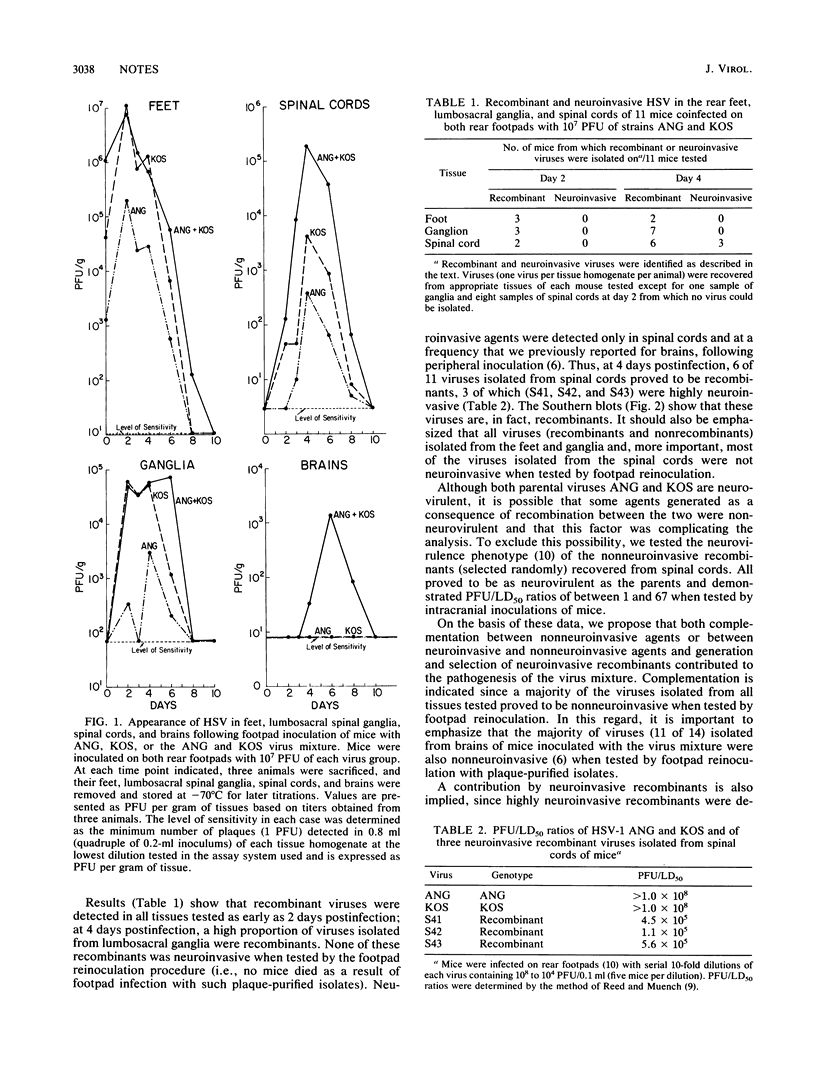
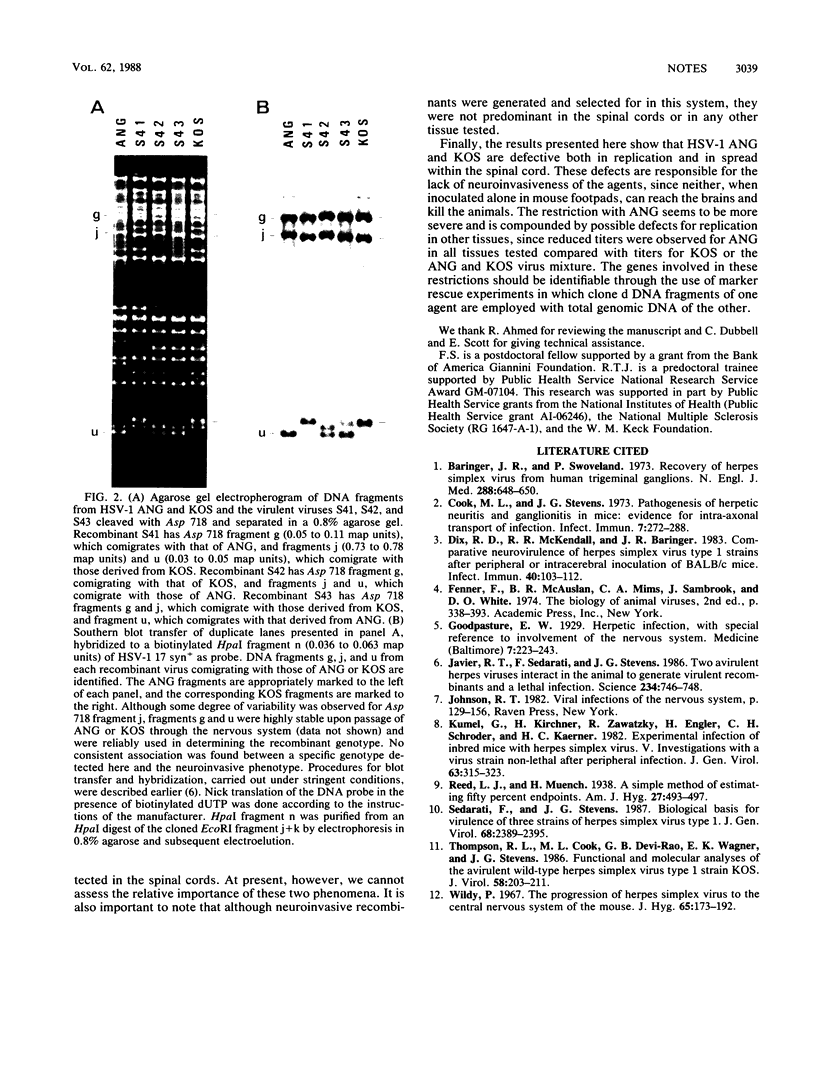
Abstract
We previously reported that simultaneous inoculation of mice on abraded rear footpads with two nonneuroinvasive viruses (herpes simplex virus type 1 ANG and KOS) resulted in the deaths of 62% of the animals (R. T. Javier, F. Sedarati, and J. G. Stevens, Science 234:746-748, 1986). In the current study, to better understand the events responsible for the pathogenesis of this virus mixture, we investigated replicative capacity and spread of the virus mixture within specific tissues. We found that, compared with neuroinvasiveness of ANG or KOS alone, neuroinvasiveness of the virus mixture related to significantly increased amounts of the virus within spinal cords and brains of the mice. This finding indicates that ANG and KOS have defects in their capacities to spread and replicate within spinal cords. We also examined whether the increased neuroinvasiveness of the virus mixture related to complementation between viruses in tissues of the nervous system, generation and selection of neuroinvasive recombinants, or both. It was found that, although neuroinvasive recombinant viruses could be detected in the spinal cords of the infected animals, most of the viruses (both recombinants and nonrecombinants) isolated from all tissues tested were nonneuroinvasive (i.e., no mice died as a result of footpad infection with high doses of such plaque-purified isolates). As a result of these findings, we propose that the virulence of the virus mixture is a consequence of the complementation as well as the generation and selection of neuroinvasive recombinants in spinal cords of these mice.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baringer J. R., Swoveland P. Recovery of herpes-simplex virus from human trigeminal ganglions. N Engl J Med. 1973 Mar 29;288(13):648–650. doi: 10.1056/NEJM197303292881303. [DOI] [PubMed] [Google Scholar]
- Cook M. L., Stevens J. G. Pathogenesis of herpetic neuritis and ganglionitis in mice: evidence for intra-axonal transport of infection. Infect Immun. 1973 Feb;7(2):272–288. doi: 10.1128/iai.7.2.272-288.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix R. D., McKendall R. R., Baringer J. R. Comparative neurovirulence of herpes simplex virus type 1 strains after peripheral or intracerebral inoculation of BALB/c mice. Infect Immun. 1983 Apr;40(1):103–112. doi: 10.1128/iai.40.1.103-112.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javier R. T., Sedarati F., Stevens J. G. Two avirulent herpes simplex viruses generate lethal recombinants in vivo. Science. 1986 Nov 7;234(4777):746–748. doi: 10.1126/science.3022376. [DOI] [PubMed] [Google Scholar]
- Kümel G., Kirchner H., Zawatzky R., Engler H., Schröder C. H., Kaerner H. C. Experimental infection of inbred mice with herpes simplex virus. V. Investigations with a virus strain non-lethal after peripheral infection. J Gen Virol. 1982 Dec;63(2):315–323. doi: 10.1099/0022-1317-63-2-315. [DOI] [PubMed] [Google Scholar]
- Sedarati F., Stevens J. G. Biological basis for virulence of three strains of herpes simplex virus type 1. J Gen Virol. 1987 Sep;68(Pt 9):2389–2395. doi: 10.1099/0022-1317-68-9-2389. [DOI] [PubMed] [Google Scholar]
- Thompson R. L., Cook M. L., Devi-Rao G. B., Wagner E. K., Stevens J. G. Functional and molecular analyses of the avirulent wild-type herpes simplex virus type 1 strain KOS. J Virol. 1986 Apr;58(1):203–211. doi: 10.1128/jvi.58.1.203-211.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]