.2004-0021. [DOI] [PubMed] [Google Scholar]
- Hess A P, Nayak N R, Giudice L C. Oviduct and endometrium: cyclic changes in the primate oviduct and endometrium. Neill J D, editor. New York, NY, USA: Elsevier Science & Technology Books; 2005:337–381. [Google Scholar]
- Ludwig H, Spornitz U M. Microarchitecture of the human endometrium by scanning electron microscopy: menstrual desquamation and remodeling. Ann N Y Acad Sci. 1991;622:28–46. doi: 10.1111/j.1749-6632.1991.tb37848.x. [DOI] [PubMed] [Google Scholar]
- Dvorak H F. Angiogenesis: update 2005. J Thromb Haemost. 2005;3:1835–1842. doi: 10.1111/j.1538-7836.2005.01361.x. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain R K. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Kerbel R S. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- Gluzman-Poltorak Z, Cohen T, Herzog Y, Neufeld G. Neuropilin-2 is a receptor for the vascular endothelial growth factor (VEGF) forms VEGF-145 and VEGF-165. J Biol Chem. 2000;275:18040–18045. doi: 10.1074/jbc.M909259199. [DOI] [PubMed] [Google Scholar]
- Soker S, Miao H Q, Nomi M, Takashima S, Klagsbrun M. VEGF165 mediates formation of complexes containing VEGFR-2 and neuropilin-1 that enhance VEGF165-receptor binding. J Cell Biochem. 2002;85:357–368. doi: 10.1002/jcb.10140. [DOI] [PubMed] [Google Scholar]
- Takashima S, Kitakaze M, Asakura M, Asanuma H, Sanada S, Tashiro F, Niwa H, Miyazaki J J, Hirota S, Kitamura Y, Kitsukawa T, Fujisawa H, Klagsbrun M, Hori M. Targeting of both mouse neuropilin-1 and neuropilin-2 genes severely impairs developmental yolk sac and embryonic angiogenesis. Proc Natl Acad Sci U S A. 2002;99:3657–3662. doi: 10.1073/pnas.022017899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder J B, Zhao X, Soker S, Paria B C, Klagsbrun M, Das S K, Dey S K. Differential expression of VEGF isoforms and VEGF(164)-specific receptor neuropilin-1 in the mouse uterus suggests a role for VEGF(164) in vascular permeability and angiogenesis during implantation. Genesis. 2000;26:213–224. [PubMed] [Google Scholar]
- Germeyer A, Hamilton A E, Laughlin L S, Lasley B L, Brenner R M, Giudice L C, Nayak N R. Cellular expression and hormonal regulation of neuropilin-1 and -2 messenger ribonucleic acid in the human and rhesus macaque endometrium. J Clin Endocrinol Metab. 2005;90:1783–1790. doi: 10.1210/jc.2004-1769. [DOI] [PubMed] [Google Scholar]
- Nayak N R, Brenner R M. Vascular proliferation and vascular endothelial growth factor expression in the rhesus macaque endometrium. J Clin Endocrinol Metab. 2002;87:1845–1855. doi: 10.1210/jcem.87.4.8413. [DOI] [PubMed] [Google Scholar]
- Charnock-Jones D S, Sharkey A M, Rajput-Williams J, Burch D, Schofield J P, Fountain S A, Boocock C A, Smith S K. Identification and localization of alternately spliced mRNAs for vascular endothelial growth factor in human uterus and estrogen regulation in endometrial carcinoma cell lines. Biol Reprod. 1993;48:1120–1128. doi: 10.1095/biolreprod48.5.1120. [DOI] [PubMed] [Google Scholar]
- Girling J E, Rogers P A. Recent advances in endometrial angiogenesis research. Angiogenesis. 2005;8:89–99. doi: 10.1007/s10456-005-9006-9. [DOI] [PubMed] [Google Scholar]
- Ferenczy A, Bertrand G, Gelfand M M. Proliferation kinetics of human endometrium during the normal menstrual cycle. Am J Obstet Gynecol. 1979;133:859–867. doi: 10.1016/0002-9378(79)90302-8. [DOI] [PubMed] [Google Scholar]
- Smith S K. Regulation of angiogenesis in the endometrium. Trends Endocrinol Metab. 2001;12:147–151. doi: 10.1016/s1043-2760(01)00379-4. [DOI] [PubMed] [Google Scholar]
- Holash J, Davis S, Papadopoulos N, Croll S D, Ho L, Russell M, Boland P, Leidich R, Hylton D, Burova E, Ioffe E, Huang T, Radziejewski C, Bailey K, Fandl J P, Daly T, Wiegand S J, Yancopoulos G D, Rudge J S. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99:11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh J A, Calkins-Adams D P, Rinker L H, Ballenger C A, Weissler M C, Fowler W C, Jr, Novotny D B, Varia M A. Hypoxia and vascular endothelial growth factor expression in human squamous cell carcinomas using pimonidazole as a hypoxia marker. Cancer Res. 1998;58:3765–3768. [PubMed] [Google Scholar]
- Cooke P S, Buchanan D L, Young P, Setiawan T, Brody J, Korach K S, Taylor J, Lubahn D B, Cunha G R. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci U S A. 1997;94:6535–6540. doi: 10.1073/pnas.94.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathi R B, Hess A P, Tulac S, Nayak N R, Conti M, Giudice L C. Dose-dependent insulin regulation of insulin-like growth factor binding protein-1 in human endometrial stromal cells is mediated by distinct signaling pathways. J Clin Endocrinol Metab. 2005;90:1599–1606. doi: 10.1210/jc.2004-1676. [DOI] [PubMed] [Google Scholar]
- Nayak N R, Kuo C J, Desai T A, Wiegand S J, Lasley B L, Giudice L C, Brenner R M. Expression, localization and hormonal control of angiopoietin-1 in the rhesus macaque endometrium: potential role in spiral artery growth. Mol Hum Reprod. 2005;11:791–799. doi: 10.1093/molehr/gah237. [DOI] [PubMed] [Google Scholar]
- Kamba T, Tam B Y, Hashizume H, Haskell A, Sennino B, Mancuso M R, Norberg S M, O'Brien S M, Davis R B, Gowen L C, Anderson K D, Thurston G, Joho S, Springer M L, Kuo C J, McDonald D M. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol. 2006;290:H560–H576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- Heryanto B, Lipson K E, Rogers P A. Effect of angiogenesis inhibitors on oestrogen-mediated endometrial endothelial cell proliferation in the ovariectomized mouse. Reproduction. 2003;125:337–346. doi: 10.1530/rep.0.1250337. [DOI] [PubMed] [Google Scholar]
- Hastings J M, Licence D R, Burton G J, Charnock-Jones D S, Smith S K. Soluble vascular endothelial growth factor receptor 1 inhibits edema and epithelial proliferation induced by 17beta-estradiol in the mouse uterus. Endocrinology. 2003;144:326–334. doi: 10.1210/en.2002-220641. [DOI] [PubMed] [Google Scholar]
- Benjamin L E, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999;103:159–165. doi: 10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer A J, Clark R A. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Rak J, Filmus J, Kerbel R S. Reciprocal paracrine interactions between tumour cells and endothelial cells: the “angiogenesis progression” hypothesis. Eur J Cancer. 1996;32A:2480–2450. doi: 10.1016/s0959-8049(96)00396-6. [DOI] [PubMed] [Google Scholar]
- Semenza G L. HIF-1: using two hands to flip the angiogenic switch. Cancer Metastasis Rev. 2000;19:59–65. doi: 10.1023/a:1026544214667. [DOI] [PubMed] [Google Scholar]
- Critchley H O, Osei J, Henderson T A, Boswell L, Sales K J, Jabbour H N, Hirani N. Hypoxia-inducible factor-1alpha expression in human endometrium and its regulation by prostaglandin E-series prostanoid receptor 2 (EP2) Endocrinology. 2006;147:744–753. doi: 10.1210/en.2005-1153. [DOI] [PubMed] [Google Scholar]
- Sharkey A M, Day K, McPherson A, Malik S, Licence D, Smith S K, Charnock-Jones D S. Vascular endothelial growth factor expression in human endometrium is regulated by hypoxia. J Clin Endocrinol Metab. 2000;85:402–409. doi: 10.1210/jcem.85.1.6229. [DOI] [PubMed] [Google Scholar]
- Popovici R M, Irwin J C, Giaccia A J, Giudice L C. Hypoxia and cAMP stimulate vascular endothelial growth factor (VEGF) in human endometrial stromal cells: potential relevance to menstruation and endometrial regeneration. J Clin Endocrinol Metab. 1999;84:2245–2248. doi: 10.1210/jcem.84.6.5886. [DOI] [PubMed] [Google Scholar]
- Zhang J, Salamonsen L A. Expression of hypoxia-inducible factors in human endometrium and suppression of matrix metalloproteinases under hypoxic conditions do not support a major role for hypoxia in regulating tissue breakdown at menstruation. Hum Reprod. 2002;17:265–274. doi: 10.1093/humrep/17.2.265. [DOI] [PubMed] [Google Scholar]
- Daikoku T, Matsumoto H, Gupta R A, Das S K, Gassmann M, DuBois R N, Dey S K. Expression of hypoxia-inducible factors in the peri-implantation mouse uterus is regulated in a cell-specific and ovarian steroid hormone-dependent manner. Evidence for differential function of HIFs during early pregnancy. J Biol Chem. 2003;278:7683–7691. doi: 10.1074/jbc.M211390200. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Franck-Lissbrant I, Häggström S, Damber J E, Bergh A. Testosterone stimulates angiogenesis and vascular regrowth in the ventral prostate in castrated adult rats. Endocrinology. 1998;139:451–456. doi: 10.1210/endo.139.2.5683. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.