Abstract
The effect of iron regulatory protein-2 (IRP2) on ferritin expression and neuronal vulnerability to hemoglobin was assessed in primary cortical cell cultures prepared from wild-type and IRP2 knockout mice. Baseline levels of H and L-ferritin subunits were significantly increased in IRP2 knockout neurons and astrocytes. Hemoglobin was toxic to wild-type neurons in mixed neuron-astrocyte cultures, with an LC50 near 3 µM for a 24 hour exposure. Neuronal death was reduced by 85–95% in knockout cultures, and also in cultures containing knockout neurons plated on wild-type astrocytes. Protein carbonylation, reactive oxygen species formation, and heme oxygenase-1 expression after hemoglobin treatment were also attenuated by IRP2 gene deletion. These results suggest that IRP2 binding activity increases the vulnerability of neurons to hemoglobin, possibly by reducing ferritin expression. Therapeutic strategies that target this regulatory mechanism may be beneficial after hemorrhagic CNS injuries.
Keywords: cell culture, free radical, iron, hemoglobin toxicity, intracerebral hemorrhage, oxidative stress, stroke
Introduction
Hemoglobin, the most abundant protein in blood, is present in millimolar concentrations in an intracerebral hematoma. Considerable experimental evidence supports the hypothesis that release of some of this hemoglobin into the extracellular space generates oxidative stress in adjacent tissue (Huang et al., 2002; Koeppen et al., 2004; Qu et al., 2007). The pro-oxidant effect of hemoglobin in vitro does not appear to be directly due to the hemoglobin molecule per se, but rather to iron (Gutteridge, 1986; Sadrzadeh et al., 1987; Levere et al., 1989; Lamb et al., 1999), which accumulates in tissue near a hematoma and persists for months (Hua et al., 2006). Consistent with an iron-mediated effect, iron chelators are protective after injection of either hemoglobin or whole blood into the rodent brain (Huang et al., 2002; Nakamura et al., 2004; Masuda et al., 2007), and mitigate hemin or hemoglobin neurotoxicity in cell culture models (Regan and Panter, 1993; Goldstein et al., 2003).
The use of exogenous iron chelators after intracerebral hemorrhage may be limited by their toxicity when administered in the absence of systemic iron overload (Porter and Huehns, 1989; Kushner et al., 2001). Enhancing the endogenous ability of CNS cells to sequester and detoxify iron may be an alternative or complementary approach. Ferritin, the primary cell iron storage protein, is a 24-mer heteropolymer constructed of H and L subunits that form a hollow protein shell with a capacity for several thousand iron atoms (Harrison and Arosio, 1996; Torti and Torti, 2002; Wagner et al., 2003). It is induced in astrocytes (Regan et al., 2002), oligodendrocytes (Qi et al., 1995) and endothelial cells (Balla et al., 1992) by treatment with nontoxic concentrations of heme; preconditioned cells are then protected from toxic doses of hemin and other oxidants. These observations indicate that ferritin is a potent endogenous antioxidant, provided that its expression is increased prior to delivery of an oxidative insult. Although the delayed release of hemoglobin from erythrocytes after intracerebral hemorrhage may provide sufficient time for ferritin induction (Xi et al., 1998), heme preconditioning may not be feasible in a clinical setting, due to the narrow range between therapeutic and toxic heme concentrations (Balla et al., 1992; Regan et al., 2002).
Iron regulatory proteins (IRP)-1 and 2 inhibit synthesis of both H and L-ferritin by binding to an iron regulatory element (IRE) in the 5' regions of their mRNA, thereby preventing translation (Harrison and Arosio, 1996; Torti and Torti, 2002). IRP2 appears to predominate in the murine brain and in most tissues, with the exceptions of kidney and brown fat (Meyron-Holtz et al., 2004). Recent studies have demonstrated that targeting IRP binding activity using pharmacologic or genetic approaches increases ferritin expression in HeLa and C6 glioma cells, and reduces the vulnerability of these cells to oxidative injury (Festa et al., 2000; Wang et al., 2007). To date, this approach has not been tested on neurons, which are rather inefficient inducers of ferritin after iron or hemoglobin exposure (Bishop and Robinson, 2001; Wu et al., 2003; Koeppen et al., 2004, also unpublished observations). It therefore remains to be determined if reducing IRP binding activity would increase neuronal ferritin sufficiently to provide a meaningful protective effect. In the present study, the therapeutic potential of targeting this regulatory pathway was examined in an established cell culture model of hemoglobin neurotoxicity. Specifically, we tested the hypotheses that ferritin expression would be significantly increased in IRP2 knockout cortical cultures, and that knockout neurons would be less vulnerable to hemoglobin than their wild-type counterparts.
Materials and Methods
Cell Culture Preparation
Founding pairs of IRP2 knockout mice (Ireb2 −/−, C57BL6/129 strain, LaVaute et al., 2001) were kindly provided by Rouault and colleagues and were bred with wild-type mice of the same strain that are maintained in our colony. All mice subsequently used for breeding were first or second generation offspring of mice heterozygous at the IRP2 locus, and were less than one year old. Genotype was determined by PCR of genomic DNA obtained from tail clippings, using the following primer sets: IRP2A: 5' - TGT TCC TGT CAG TCC TCG TG - 3'; IRP2B: 5' - GGC CAG ACT GGT CTT CAG AG - 3'; Neo 1 insert: 5' - GAT CTC CTG TCA TCT CAC CT-3'; Neo 2 insert: 5' - TCA GAA GAA CTC GTC AAG AA-3'. Reactions to detect the presence of the knockout and wild-type genes were run separately.
Mixed cortical cell cultures containing both neurons and astrocytes were prepared from IRP2 knockout or wild-type mice as previously described in detail (Regan and Choi, 1994). Plating medium contained Eagle's minimal essential medium (MEM, Gibco/Invitrogen, Grand Island, NY, USA, Product No.11430), 5% heat inactivated fetal bovine serum (Hyclone, Logan, UT, USA), 5% heat inactivated equine serum (Hyclone), glutamine (2mM), and glucose (23mM). Astrocyte cultures were prepared in a similar fashion from 1–3 day postnatal mice, using plating medium containing MEM supplemented with 10% equine serum, 10% fetal bovine serum, 2 mM glutamine, and 10 ng/ml epidermal growth factor (Sigma Aldrich, St. Louis, MO, USA, Product No. E4127). Pure neuronal cultures were plated on 24 well plates coated with 50 µg/ml poly-D-lysine, in Neurobasal medium (Gibco/Invitrogen) containing B27 supplement without antioxidants (Gibco/Invitrogen, Product No. 10889) and 1 mM glutamine. This serum-free medium supports the growth of neurons but not other cell types, resulting in cultures that are largely free of glial cells (Brewer, 1995). The viability of IRP2 knockout neurons in mixed or pure neuron cultures was similar to that of wild-type cells.
RT-PCR
Brain total RNA was isolated using TriZol reagent (Invitrogen, Carlsbad, CA), following the manufacturer’s instructions. Complementary DNA was then synthesized from 5µg total RNA (SuperScript TM III Reverse Transcriptase Kit, Invitrogen, Oligo(dT) primers). The presence of the wild-type gene was then confirmed using the following primer pair that spans the neomycin gene insertion site: Forward 5' - TCC GAC AGA TCT CAC AGT GG -3'; Reverse 5' -TGA GTT CCG GCT TAG CTC TC -3'. Control primers detecting glyceraldehyde 3-phosphate dehydrogenase had the following sequences: Forward 5'- ACCACAGTCCATGCCATCAC -3'; Reverse 5'- TCCACCACCCTGTTGCTGTA-3'.
Hemoglobin Exposure
Purified, endotoxin-free human hemoglobin A was provided as a gift from Hemosol, Inc. (Toronto, Ontario, Canada), and was stored in aliquots at −70°C until used. Astrocyte and mixed neuron/astrocyte cultures were exposed to hemoglobin in serum-free MEM containing 10mM glucose (MEM10). Pure neuron cultures were exposed to hemoglobin in Neurobasal/B27 medium without antioxidants, since these cultures do not tolerate MEM10 per se. All exposures were conducted at 37°C in a 5% CO2 atmosphere. Hemoglobin is expressed as the molar concentration of the tetramer.
Assessment of Injury
Cell death was quantified by measurement of lactate dehydrogenase (LDH) activity in the culture medium, which is an accurate marker of both necrotic and apoptotic death in this culture system (Koh and Choi, 1988; Gwag et al., 1995). Stock solutions of 100 mM potassium phosphate buffer (pH 7.4) and 27.2 mM sodium pyruvate in potassium phosphate buffer were prepared in advance and stored at 4°C until used. Medium samples (25 µl) were removed from each culture and were placed into a 96 well assay plate. Ten minutes after addition of 125 µl phosphate buffer and 100 µl of a fresh solution of NADH (0.3 mg/ml in phosphate buffer) into each well, 25 µl sodium pyruvate was rapidly added using an Eppendorf repeater pipette with 8-well adapter. The absorbance of the reaction mixture at 340 nm was determined at 9 second intervals for 2 minutes, using a kinetic plate reader (Molecular Devices, Sunnyvale, CA). The linearity of the assay under the experimental conditions was verified by testing sequential dilutions of an LDH control solution (Sigma). In order to control for variability in LDH content in cultures prepared in different platings or at different days in vitro, LDH values were scaled to those in sister cultures treated concomitantly with 300 µM NMDA, which releases essentially all neuronal LDH in mixed cultures without injuring astrocytes (Koh and Choi, 1988). In experiments using pure astrocyte cultures, LDH values were scaled to those in cultures treated with 0.1% Triton X-100, which rapidly lyses all cells. In all experiments, the LDH activity in sister cultures subjected to medium exchange (sham wash) only was subtracted from all values to quantify the signal specific to hemoglobin, ferrous sulfate or NMDA neurotoxicity.
Reactive oxygen species (ROS) were quantified by staining with dihydrorhodamine 123 (DHR, Molecular Probes/Invitrogen), which is a non-fluorescent compound that is oxidized within cells to the fluorescent dye rhodamine 123 (Royall and Ischiropoulos, 1993). Cultures were washed free of hemoglobin prior to staining, in order to prevent direct DHR oxidation by any hemin that may be present in the medium (Ohashi et al., 2002). After incubation for 15 minutes with 20 µM DHR in MEM10, the medium was replaced with a HEPES-buffered salt solution containing (in mM): NaCl, 120; KCl, 5.4; MgCl2, 0.8; CaCl2, 1.8; HEPES, 20; glucose, 5.5, (pH 7.4). A single 100X field at the center of each well was captured immediately after illumination (25 msec exposure) in order to minimize photo-oxidation by the high-intensity light source. Images were analyzed with IPLab software (Scanalytics, Inc., Fairfax, VA). Background fluorescence from cultures subjected to sham wash and dye incubation only were subtracted from each recording to define the signal due to hemoglobin exposure.
Protein oxidation was detected by assaying for carbonyl groups, using a kit (Oxyblot, Chemicon/Millipore, Billerica, MA) and following the manufacturer's instructions. Culture medium was aspirated, and cultures were then washed once with 1ml MEM10. After aspiration, 100 µl ice-cold lysis buffer (210 mM mannitol, 70 mM sucrose, 5 mM HEPES, 1 mM EDTA, 0.1 % sodium dodecyl sulfate, 0.1 % Triton X-100) was added. Wells were gently scraped, and the lysate was collected, sonicated on ice, and centrifuged. A supernatant sample was then removed for protein assay (BCA method, Pierce Biotechnology, Rockford, IL); 2-mercaptoethanol (final concentration 1%) was added to the remainder to prevent further oxidation. After derivitization of carbonyl groups by treatment with 2, 4-dinitrophenylhydrazine, proteins were separated on 12% SDS-PAGE gels and were transferred to a polyvinylidene difluoride (PVDF) transfer membrane (Immobilon-P, Millipore). Membranes were then treated with anti-dinitrophenyl primary antibody (1:150) followed by goat anti-rabbit IgG (1:300). Immunoreactive proteins were visualized using Super Signal West Femto Reagent (Pierce, Rockford, IL) and Kodak Gel Logic 2200.
Immunoblotting
Cells were washed and lysed as described above for protein oxidation assay. Protein samples (20 µg in 30 µL) were then diluted with 10 µL 4X loading buffer (Tris-Cl 240 mmol/L, β-mercaptoethanol 20%, sodium dodecyl sulfate 8%, glycerol 40%, and bromophenol blue 0.2%) and heated to 95°C for 3–5 minutes. Proteins were separated on 12% (for detecting ferritin and heme oxygenase-1) or 7.5% (for detecting IRP2) SDS-PAGE gels (Ready Gel, Bio-Rad), and were then transferred to a PVDF transfer membrane. After washing, nonspecific sites were blocked with 5% nonfat dry milk in a buffer containing 20 mM Tris, 500 mM NaCl, and 0.1% Tween 20 (pH 7.5) for 1 h at 37°C. Membranes were incubated at 4°C overnight with one of the following primary antibodies: 1) goat anti-L-ferritin, Santa Cruz Biotechnology, Santa Cruz, CA, USA, Product No. SC-14420, 1:200 dilution; 2) rabbit anti-H-ferritin, gift of Dr. James Connor, Pennsylvania State University, 1:10,000; 3) rabbit anti-IRP2, Novus Biologicals, Littleton, CO, USA, Product No. NB100-1798, 1:500; 4) rabbit anti-HO-1, Assay Designs, Ann Arbor, MI, Product No. SPA-895, 1:5000; 5) rabbit anti-horse spleen ferritin, Sigma Aldrich, Product No. F5762, 1:250; 6) rabbit anti-actin (gel loading control), Sigma Aldrich Product No. A2066. 1:400. After treating membranes for one hour with appropriate secondary antibody (Pierce goat anti-rabbit IgG-HRP, Product # 1858415 or Santa Cruz Biotechnology donkey anti-goat IgG-HRP, Product # SC-2020. 1:3000), immunoreactive proteins were visualized as described above.
Statistical Analysis
Data were analyzed with one-way analysis of variance. Differences between groups were assessed with the Bonferroni multiple comparisons test.
Results
Genotyping
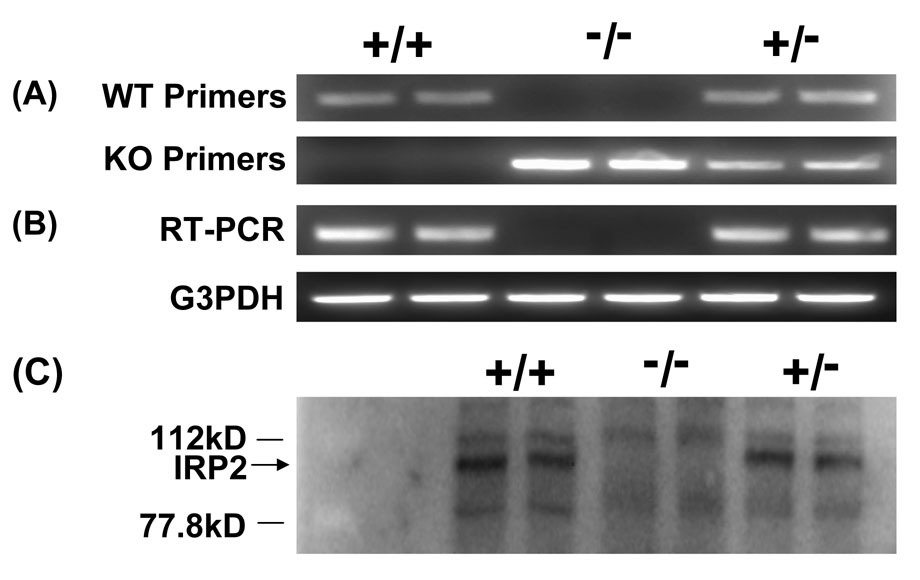
PCR genotyping demonstrated an approximately 500 bp product for the IRP2 KO gene and an approximately 250 bp product for the wild-type (Fig. 1A). Absence of wild-type mRNA expression in mice identified as knockouts by this method was demonstrated by RT-PCR (Fig. 1B). Immunoblotting confirmed the presence of IRP2 in mice identified as wild-type, and its absence in homozygous knockouts (Fig. 1C).
Figure 1.
A) Representative 2% agarose gels showing PCR products from wild-type (+/+), IRP2 knockout (−/−), and heterozygous (+/−) mice. B) Representative 2% agarose gels showing RT-PCR products using IRP2 or glyceraldehyde 3-phosphate dehydrogenase (G3PDH) control primers. C) immunoblot stained with anti-IRP2.
Effect of IRP2 gene deletion on ferritin expression in neurons and astrocytes
The ferritin level in mixed neuron/astrocyte knockout cultures subjected to medium exchange (sham wash) only was 21-fold higher than that in wild-type cultures, when quantitatively assessed with a polyclonal antibody raised against horse spleen ferritin (Fig. 2). Hemoglobin treatment increased cell ferritin in both WT and KO cultures; relative expression remained significantly greater in knockouts at all hemoglobin concentrations tested. Although murine H and L-ferritin migrate separately on SDS-PAGE gels (Beaumont et al., 1989), only a single ferritin band with an estimated molecular weight of approximately 21 kDa was detected with this antibody.
Figure 2.
Increased ferritin expression in mixed neuron/astrocyte IRP2 knockout (KO) cultures. Representative immunoblots from mixed neuron-astrocyte cultures treated with indicated hemoglobin (Hb) concentrations (µM) for 24 h, or subjected to medium exchange (SW, sham-wash) only, stained with anti-horse spleen ferritin (Ferritin), anti-H-ferritin, or anti-L-ferritin. Bars represent mean band densities (± S.E.M., n = 5/condition) from immunoblots stained with anti-horse spleen ferritin, scaled to that in wild type (WT) sham-washed cultures (= 1). *P < 0.05, ***P < 0.001 v. corresponding WT value, Bonferroni multiple comparisons test.
Additional experiments were conducted using primary antibodies specific for either H or L-ferritin. In mixed cultures, immunoblots stained with these antibodies were similar to those stained with anti-horse spleen ferritin (Fig. 2). In wild-type pure neuronal cultures, H-ferritin was barely detectable after sham wash only (Fig. 3A). From this very low level, it was increased by 3.7-fold in IRP2 knockouts, and by approximately 9.3-fold in IRP2 knockouts treated with hemoglobin. In astrocyte cultures, H-ferritin was easily detectable in sham-washed wild-type cells and was increased sixfold in knockouts, with further increases observed in cultures treated with hemoglobin. In pure neuron cultures, L-ferritin was expressed by sham-washed wild-type cells and was increased threefold by IRP2 gene deletion (Fig. 3B). L-ferritin was expressed at a low level by WT sham-washed astrocytes, but was robustly increased by IRP2 knockout and/or hemoglobin treatment.
Figure 3.
IRP2 gene deletion increases ferritin subunit expression in neurons and astrocytes. Cultures containing either neurons or astrocytes were treated with 10 µM hemoglobin for 6 h before lysis, or were subjected to sham-wash (SW); immunoblots were stained with antibody to the ferritin H (Fig. 3A) or L (Fig 3B) subunits. Bars represent mean band densities, scaled to the density in wild-type sham-washed astrocyte cultures (= 1.0), of immunoblots from 6–8 cultures/condition. *P < 0.05, ***P < 0.001 v. corresponding WT value, Bonferroni multiple comparisons test.
IRP2 knockout neurons are less vulnerable to hemoglobin
Cytotoxicity experiments were conducted on mixed neuron-astrocyte cultures, since primary neurons in astrocyte-free cultures require Neurobasal Medium with B27 supplement for survival, and components of the latter protect against hemoglobin neurotoxicity. Wild-type cultures treated with low micromolar concentrations of hemoglobin sustained widespread neuronal death, without apparent injury to the astrocyte monolayer. The LC50 for neuronal death, as quantified by LDH release assay, was near 3 µM (Fig. 4). The same exposure produced significantly less injury in IRP2 knockout cultures. A protective effect was also observed in cultures containing knockout neurons plated on a monolayer of wild-type astrocytes. As previously reported (Regan and Panter, 1993; Chen-Roetling and Regan, 2006), astrocytes were much less sensitive to hemoglobin than neurons. Wild-type astrocyte cultures treated with 3 µM hemoglobin released 5.7±1.6% of culture LDH by 24h, while IRP2 knockouts released 2.8±2.7% (P > 0.05, WT v. KO and both v. sham-wash).
Figure 4.
IRP2 knockout neurons are less vulnerable to hemoglobin. Bars represent mean culture medium LDH (± S.E.M.) in mixed neuron/astrocyte cultures treated with indicated concentrations of hemoglobin for 24 hours. LDH values are expressed as a percentage of the mean value in sister cultures exposed to 300 µM NMDA (=100), which releases essentially all neuronal LDH without injuring astrocytes. The LDH activity in sister cultures subjected to medium exchange (sham wash) only was subtracted from all values to quantify the signal specific to hemoglobin or NMDA neurotoxicity. *P < 0.05, ***P < 0.001 v. corresponding WT value, Bonferroni multiple comparisons test, n = 7–15/condition.
ROS levels are reduced in IRP2 knockout cultures
Consistent with prior observations (Rogers et al., 2003), fluorescence intensity was increased in mixed wild-type cultures treated with hemoglobin for 4–6 hours and then stained with the oxidation-sensitive dye dihydrorhodamine 123 (Fig. 5). This fluorescence was observed predominantly in cells with elliptical or round cell bodies and extensive processes that aggregated in groups, which is the typical appearance of primary cultured neurons. It was minimal in the background astrocyte monolayer. Fluorescence intensity in IRP2 knockout cultures treated with hemoglobin was markedly less than that in wild-type cultures. Quantification of ROS levels after hemoglobin exposures longer than six hours is not feasible with this method, since membrane disruption in wild-type cultures after this time point reduces dye retention and the fluorescent signal.
Figure 5.
Reduced ROS formation after hemoglobin treatment in IRP2 knockout cultures. Fluorescence photomicrographs of mixed wild type (WT) and IRP2 knockout (KO) cultures treated with 10 µM hemoglobin for 6 hours, then stained with dihydrorhodamine 123, which fluoresces when oxidized. KO photomicrograph on right is same field as left KO photomicrograph, but image brightness and contrast have been increased to outline the area covered by neurons, which have a baseline level of fluorescence that exceeds that of astrocytes. Scale bar = 100 µm. Bar chart represents mean fluorescence intensity ± S.E.M. ***P < 0.001 v. corresponding wild-type value, Bonferroni multiple comparisons test, n = 7–8/condition.
IRP2 KO reduces protein oxidation and heme oxygenase (HO)-1 expression
Protein carbonylation is a sensitive marker of heme-mediated oxidative injury in this culture system (Regan et al., 2004). In mixed wild-type cultures, the carbonyl signal was increased approximately fourfold by 24 hour treatment with 3 or 10 µM hemoglobin. It was significantly less at both concentrations in cultures prepared from IRP2 knockout mice (Fig. 6).
Figure 6.
Protein oxidation after hemoglobin treatment is attenuated by IRP2 gene deletion. Immunoblot of proteins from mixed wild type (WT) and IRP2 knockout (KO) cultures treated with 1, 3, or 10 µM hemoglobin (Hb) for 24 h, or subjected to medium exchange (SW, sham wash) only, stained with antibody to derivatized carbonyl groups. Bar chart represents mean lane densities after background subtraction. ***P < 0.001 v. mean signal in KO cultures exposed to same hemoglobin concentrations, n = 5/condition.
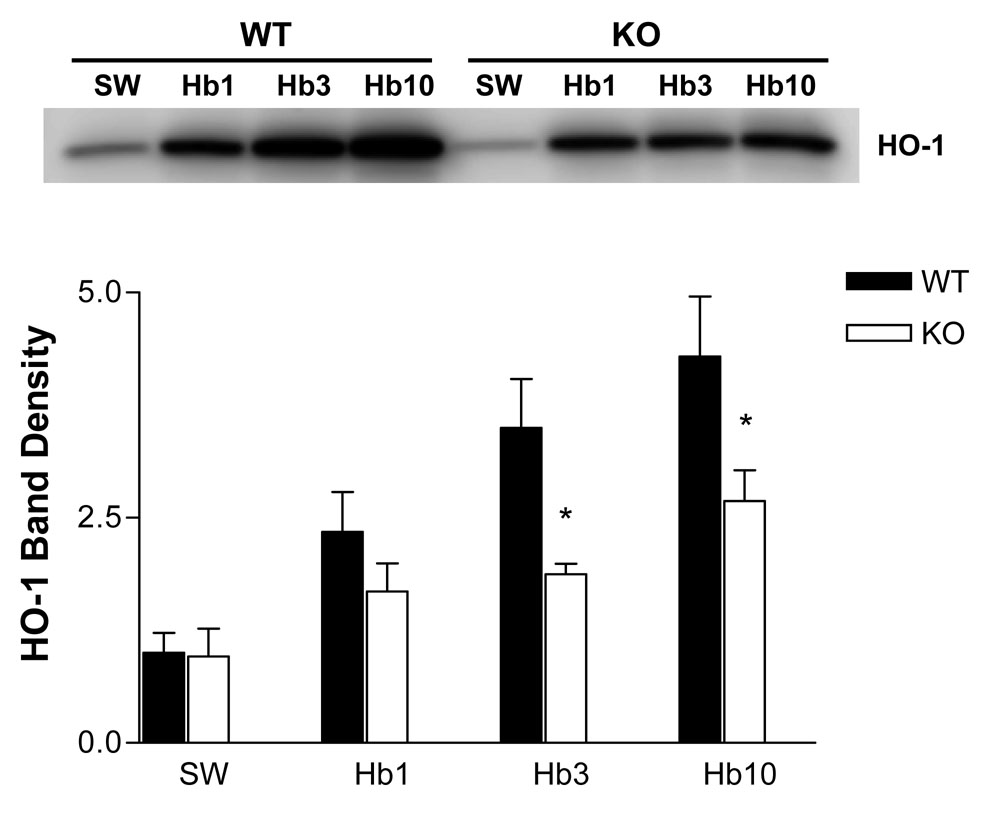
The HO-1 gene has an antioxidant response element in its promoter region, and is rapidly induced by oxidative stress in most cell types. Its induction is an early marker of CNS oxidative injury due to hemoglobin or excitotoxins (Muñoz et al., 2005; Qu et al., 2005). HO-1 protein levels were significantly higher in mixed wild-type cultures treated with 3–10 µM hemoglobin for 24 hours than in IRP2 knockout cultures, consistent with a higher level of oxidative stress in the former (Fig. 7).
Figure 7.
HO-1 induction is attenuated in IRP2 knockout mice. Immunoblot demonstrating HO-1 expression in mixed WT and IRP2 KO cultures treated with indicated hemoglobin concentrations for 24 hours. Cultures in sham wash (SW) condition were subjected to medium exchange only. Bars represent mean band densities (n = 4/condition). *P < 0.05 v. corresponding knockout condition, Bonferroni multiple comparisons test.
IRP2 knockout neurons are less sensitive to iron
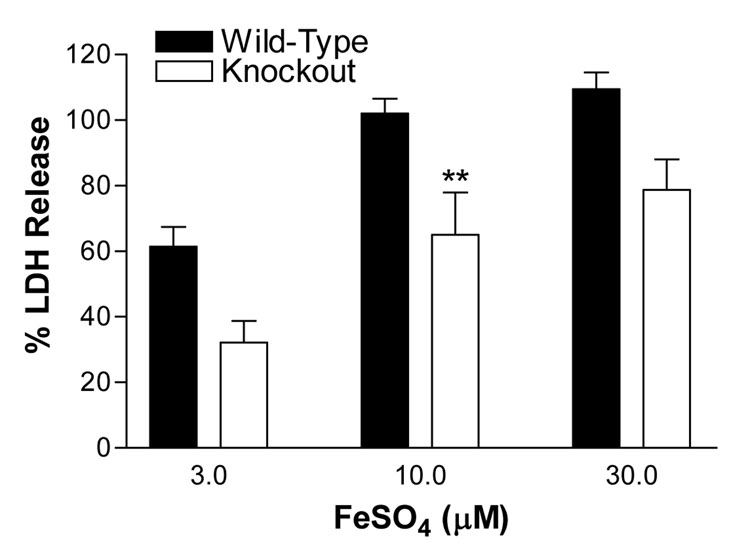
We have previously reported that wild-type neurons in mixed neuron-astrocyte cultures were very sensitive to low micromolar concentrations of ferrous sulfate, while the feeder astrocyte monolayer was not injured after a 24h exposure (Rogers et al., 2003; Chen and Regan, 2004). Treating mixed wild-type cultures with 10–30 µM ferrous sulfate resulted in release of all neuronal LDH. Less cell death was observed in IRP2 knockout mixed cultures, but the difference reached statistical significance only at 10 µM (Fig. 8).
Figure 8.
IRP2 knockout neurons are less sensitive to ferrous sulfate. Cultures were treated with indicated concentrations of ferrous sulfate for 24 h. LDH values are expressed as a percentage of the mean value in sister cultures exposed to 300 µM NMDA (=100), which releases essentially all neuronal LDH without injuring astrocytes. The LDH activity in sister cultures subjected to medium exchange (sham wash) only was subtracted from all values to quantify the signal specific to ferrous sulfate or NMDA neurotoxicity. **P < 0.01 v. value in corresponding wild-type condition, Bonferroni multiple comparisons test.
Discussion
The results of this study suggest the following: 1) Ferritin levels are negatively regulated by IRP2 in cultured cortical neurons and astrocytes, resulting in significantly increased expression of this cytoprotective protein in IRP2 knockout cells; 2) Cultured cortical astrocytes express more H-ferritin at baseline and after hemoglobin treatment than cortical neurons; 3) neurons in mixed neuron/astrocyte IRP2 knockout cultures are highly resistant to the toxicity of hemoglobin; since this resistance is also observed in cultures containing knockout neurons on a monolayer of wild-type astrocytes, it is likely mediated at least in part by an increase in neuronal ferritin.
In vivo, IRP2 knockout increases ferritin in the forebrain and cerebellum, while IRP1 knockout per se has no apparent effect (Meyron-Holtz et al., 2004). The present results suggest a similar regulatory mechanism in this culture system, although the precise role of IRP1 will be determined in future studies using these mutants. It is noteworthy that IRP2 knockout cells responded to hemoglobin with a further increase in ferritin, which may indicate a regulatory role for IRP1 in the absence of IRP2, as recently reported after IRP2 knockdown in HeLa cells (Wang et al., 2007). Alternatively, transcriptional regulation may account for this increase, since ferritin genes contain antioxidant response element sequences in their promoter regions that are positively regulated by hemoglobin, hemin or protoporphyrin IX, but are relatively insensitive to iron (Hintze and Theil, 2005; Iwasaki et al., 2006).
The observation that cultured cortical neurons express L-ferritin is consistent with observations by Malecki et al. (2002) in the 12 week-old mouse brain. The minimal H-ferritin expression in wild-type neurons differs from results of Cheepsunthorn et al. (1998), who noted H-ferritin immunoreactivity in the nuclei of neurons throughout the cerebral cortex of postnatal rats, and from those of Malecki et al. (2002), who observed H-ferritin immunoreactivity in some cortical neuronal nuclei in 12 week BALB/cj mice. A number of factors may contribute to this discrepancy, including the younger age of tissue samples used to prepare these cultures (gestational age 15–16 days), strain differences, and expression changes associated with tissue dissociation and culture preparation. It should also be noted that some variability in ferritin subunit expression in the intact rodent brain has been reported to date. In ten week-old rats, Hansen et al. observed ferritin immunoreactivity, as detected with a polyclonal antibody raised to liver ferritin, mainly in glial cells; neuronal expression was largely limited to the globus pallidus, medial habenular nucleus, and interpedunculate nucleus, and was seen only in the cytoplasm (Hansen et al., 1999; Moos and Morgan, 2004). In adult rats, Wu et al. (2003) observed increased expression of both H and L ferritin in the basal ganglia after experimental intracerebral hemorrhage. However, induction was predominantly in astrocytes and microglia, rather than in neurons. Further definition of ferritin subunit expression in the developing and adult brain at baseline and after injury seems warranted.
The greater expression of H-ferritin by cultured astrocytes may contribute to the resistance of this cell population to the toxic effects of hemoglobin and iron (Regan and Panter, 1993; Kress et al., 2002). Although the relationship between the subunit composition of ferritin and its effect on oxidative injury has not been intensively investigated, evidence to date suggests that H-rich ferritin is a more potent antioxidant than L-rich ferritin (Arosio and Levi, 2002). H-ferritin knockdown markedly increased HeLa cell vulnerability to hydrogen peroxide, while upregulation was protective; modulation of L-ferritin levels had no significant effect (Cozzi et al., 2004). Similar potentiation of oxidative injury was demonstrated by H-ferritin knockdown in human HL-60 cells (Lin and Girotti, 1998), while pretreatment with recombinant H-ferritin protected endothelial cells from hemin-catalyzed hydrogen peroxide toxicity (Balla et al., 1992). One key functional difference between H and L-ferritin is the ferroxidase activity of the former, which oxidizes ferrous to ferric iron. Since only ferric iron can be deposited in the ferritin nanocage (Levi et al., 1992), and since breakdown of heme or hemin by the heme oxygenases releases ferrous iron (Yoshida and Migita, 2000), adequate ferroxidase activity may be particularly essential to protect cells from supraphysiologic heme concentrations. Consistent with this hypothesis, Balla et al. (1992) observed that the cytoprotection provided by recombinant H-ferritin against hemin/H2O2 was lost when its ferroxidase activity was deleted by site-directed mutagenesis.
These results provide additional evidence that wild-type neurons are vulnerable to low concentrations of hemoglobin primarily due to their limited capacity to sequester and detoxify iron. Prior studies demonstrated that the oxidative neurotoxicity of hemoglobin is mediated by iron release, since it could be effectively prevented by iron-chelating antioxidants in cell culture and in vivo (Regan and Rogers, 2003; Song et al., 2007). The beneficial effect of IRP2 gene deletion suggests that increasing ferritin has a similar effect. Putative mechanisms that mediate the cytotoxicity of chelatable iron in vitro include generation of highly-reactive hydroxyl radicals via the Fenton reaction, and initiation of lipid peroxidation chain reactions by direct decomposition of membrane peroxides (Halliwell and Gutteridge, 1999; Kakhlon and Cabantchik, 2002). The relevance of IRP2 binding activity to hemorrhagic CNS injuries remains to be determined. However, iron deposition and ferritin hyper-expression are prominent histological features in tissue surrounding an experimental intracerebral hemorrhage, and are consistent with loss of cellular iron homeostasis (Koeppen et al., 1995; Wu et al., 2003). The protective effect of iron chelators in vivo (Huang et al., 2002; Nakamura et al., 2004; Masuda et al., 2007) suggests that iron toxicity contributes to tissue injury, and that IRP2 gene deletion may also confer benefit.
IRP2 gene knockout was considerably more protective in cultures treated with hemoglobin than in those treated with ferrous sulfate, which likely reflects a greater contribution of intracellular iron to the injury produced by the former. Prior studies suggest that the iron-dependent neurotoxicity of hemoglobin is accelerated by intracellular breakdown of heme by the heme oxygenases, particularly heme oxygenase-2, the predominant neuronal isoform (Rogers et al., 2003). While iron transport across the cell membrane may also contribute to the toxicity of iron salts, low molecular weight iron has a very high affinity for cell membrane phospholipids, and once bound can catalyze lipid peroxidation chain reactions in this cell compartment, which is less accessible to cytosolic ferritin (Browne et al., 1998; Tampo, 2000).
The inverse relationship between ferritin expression and neuronal vulnerability to heme-mediated injury complements observations in other studies that modulated ferritin levels by hemin preconditioning or exogenous apoferritin treatment (Balla et al., 1992; Regan et al., 2002). Although the present results provide additional evidence that ferritin is a key intracellular antioxidant, the nonspecificity of an IRP2 knockout approach is acknowledged. In addition to its effects on H and L-ferritin translation, IRP2 regulates expression of transferrin receptor (TfR)-1, ferroportin, divalent metal transporter-1 (DMT-1), and perhaps other CNS proteins. Since cells were exposed to hemoglobin in transferrin-free culture medium, it is unlikely that differences in TfR-1 expression directly contributed to reduced cell injury in knockout cultures. The effects of ferroportin and DMT-1 in this and other oxidative injury models remain undefined, and their altered expression may have mitigated oxidative stress after hemoglobin treatment. It is also possible that IRP2 gene deletion produced constitutive changes in iron transport and storage that reduced the labile iron pool, leading to a lower basal level of oxidative stress and a larger reserve of endogenous antioxidants than in wild-type cells. The very similar protein carbonyl signal in sham-washed knockout and wild-type cultures does not support this hypothesis; however, an effect on other oxidative injury markers cannot be excluded.
Regardless of the precise mechanisms that protect IRP2 knockout neurons, these results suggest that IRP2 binding activity may be a novel therapeutic target after hemorrhagic CNS injuries. Wang et al. (2007) have recently reported the successful knockdown of IRP2 in cultured HeLa cells with small interfering RNA, resulting in increased ferritin levels and reduced vulnerability to hydrogen peroxide. Alternatively, pharmacologic inhibition of IRP2 binding activity may be feasible. Festa et al. (2000) reported that the aconitase inhibitor oxalamalate reduced both IRP1 and IRP2 binding activities in cell culture and in vivo. This effect was sufficient to increase ferritin two- to threefold in SH-SY5Y and C6 glioma cells, and to reduce levels of ROS and malondialdehyde after ferric ammonium citrate treatment (Santamaria et al., 2004). Further exploration of these and related therapeutic strategies in hemorrhagic stroke models is warranted.
Acknowledgements
This study was supported by a grant from the National Institutes of Health (NS042273) to R.F.R, and by a postdoctoral fellowship from the Pennsylvania/Delaware affiliate of the American Heart Association to L.B.Z. The authors thank Dr. Tracey Rouault for providing the IRP2 knockout mice that were used to establish our colony and Dr. James Connor for providing anti-H-ferritin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arosio P, Levi S. Ferritin, iron homeostasis, and oxidative damage. Free Radic Biol Med. 2002;33:457–463. doi: 10.1016/s0891-5849(02)00842-0. [DOI] [PubMed] [Google Scholar]
- Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, Eaton JW, Vercellotti GM. Ferritin: A cytoprotective strategem of endothelium. J. Biol. Chem. 1992;267:18148–18153. [PubMed] [Google Scholar]
- Beaumont C, Dugast I, Renaudie F, Souroujon M, Grandchamp B. Transcriptional regulation of ferritin H and L subunits in adult erythroid and liver cells from the mouse. Unambiguous identification of mouse ferritin subunits and in vitro formation of ferritin shells. J. Biol. Chem. 1989;264:7498–7504. [PubMed] [Google Scholar]
- Bishop GM, Robinson SR. Quantitative analysis of cell death and ferritin expression in response to cortical iron: implications for hypoxia-ischemia and stroke. Brain Res. 2001;907:175–187. doi: 10.1016/s0006-8993(01)02303-4. [DOI] [PubMed] [Google Scholar]
- Brewer GJ. Serum-free B27/neurobasal medium supports differentiated growth of neurons from the striatum, substantia nigra, septum, cerebral cortex, cerebellum, and dentate gyrus. J Neurosci Res. 1995;42:674–683. doi: 10.1002/jnr.490420510. [DOI] [PubMed] [Google Scholar]
- Browne P, Shalev O, Hebbel RP. The molecular pathobiology of cell membrane iron: the sickle red cell as a model. Free Radic Biol Med. 1998;24:1040–1048. doi: 10.1016/s0891-5849(97)00391-2. [DOI] [PubMed] [Google Scholar]
- Cheepsunthorn P, Palmer C, Connor JR. Cellular distribution of ferritin subunits in postnatal rat brain. J. Comp. Neurol. 1998;400:73–86. [PubMed] [Google Scholar]
- Chen J, Regan RF. Heme oxygenase-2 gene deletion increases astrocyte vulnerability to hemin. Biochem. Biophys. Res. Commun. 2004;318:88–94. doi: 10.1016/j.bbrc.2004.03.187. [DOI] [PubMed] [Google Scholar]
- Chen-Roetling J, Regan RF. Effect of heme oxygenase-1 on the vulnerability of astrocytes and neurons to hemoglobin. Biochem Biophys Res Commun. 2006;350:233–237. doi: 10.1016/j.bbrc.2006.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzi A, Corsi B, Levi S, Santambrogio P, Biasiotto G, Arosio P. Analysis of the biologic functions of H- and L-ferritins in HeLa cells by transfection with siRNAs and cDNAs: evidence for a proliferative role of L-ferritin. Blood. 2004;103:2377–2383. doi: 10.1182/blood-2003-06-1842. [DOI] [PubMed] [Google Scholar]
- Festa M, Colonna A, Pietropaolo C, Ruffo A. Oxalomalate, a competitive inhibitor of aconitase, modulates the RNA-binding activity of iron-regulatory proteins. Biochem J. 2000;348(Pt 2):315–320. [PMC free article] [PubMed] [Google Scholar]
- Goldstein L, Teng ZP, Zeserson E, Patel M, Regan RF. Hemin induces an iron-dependent, oxidative injury on human neuron-like cells. J. Neurosci. Res. 2003;73:113–121. doi: 10.1002/jnr.10633. [DOI] [PubMed] [Google Scholar]
- Gutteridge JMC. Iron promoters of the Fenton reaction and lipid peroxidation can be released from haemoglobin by peroxides. FEBS Letters. 1986;201:291–295. doi: 10.1016/0014-5793(86)80626-3. [DOI] [PubMed] [Google Scholar]
- Gwag BJ, Lobner D, Koh JY, Wie MB, Choi DW. Blockade of glutamate receptors unmasks neuronal apoptosis after oxygen-glucose deprivation in vitro. Neuroscience. 1995;68:615–619. doi: 10.1016/0306-4522(95)00232-8. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford: Oxford University Press; 1999. p. 396. [Google Scholar]
- Hansen TM, Nielsen H, Bernth N, Moos T. Expression of ferritin protein and subunit mRNAs in normal and iron deficient rat brain. Brain Res Mol Brain Res. 1999;65:186–197. doi: 10.1016/s0169-328x(99)00011-x. [DOI] [PubMed] [Google Scholar]
- Harrison PM, Arosio P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta. 1996;1275:161–203. doi: 10.1016/0005-2728(96)00022-9. [DOI] [PubMed] [Google Scholar]
- Hintze KJ, Theil EC. DNA and mRNA elements with complementary responses to hemin, antioxidant inducers, and iron control ferritin-L expression. Proc Natl Acad Sci U S A. 2005;102:15048–15052. doi: 10.1073/pnas.0505148102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Y, Nakamura T, Keep RF, Wu J, Schallert T, Hoff JT, Xi G. Long-term effects of experimental intracerebral hemorrhage: the role of iron. J Neurosurg. 2006;104:305–312. doi: 10.3171/jns.2006.104.2.305. [DOI] [PubMed] [Google Scholar]
- Huang FP, Xi G, Keep RF, Hua Y, Nemoianu A, Hoff JT. Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. J. Neurosurg. 2002;96:287–293. doi: 10.3171/jns.2002.96.2.0287. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Mackenzie EL, Hailemariam K, Sakamoto K, Tsuji Y. Hemin-mediated regulation of an antioxidant-responsive element of the human ferritin H gene and role of Ref-1 during erythroid differentiation of K562 cells. Mol Cell Biol. 2006;26:2845–2856. doi: 10.1128/MCB.26.7.2845-2856.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakhlon O, Cabantchik ZI. The labile iron pool: characterization, measurement, and participation in cellular processes(1) Free Radic Biol Med. 2002;33:1037–1046. doi: 10.1016/s0891-5849(02)01006-7. [DOI] [PubMed] [Google Scholar]
- Koeppen AH, Dickson AC, McEvoy JA. The cellular reactions to experimental intracerebral hemorrhage. J Neurol Sci. 1995;134 Suppl:102–112. doi: 10.1016/0022-510x(95)00215-n. [DOI] [PubMed] [Google Scholar]
- Koeppen AH, Dickson AC, Smith J. Heme oxygenase in experimental intracerebral hemorrhage: the benefit of tin-mesoporphyrin. J. Neuropathol. Exp. Neurol. 2004;63:587–597. doi: 10.1093/jnen/63.6.587. [DOI] [PubMed] [Google Scholar]
- Koh JY, Choi DW. Vulnerability of cultured cortical neurons to damage by excitotoxins: Differential susceptibility of neurons containing NADPH-diaphorase. J. Neurosci. 1988;8:2153–2163. doi: 10.1523/JNEUROSCI.08-06-02153.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress GJ, Dineley KE, Reynolds IJ. The relationship between intracellular free iron and cell injury in cultured neurons, astrocytes, and oligodendrocytes. J Neurosci. 2002;22:5848–5855. doi: 10.1523/JNEUROSCI.22-14-05848.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner JP, Porter JP, Olivieri NF. Secondary Iron Overload. Hematology. 2001;2001:47–61. doi: 10.1182/asheducation-2001.1.47. [DOI] [PubMed] [Google Scholar]
- Lamb NJ, Quinlan GJ, Mumby S, Evans TW, Gutteridge JMC. Haem oxygenase shows pro-oxidant activity in microsomal and cellular systems: implications for the release of low-molecular-mass iron. Biochem. J. 1999;344:153–158. [PMC free article] [PubMed] [Google Scholar]
- LaVaute T, Smith S, Cooperman S, Iwai K, Land W, Meyron-Holtz E, Drake SK, Miller G, Abu-Asab M, Tsokos M, Switzer R, 3rd, Grinberg A, Love P, Tresser N, Rouault TA. Targeted deletion of the gene encoding iron regulatory protein-2 causes misregulation of iron metabolism and neurodegenerative disease in mice. Nat Genet. 2001;27:209–214. doi: 10.1038/84859. [DOI] [PubMed] [Google Scholar]
- Levere RD, Escalante B, Schwartzman ML, Abraham NG. Role of heme oxygenase in heme-mediated inhibition of rat brain Na+-K+ -ATPase: protection by tin protoporphyrin. Neurochem. Res. 1989;14:861–864. doi: 10.1007/BF00964815. [DOI] [PubMed] [Google Scholar]
- Levi S, Yewdall SJ, Harrison PM, Santambrogio P, Cozzi A, Rovida E, Albertini A, Arosio P. Evidence of H- and L- chains have cooperative roles in the iron-uptake mechanism of human ferritin. Biochem. J. 1992;288:591–596. doi: 10.1042/bj2880591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Girotti AW. Hemin-enhanced resistance of human leukemia cells to oxidative killing: antisense determination of ferritin involvement. Arch Biochem Biophys. 1998;352:51–58. doi: 10.1006/abbi.1998.0588. [DOI] [PubMed] [Google Scholar]
- Malecki EA, Cable EE, Isom HC, Connor JR. The lipophilic iron compound TMH-ferrocene [(3,5,5-trimethylhexanoyl)ferrocene] increases iron concentrations, neuronal L-ferritin, and heme oxygenase in brains of BALB/c mice. Biol Trace Elem Res. 2002;86:73–84. doi: 10.1385/BTER:86:1:73. [DOI] [PubMed] [Google Scholar]
- Masuda T, Hida H, Kanda Y, Aihara N, Ohta K, Yamada K, Nishino H. Oral administration of metal chelator ameliorates motor dysfunction after a small hemorrhage near the internal capsule in rat. J Neurosci Res. 2007;85:213–222. doi: 10.1002/jnr.21089. [DOI] [PubMed] [Google Scholar]
- Meyron-Holtz EG, Ghosh MC, Iwai K, LaVaute T, Brazzolotto X, Berger UV, Land W, Ollivierre-Wilson H, Grinberg A, Love P, Rouault TA. Genetic ablations of iron regulatory proteins 1 and 2 reveal why iron regulatory protein 2 dominates iron homeostasis. Embo J. 2004;23:386–395. doi: 10.1038/sj.emboj.7600041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos T, Morgan EH. The metabolism of neuronal iron and its pathogenic role in neurological disease: review. Ann N Y Acad Sci. 2004;1012:14–26. doi: 10.1196/annals.1306.002. [DOI] [PubMed] [Google Scholar]
- Muñoz AM, Rey P, Parga J, Guerra MJ, Labandeira-Garcia JL. Glial overexpression of heme oxygenase-1: a histochemical marker for early stages of striatal damage. J Chem Neuroanat. 2005;29:113–126. doi: 10.1016/j.jchemneu.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Keep RF, Hua Y, Schallert T, Hoff JT, Xi G. Deferoxamine-induced attenuation of brain edema and neurological deficits in a rat model of intracerebral hemorrhage. J Neurosurg. 2004;100:672–678. doi: 10.3171/jns.2004.100.4.0672. [DOI] [PubMed] [Google Scholar]
- Ohashi T, Mizutani A, Murakami A, Kojo S, Ishii T, Taketani S. Rapid oxidation of dichlorofluorescin with heme and hemoproteins: formation of fluorescein is independent of the generation of reactive oxygen species. FEBS Lett. 2002;511:21–27. doi: 10.1016/s0014-5793(01)03262-8. [DOI] [PubMed] [Google Scholar]
- Porter JB, Huehns ER. The toxic effects of desferrioxamine. Baillieres Clin Haematol. 1989;2:459–474. doi: 10.1016/s0950-3536(89)80027-7. [DOI] [PubMed] [Google Scholar]
- Qi Y, Jamindar TM, Dawson G. Hypoxia alters iron homeostasis and induces ferritin synthesis in oligodendrocytes. J Neurochem. 1995;64:2458–2464. doi: 10.1046/j.1471-4159.1995.64062458.x. [DOI] [PubMed] [Google Scholar]
- Qu Y, Chen J, Benvenisti-Zarom L, Ma X, Regan RF. Effect of targeted deletion of the heme oxygenase-2 gene on hemoglobin toxicity in the striatum. J. Cereb. Blood Flow Metab. 2005;25:1466–1475. doi: 10.1038/sj.jcbfm.9600143. [DOI] [PubMed] [Google Scholar]
- Qu Y, Chen-Roetling J, Benvenisti-Zarom L, Regan RF. Attenuation of oxidative injury after induction of experimental intracerebral hemorrhage in heme oxygenase-2 knockout mice. J. Neurosurg. 2007;106:428–435. doi: 10.3171/jns.2007.106.3.428. [DOI] [PubMed] [Google Scholar]
- Regan RF, Chen J, Benvenisti-Zarom L. Heme oxygenase-2 gene deletion attenuates oxidative stress in neurons exposed to extracellular hemin. BMC Neurosci. 2004;5:34. doi: 10.1186/1471-2202-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan RF, Choi DW. The effect of NMDA, AMPA/kainate, and calcium channel antagonists on traumatic cortical neuronal injury in culture. Brain Res. 1994;633:236–242. doi: 10.1016/0006-8993(94)91544-x. [DOI] [PubMed] [Google Scholar]
- Regan RF, Kumar N, Gao F, Guo YP. Ferritin induction protects cortical astrocytes from heme-mediated oxidative injury. Neuroscience. 2002;113:985–994. doi: 10.1016/s0306-4522(02)00243-9. [DOI] [PubMed] [Google Scholar]
- Regan RF, Panter SS. Neurotoxicity of hemoglobin in cortical cell culture. Neurosci. Lett. 1993;153:219–222. doi: 10.1016/0304-3940(93)90326-g. [DOI] [PubMed] [Google Scholar]
- Regan RF, Rogers B. Delayed treatment of hemoglobin neurotoxicity. J. Neurotrauma. 2003;20:111–120. doi: 10.1089/08977150360517236. [DOI] [PubMed] [Google Scholar]
- Rogers B, Yakopson V, Teng ZP, Guo Y, Regan RF. Heme oxygenase-2 knockout neurons are less vulnerable to hemoglobin toxicity. Free Rad. Biol. Med. 2003;35:872–881. doi: 10.1016/s0891-5849(03)00431-3. [DOI] [PubMed] [Google Scholar]
- Royall JA, Ischiropoulos H. Evaluation of 2',7'-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch. Biochem. Biophys. 1993;302:348–355. doi: 10.1006/abbi.1993.1222. [DOI] [PubMed] [Google Scholar]
- Sadrzadeh SMH, Anderson DK, Panter SS, Hallaway PE, Eaton JW. Hemoglobin potentiates central nervous system damage. J. Clin. Invest. 1987;79:662–664. doi: 10.1172/JCI112865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria R, Irace C, Festa M, Maffettone C, Colonna A. Induction of ferritin expression by oxalomalate. Biochim Biophys Acta. 2004;1691:151–159. doi: 10.1016/j.bbamcr.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Song S, Hua Y, Keep RF, Hoff JT, Xi G. A new hippocampal model for examining intracerebral hemorrhage-related neuronal death: effects of deferoxamine on hemoglobin-induced neuronal death. Stroke. 2007;38:2861–2863. doi: 10.1161/STROKEAHA.107.488015. [DOI] [PubMed] [Google Scholar]
- Tampo Y. [Studies on membrane factors in iron-supported lipid peroxidation] Yakugaku Zasshi. 2000;120:387–396. doi: 10.1248/yakushi1947.120.4_387. [DOI] [PubMed] [Google Scholar]
- Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002;99:3505–3516. doi: 10.1182/blood.v99.10.3505. [DOI] [PubMed] [Google Scholar]
- Wagner KR, Sharp FR, Ardizzone TD, Lu A, Clark JF. Heme and iron metabolism: role in cerebral hemorrhage. J Cereb Blood Flow Metab. 2003;23:629–652. doi: 10.1097/01.WCB.0000073905.87928.6D. [DOI] [PubMed] [Google Scholar]
- Wang W, Di X, D'Agostino RB, Jr, Torti SV, Torti FM. Excess capacity of the iron regulatory protein system. J Biol Chem. 2007;282:24650–24659. doi: 10.1074/jbc.M703167200. [DOI] [PubMed] [Google Scholar]
- Wu J, Hua Y, Keep RF, Nakemura T, Hoff JT, Xi G. Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke. 2003;34:2964–2969. doi: 10.1161/01.STR.0000103140.52838.45. [DOI] [PubMed] [Google Scholar]
- Xi GH, Keep RF, Hoff JT. Erythrocytes and delayed brain edema formation following intracerebral hemorrhage in rats. J. Neurosurg. 1998;89:991–996. doi: 10.3171/jns.1998.89.6.0991. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Migita CT. Mechanism of heme degradation by heme oxygenase. J Inorg Biochem. 2000;82:33–41. doi: 10.1016/s0162-0134(00)00156-2. [DOI] [PubMed] [Google Scholar]