Abstract
Dephosphorylation of the natriuretic peptide receptor-A (NPR-A) is hypothesized to mediate its desensitization in response to atrial natriuretic peptide (ANP) binding. Recently, we identified six phosphorylation sites within the kinase homology domain of NPR-A and determined that the conversion of these residues to alanine abolished the ability of the receptor to be phosphorylated or to be activated by ANP and ATP. In an attempt to generate a form of NPR-A that mimics a fully phosphorylated receptor but that is resistant to dephosphorylation, we engineered a receptor variant (NPR-A-6E) containing glutamate substitutions at all six phosphorylation sites. Consistent with the known ability of negatively charged glutamate residues to substitute functionally, in some cases, for phosphorylated residues, we found that NPR-A-6E was activated 10-fold by ANP and ATP. As determined by guanylyl cyclase assays, the hormone-stimulated activity of the wild-type receptor declined over time in membrane preparations in vitro, and this loss was blocked by the serine/threonine protein phosphatase inhibitor microcystin. In contrast, the activity of NPR-A-6E was more linear with time and was unaffected by microcystin. The nonhydrolyzable ATP analogue adenosine 5′-(β,γ-imino)-triphosphate was half as effective as ATP in stimulating the wild-type receptor but was equally as potent in stimulating NPR-A-6E, suggesting that ATP is required to keep the wild-type but not 6E variant phosphorylated. Finally, the desensitization of NPR-A-6E in whole cells was markedly blunted compared with that of the wild-type receptor, consistent with its inability to shed the negative charge from its kinase homology domain via dephosphorylation. These data provide the first direct test of the requirement for dephosphorylation in guanylyl cyclase desensitization and they indicate that it is an essential component of this process.
INTRODUCTION
Cell surface guanylyl cyclase receptors are conserved in organisms as varied as worms (Yu et al., 1997), sea urchins (Thorpe and Garbers, 1989), eels (Katafuchi et al., 1994), flies (Liu et al., 1995), rats (Chinkers et al., 1989), and humans (Lowe et al., 1989). The basic topology of these receptors is maintained in all species. It consists of an extracellular ligand-binding domain, a single membrane-spanning region, a juxtamembrane regulatory region called the kinase homology domain (KHD), and a carboxyl-terminal guanylyl cyclase catalytic domain (Garbers and Lowe, 1994). The activation of these receptors is achieved by the binding of a unique peptide ligand to their extracellular domains. The quintessential example of this receptor class is the mammalian natriuretic peptide receptor-A (NPR-A), which is also known as guanylyl cyclase-A (GC-A) (Chinkers and Garbers, 1989; Lowe et al., 1989). One activator of NPR-A is atrial natriuretic peptide (ANP), a cardiac hormone that regulates vascular smooth muscle tone, renal fluid balance, and cell proliferation (Ruskoaho, 1992; Drewett and Garbers, 1994). In intact cells and crude membrane preparations, the addition of ANP results in the rapid production of cGMP. However, after an initial burst, the guanylyl cyclase activity of the enzyme is attenuated with a t½ of ∼30 min (Woods and Houslay, 1991; Yasunari et al., 1992). This process is known as homologous desensitization and is characterized by the waning of enzymatic activity in the continuing presence of hormone (Sibley and Lefkowitz, 1985).
A similar desensitization response has been observed with various sea urchin guanylyl cyclase receptors (Ward et al., 1986; Garbers, 1989). In this system, the most active form of the enzyme has been correlated with a slower migrating form of the cyclase on SDS-PAGE. The addition of its activating peptide results in a very rapid initial burst of enzymatic activity followed by a subsequent equally rapid decline in activity (Bentley et al., 1986). The activity reductions have been shown to be coincident with an increase in the electrophoretic mobility of the cyclase, and this increase was shown to result from receptor dephosphorylation with the stoichiometry of phosphorylation decreasing from 15–17 mol of phosphate/mol of receptor in the absence of egg peptide to 2 mol of phosphate/mol of receptor in the presence of the peptide (Vacquier and Moy, 1986; Ramarao and Garbers, 1988). Similarly, NPR-A and the closely related NPR-B have been shown to be constitutively phosphorylated when isolated from transfected tissue culture cells (Potter and Garbers, 1992, 1994; Potter, 1998; Koller et al., 1993). Exposure of these receptors to the appropriate natriuretic peptides results in their desensitization, and the reduced activity correlates with receptor dephosphorylation (Potter and Garbers, 1992; Potter, 1998). Furthermore, the treatment of NPRs either in crude membranes (Potter and Garbers, 1992; Potter, 1998) or highly purified preparations of sea urchin cyclase (Ramarao and Garbers, 1988) with protein phosphatase (PP) results in both receptor dephosphorylation and desensitization. More recently, Foster and Garbers (1998) showed that NPR-A could be desensitized and then resensitized in crude membranes if adenosine 5′-O-(thiotriphosphate) was included in the preparations. They also demonstrated that a serine/threonine PP inhibitor, microcystin, could block the in vitro desensitization (Foster and Garbers, 1998). The target of the phosphatase was not identified, but the phosphorylation state of NPR-A was shown to be modulated by the phosphatase inhibitor, which is consistent with the idea of NPR-A being desensitized by a microcystin-sensitive PP. They concluded that ATP increased the activity of NPR-A in vitro by allosterically activating it but also by serving as a substrate for a protein kinase. Hence, adenosine 5′-O-(thiotriphosphate) is a better activator than ATP, because thiophosphorylated proteins are more resistant to dephosphorylation.
Based on these and other data, it has been proposed that receptor guanylyl cyclase desensitization is mediated by dephosphorylation. To test this hypothesis, we identified six major phosphorylation sites located within the KHD of NPR-A (Potter and Hunter, 1998b). The mutation of any single site to alanine resulted in decreased ANP-dependent cyclase activity, and the conservative replacement of four or more of these sites with alanine yielded a receptor mutant that was completely unresponsive to ANP. Recently, we showed that the removal of the major NPR-B phosphorylation sites also results in a dramatic reduction in its hormone-dependent activity (Potter and Hunter, 1998a).
The alanine mutants of NPR-A and NPR-B were instructive regarding the requirement for phosphorylation in the activation of these receptors, but they could not be used to test whether dephosphorylation is required for homologous desensitization response because they are completely unresponsive to hormone. Indeed, these mutants appear to represent the completely desensitized forms of NPR-A and -B. In an attempt to generate an NPR-A mutant that would mimic the phosphorylated state but could not be dephosphorylated, we tested whether glutamate substitutions at the known NPR-A phosphorylation sites would yield a hormonally responsive enzyme, because in some cases acidic amino acid substitutions for serines and threonines have been shown to substitute functionally for phosphorylated versions of these residues (Thorsness and Koshland, 1987; Yan and Templeton, 1994). We found that the conversion of all six sites to glutamate yielded a receptor variant (NPR-A-6E) that was not phosphorylated but was activated almost 10-fold by ANP. Using this constitutively “phosphorylated” hormonally responsive receptor, we were then able to test directly the “desensitization by dephosphorylation” hypothesis. We observed that the desensitization of the phosphate-negative NPR-A mutant both in vitro and in whole cells was markedly diminished compared with the wild-type receptor.
MATERIALS AND METHODS
Vector Construction
The phosphorylation site mutations were generated on the ∼700-bp BamHI–XbaI fragment of NPR-A, which was subcloned into pBluescript II (Stratagene, San Diego, CA). The mutations were generated using the Quikchange kit from Stratagene according to the manufacturer’s protocols. The mutant BamHI–XbaI fragments were then subcloned back into the corresponding region of the expression plasmid pCMV3-GC-A (Potter and Garbers, 1992). All indicated mutations and the absence of unwanted mutations were confirmed by manual or automated nucleic acid sequencing.
Cell Culture and Transient Transfections
The 293 cell line stably expressing NPR-A (293-GC-A) and the 293 cells used for transient transfections (293neo) have been previously described (Potter and Garbers, 1992). Neither cell line endogenously expresses NPR-A or NPR-B based on natriuretic peptide–dependent cGMP elevations in whole cells (Potter and Garbers, 1992). For transfections, the cells were grown to 40–50% confluence in 10-cm dishes that had been precoated with a 50 μg/ml solution of poly-l-lysine. Twenty-four hours later, the cells were transfected with 5 μg of the various pCMV3-NPR-A constructs using the BES-buffered calcium phosphate coprecipitation method. Forty-eight to 72 h later, the cells were harvested for membrane preparation. Transfections with a cytomegalovirus (CMV)-driven green fluorescent protein reporter plasmid indicated that the transfection efficiency was between 30 and 60%.
Whole-Cell Cyclic GMP Elevations
Twenty-four hours after transfections, the cells were split into 12-well dishes and incubated overnight. The next day these cells were ∼50–75% confluent and were washed once with 1 ml of Dulbecco’s modified Eagle’s medium (DMEM). The plates were then moved to ambient temperature and incubated with 0.5 ml of DMEM containing 0.5 mM 1-methyl-3-isobutylxanthine (a phosphodiesterase inhibitor used to block cGMP degradation) and 200 μM ANP for various periods. cGMP production was terminated by adding 0.5 ml of 6% trichloroacetic acid to each well at the appropriate time. The amount of cGMP contained in each well (cells and medium) was determined using a cGMP radioimmunoassay kit from DuPont NEN (Boston, MA) according to the manufacturer’s protocol.
Membrane Preparation
Ten-centimeter plates of transiently or stably transfected 293 cells were washed once with 10 ml of PBS and then scraped off the plate in 0.5 ml of phosphatase inhibitor buffer [50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, pH 7.4, 20% glycerol, 50 mM NaCl, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 1 μg/ml pepstatin, 10 mM NaPO4, pH 7.0, 0.1 M NaF, 1 mM Na3VO4, 80 μM β-glycerol phosphate, and 0.1 μM okadaic acid), sonicated with a Branson (Plainview, NY) Sonifier cell disrupter at 4°C, and centrifuged at 15,800 × g for 20 min at 2°C. The resulting membrane pellet was resuspended in HGPB [50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, pH 7.4, 10% glycerol, 50 mM NaCl, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 1 μg/ml pepstatin] at a protein concentration of between 1.0 and 2.5 mg/ml as estimated by the BCA protein assay (Pierce, Rockford, IL). For experiments in which we did not wish to preserve the phosphorylation state of NPR-A (see Figures 4 and 5), we lysed the cells in HGPB with no phosphatase inhibitors.
Figure 4.
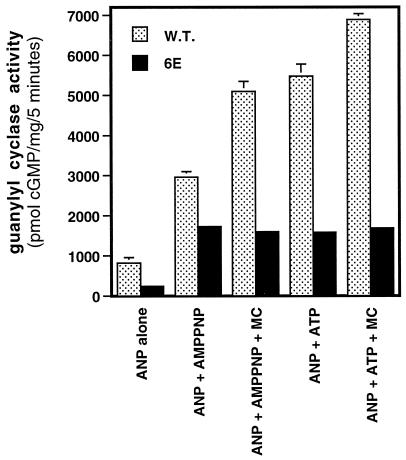
ATP and AMPPNP equivalently activate NPR-A-6E but not the wild-type receptor. Human 293 cells were transiently transfected with either wild-type NPR-A or NPR-A-6E expression constructs; 48 h later, crude membranes were prepared, and guanylyl cyclase activities were determined in the presence of 1 μM ANP and 1 mM adenine nucleotide (AMPPNP or ATP) and in the presence or absence of 1 μM microcystin (MC) for 5 min at 37°C. The vertical bars centered above the columns represent the range of values obtained from two separate determinations, which were determined in duplicate.
Figure 5.
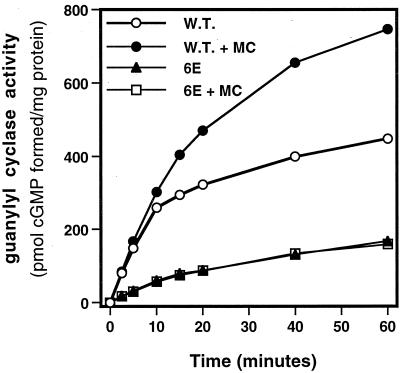
Microcystin blocks the decline in the activity of wild-type NPR-A but not NPR-A-6E in vitro. Human 293 cells were transiently transfected with either the NPR-A (W.T., squares) or NPR-A-6E (6E, circles) expression constructs; 48 h later, crude membranes were prepared, and hormone-dependent guanylyl cyclase activity (ANP, ATP, and Mg2+-GTP) was determined for the indicated periods in the presence (filled symbols) or absence (open symbols) of 1 μM microcystin.
Immunoblot Analysis
Crude membranes containing NPR-A were separated by SDS-PAGE and electroblotted onto an Immobilon-P membrane (Millipore, Bedford, MA) as previously described (Potter and Hunter, 1998b). The membrane was then blocked for 1 h in TBST (20 mM Tris, 500 mM NaCl, and 0.05% polyoxyethylene sorbitan monolaurate, pH 7.5) containing 3% BSA, washed three times for 5 min with TBST, and then incubated with rabbit polyclonal antiserum R1215 (Potter and Garbers, 1992) diluted 1:200 in TBST containing 1% BSA for 2 h at 25°C. The membrane was washed three times for 10 min with TBST and incubated for 45 min at 25°C with an affinity-purified 125I-coupled donkey anti-rabbit–directed secondary antibody. The membrane was then washed once for 15 min and twice for 5 min in TBST. The NPR-A–antibody complex was visualized by autoradiography, and then the corresponding bands were cut out of the membrane and quantitated in a γ counter.
Guanylyl Cyclase Assays
All guanylyl cyclase assays were conducted at 37°C in the presence of 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, pH 7.4, 50 mM NaCl, 0.25 mM 1-methyl-3-isobutylxanthine, 0.1% BSA, 5 mM creatine phosphate, 5–10 U/assay creatine phosphokinase, 1 mM GTP, and 0.1–0.2 μCi of [α-32P]GTP. Five millimolar MgCl2, 1 mM ATP, and 1 μM ANP or 1% Triton X-100 and 3 mM MnCl2 were also included in the reaction mixtures. Basal activity levels were determined in the presence of only 5 mM MgCl2. Assays were initiated by the addition of a solution of the above reagents to crude membranes in a total volume of 0.1 ml. The experiment described in Figure 5 was initiated by the addition of 680 μl of prewarmed reaction mixture to 120 μl of crude membranes. The reactions were terminated at the appropriate time points by transferring 100-μl aliquots to 0.5 ml of 110 mM zinc acetate on ice. cGMP accumulation was quantitated as described by Domino et al. (1991).
RESULTS
NPR-A Desensitization Is Biphasic
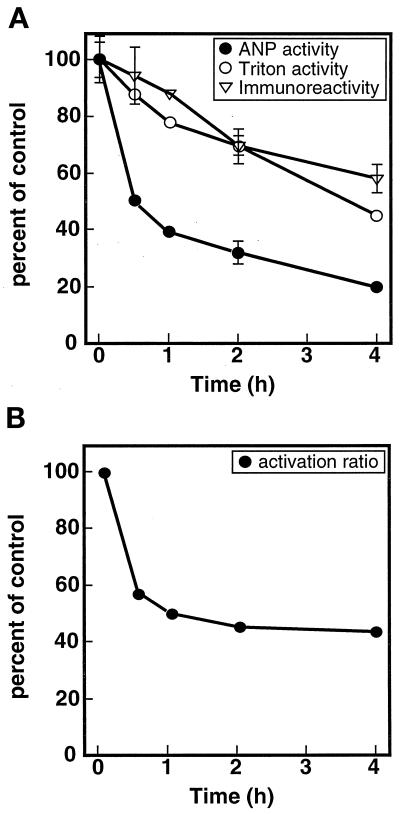
When 293 cells stably expressing NPR-A (Potter and Garbers, 1992) were incubated with ANP for increasing periods, hormone-dependent guanylyl cyclase activity assayed in crude membranes derived from these cells declined in a biphasic manner (Figure 1, filled circles). Within the first 30 min after ANP addition to the medium, hormone-dependent activity dropped to ∼50% of the original level (Figure 1A). This decrease has been shown to be tightly correlated with receptor dephosphorylation (Potter and Garbers, 1992). After the initial acute phase, the rate of decline subsided, and the loss of activity correlated with decreases in ANP-independent activity, as indicated by guanylyl cyclase activity determinations in the presence of detergent (Figure 1, open circles) and in the amount of NPR-A immunoreactivity detected in membrane preparations (Figure 1, triangles). Both of these measurements are indicators of the total amount of NPR-A protein present in the membrane preparations. The transient nature of the acute desensitization response is revealed by plotting the ratio of the two guanylyl cyclase determinations (ANP-dependent activity/detergent-dependent activity) versus the time of ANP incubation (Figure 1B). This graph indicates that the rapid response is essentially complete after 30 min and suggests that the additional decreases in hormone-dependent activity observed with longer incubations are due to receptor degradation. Thus, it appears that dephosphorylation and down-regulation acutely and chronically regulate NPR-A, respectively.
Figure 1.
NPR-A undergoes agonist-dependent (homologous) desensitization. (A) NPR-A desensitization is biphasic. 293 cells stably expressing NPR-A were treated with 1 μM ANP for increasing periods, and then crude membranes were prepared and assayed for guanylyl cyclase activity in the presence of the activators indicated or fractionated by SDS-PAGE, blotted to Immobilon membrane, and probed with a NPR-A–specific antiserum. The antigen–antibody complex was quantitated by incubating with an 125I-coupled donkey anti-rabbit–directed secondary antibody. Control equals no ANP incubation. (B) Long-term declines in ANP-dependent activity are explained by decreases in NPR-A protein. The ratio of ANP/ATP/Mg2+-dependent over Mn2+ and Triton X-100-dependent guanylyl cyclase activities from A (ordinate) was plotted versus time of ANP incubation (abscissa). The vertical bars contained within each symbol represent the range of values obtained from two separated samples, which were determined in duplicate. For some points the range is smaller than the diameter of the symbol and is not visible.
Substitution of the Phosphorylated Residues with Glutamate Yields Hormonally Responsive Receptors
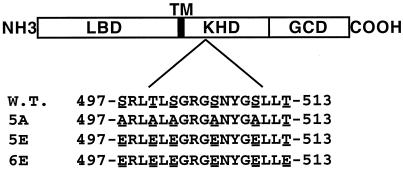
Because the conversion of four or more of the phosphorylated residues within NPR-A to alanine resulted in a hormonally insensitive (i.e., fully desensitized) enzymes, we were unable to test the effect of removing these sites on the desensitization process (Potter and Hunter, 1998b). However, because we were able to functionally substitute a glutamate residue for serine-497 in NPR-A (Potter and Hunter, 1998b), we asked whether the conversion of the remaining phosphorylatable residues to glutamate might result in a receptor lacking phosphate but still hormonally responsive. To this end, we constructed two glutamate-substituted NPR-A mutants (Figure 2). The first one (5E) was made before we had determined that threonine 513 was phosphorylated, and it contains glutamate substitutions at all of the known NPR-A phosphorylation sites except Thr-513. The second one (6E) is identical to the first, except Thr-513 is also converted to glutamate. Neither the 5E nor the 6E mutant is detectably phosphorylated when isolated from 32P-labeled cells.
Figure 2.
Description of wild-type and phosphorylation site mutant NPR-A receptors. LBD, ligand-binding domain; TM, transmembrane domain; GCD, guanylyl cyclase domain.
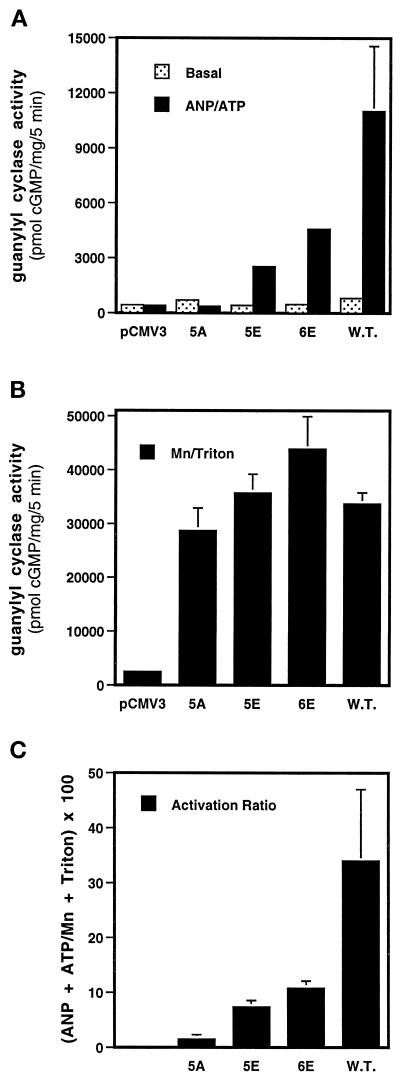
We compared the activities of the 5E and 6E mutants to the wild-type NPR-A and the 5A mutant, which contains alanine substitutions at the first five KHD NPR-A phosphorylation sites and which is completely unresponsive to ANP (Potter and Hunter, 1998b). When basal measurements were determined in the absence of ANP and ATP (Mg2+-GTP only), the guanylyl cyclase activities of the glutamate (5E and 6E) mutants were similar to those obtained for the 5A and wild-type receptors (Figure 3A). However, because these activities were not significantly higher than those obtained from vector alone–transfected cells, we could not be sure that the expression level of each mutant protein was similar. Therefore, we repeated the assay in the presence of Triton X-100 and Mg2+-GTP (Figure 3B). These conditions artificially stimulate NPR-A to maximum levels in an ANP-independent manner (Potter and Garbers, 1992). Again, we found that the activities of the mutant receptors were relatively similar. We next tested whether the glutamate-substituted receptors were responsive to physiological activation by assaying in the presence of ANP, ATP, and Mg2+-GTP. Under these conditions, the 5E and 6E mutant receptors were activated 7- and 10-fold over their corresponding basal levels, respectively (Figure 3A). In contrast, the 5A mutant was completely insensitive to ANP activation. Based on these observations, it appears that one major function of these phosphorylation sites is to provide a region of localized negative charge within the KHD that is required for ANP responsiveness. However, it should be noted that under the same conditions the wild-type receptor was stimulated almost 14-fold over its respective basal level (Figure 3A), and its activation ratio is markedly higher than those of the 5E or 6E receptors (Figure 3C). Hence, the glutamate substitutions should not be considered completely equivalent to the phosphorylated wild-type residues.
Figure 3.
Glutamate but not alanine phosphorylation site substitutions yield hormonally responsive receptors. (A) Glutamate-substituted receptors are activated by ANP and ATP. Human 293 cells were transiently transfected with the indicated NPR-A expression constructs; 48 h later, crude membranes were prepared and assayed for guanylyl cyclase activity in the presence of Mg2+-GTP only (basal) or ANP, ATP, and Mg2+-GTP (ANP/ATP). (B) The hormone-dependent activities of the glutamate-substituted receptors are not explained by increased protein levels. Crude membranes were prepared as described above and assayed for guanylyl cyclase activity in the presence of Triton X-100 and Mn2+-GTP. (C) The glutamate substitutions are not equivalent to the wild-type receptor. The activity ratio of the wild-type and mutant receptors was determined by dividing the hormone-dependent activity by the detergent activity and multiplying this number by 100. The vertical bars centered above the columns represent the range of values obtained from four separate determinations, which were determined in two separate experiments.
Equivalent Activation of NPR-A-6E by Adenine Nucleotides
Recently it was reported that ATP serves a dual role in the activation of NPR-A (Foster and Garbers, 1998). It was suggested that ATP is both an allosteric regulator of NPR-A as well as a substrate in a protein kinase reaction. The substrate of the kinase reaction was not determined, but microcystin was shown to increase the 32P content of NPR-A when [γ-32P]ATP was included in guanylyl cyclase assays. To assess whether the phosphorylation of the KHD of NPR-A is sufficient to mediate the differing effects of various adenine nucleotides, we tested the effect of ATP and the nonhydrolyzable analogue adenosine 5′-(β,γ-imino)triphosphate (AMPPNP) on the activation of NPR-A and NPR-A-6E in the presence of saturating concentrations (1 μM) of ANP. We reasoned that if NPR-A is the target of this phosphorylation reaction, then ATP and AMPPNP should be equivalent activators of NPR-A-6E, because this receptor is unable to be phosphorylated. We observed that the wild-type receptor was activated 3.6-fold with AMPPNP, and this activation was almost doubled by the addition of microcystin (Figure 4). ATP alone activated the wild-type receptor 6.7-fold, and microcystin increased the activation to 8.4-fold. In contrast, AMPPNP was as efficacious as ATP in activating NPR-A-6E (∼7 fold), and the inclusion of microcystin had no additional effect on NPR-A-6E activity assayed in the presence of either ATP or AMPPNP. These data suggest that the KHD of NPR-A is a target for a protein kinase that is present in these membrane preparations, and that the ability of ATP, but not AMPPNP, to serve as a substrate in the phosphorylation of NPR-A is sufficient to account for their differing potencies.
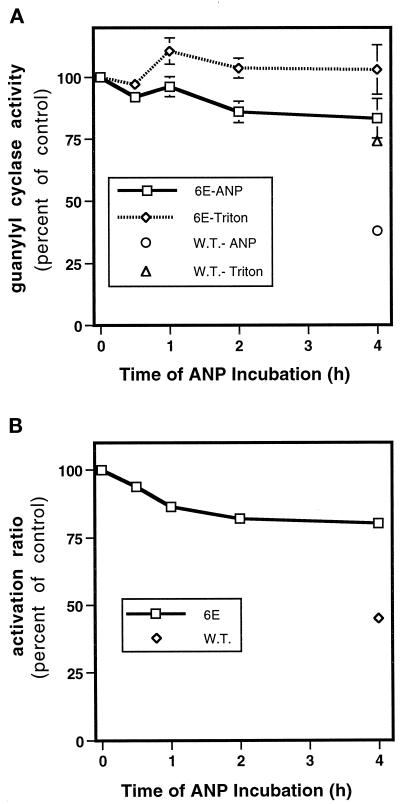
NPR-A-6E Is Resistant to Desensitization and Microcystin In Vitro
We next asked whether NPR-A-6E was resistant to desensitization in guanylyl cyclase assays. Because it has recently been shown that microcystin, a known inhibitor of PP1, PP2A, PP4, and PP5, can preserve the ANP- and ATP-dependent activity of NPR-A in guanylyl cyclase assays conducted in crude membranes (Foster and Garbers, 1998), we asked whether this PP inhibitor was preventing the dephosphorylation of NPR-A or some other protein. We reasoned that if NPR-A were the target molecule, then NPR-A-6E should be unaffected by microcystin, because it cannot be dephosphorylated. When we measured the hormone-dependent guanylyl cyclase activity of wild-type NPR-A in membranes, we found that product formation was almost linear for the first 5 min but began to fall off thereafter (Figure 5). As previously reported (Foster and Garbers, 1998), the inclusion of microcystin in the assay blocked this loss of activity, although the block is not as complete in these cells as was demonstrated for NPR-A expressed in NIH3T3 cells. In contrast to wild-type NPR-A, microcystin had absolutely no effect on the ANP-stimulated activity of NPR-A-6E, which was lower than that of the ANP-stimulated wild-type receptor (Figure 5). These data indicate that NPR-A is the target of the microcystin-sensitive phosphatase.
NPR-A-6E Displays a Blunted Whole-Cell Desensitization Response
With the construction of NPR-A-6E, we were now able to test directly the role of dephosphorylation in the whole cell desensitization process. To this end, we repeated the experiment described in Figure 1 by transiently transfecting the same parental 293 cell line with the NPR-A-6E construct. As can be seen by comparing Figures 1A and 6A, losses in subsequent hormone-dependent guanylyl cyclase activities of the 6E mutant were markedly diminished compared with the wild-type receptor. Likewise, the ANP-dependent reductions in the activity ratio of the 6E variant were also reduced in comparison with the wild-type receptor (compare Figures 1B and 6B). The diminished desensitization response of the 6E variant was not a function of the transient transfection procedure or experiment-to-experiment variation, because a 4-h ANP treatment reduced the activity of the wild-type receptor to 38% of its untreated value, whereas the identical treatment of the 6E receptor (performed in the same experiment) only reduced its activity to 83% (Figure 4A). Additionally, the activity ratio of the 6E variant was reduced to only 80% compared with 50% for the wild-type receptor in the transiently transfected 293 cells (Figure 4B). Thus, by blocking the ability of NPR-A to shed its negative charge via dephosphorylation, we were able to block ∼60% of the desensitization response in these cells.
Figure 6.
NPR-A-6E displays a blunted desensitization response in whole cells. (A) Effect of ANP incubation on subsequent ANP- and detergent-dependent guanylyl cyclase activities. Human 293 cells were transiently transfected with either NPR-A (W.T.) or NPR-A-6E (6E) expression constructs. Two days later, the cells were incubated with 0.5 μM ANP for the indicated periods. The cells were then washed twice with PBS, and crude membranes were prepared and assayed for guanylyl cyclase activity in the presence of ANP, ATP, and Mg2+-GTP or Triton X-100 and Mn2+-GTP. (B) Effect of ANP incubation on the activation ratio of NPR-A-6E. The ratios of hormone-dependent over detergent-dependent guanylyl cyclase activities (ordinate) were plotted versus time of ANP incubation (abscissa). The vertical bars contained within each symbol represent the range of two separate determinations.
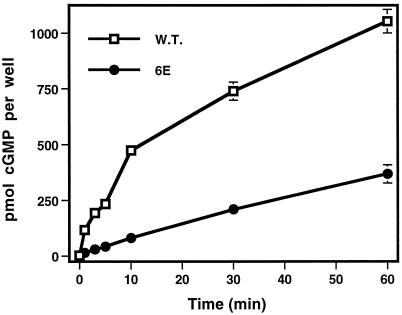
To confirm the results obtained by guanylyl cyclase assays, we asked whether the ANP-dependent cGMP accumulations in whole cells transfected with the 6E mutant receptor were more linear than those observed in cells transfected with the wild-type receptor. We found that cells expressing the wild-type receptor accumulate cGMP very rapidly initially, but after a few minutes the rate of accumulation declines (Figure 7). Cells expressing the 6E mutant had decreased ANP-dependent cGMP accumulation compared with cells expressing the wild-type receptor, which is consistent with the diminished ANP-dependent guanylyl cyclase activity of NPR-A-6E (Figure 3). However, in contrast to the cells expressing the wild-type receptor, the rate of cGMP accumulation in these cells only declined slightly over the course of 1 h (Figure 7). These data indicate that NPR-A undergoes homologous desensitization in whole cells, and that this desensitization is mediated, at least in part, by dephosphorylation of the sites in the KHD.
Figure 7.
Cells expressing NPR-A-6E but not wild-type NPR-A demonstrate linear ANP-dependent cGMP accumulation. Human 293 cells were transiently transfected with either NPR-A (W.T.) or NPR-A-6E (6E) expression constructs and then split into 12-well plates 24 h later. The next day these cells were moved to ambient temperature and incubated with 0.5 ml of DMEM containing 0.5 mM 1-methyl-3-isobutylxanthine and 200 μM ANP for the periods shown. cGMP production was terminated by adding 0.5 ml of 6% trichloroacetic acid to each well at the indicated time and assayed as described in MATERIALS AND METHODS. The vertical bars contained within each symbol represent the SEM, where n = 4.
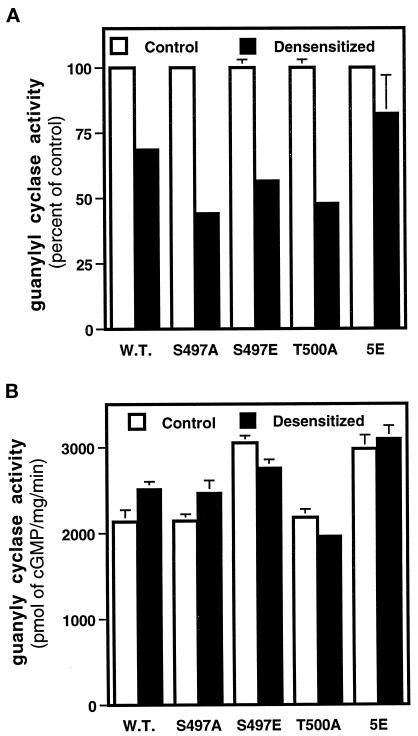
Mutation of Ser-497 or Thr-500 Alone Does Not Block Desensitization
Finally, we asked whether the dephosphorylation of a single residue was sufficient to cause desensitization or whether the desensitization was mediated by a coordinated dephosphorylation of all six residues. The homologous but not heterologous desensitization of both NPR-A and B has been suggested to be mediated by the simultaneous dephosphorylation of all the receptor phosphorylation sites, because tryptic phosphopeptide maps of the desensitized receptors are identical to those of the wild-type receptors, even though they contain markedly less phosphate (Potter and Garbers, 1992; Potter, 1998). Because the mutation of serine 497 to alanine (S497A) results in the most dramatic decrease in hormone-dependent guanylyl cyclase activity of NPR-A, and because we were able to restore activity by mutating this residue to glutamate (S497E), we asked whether either of these mutations would block the desensitization of NPR-A. We tested the ability of a receptor containing an alanine in place of threonine 500 (T500A) and the 5E mutant as well. We found that the incubation of cells expressing the wild-type receptor with 200 nM ANP for 1 h resulted in membranes that contained reduced hormone-dependent guanylyl cyclase activity (Figure 8). However, the S497A, S487E, and T500A mutants desensitized to equal or greater levels than the wild-type receptor. In contrast, the 5E receptor displayed a muted desensitization response. These data are consistent with the notion that homologous desensitization is mediated by a coordinated dephosphorylation of all the phosphorylation sites in the NPR-A KHD.
Figure 8.
Mutation of Ser-497 or Thr-500 does not block the desensitization of NPR-A. Human 293 cells were transiently transfected with the indicated NPR-A expression constructs; 48 h later the cells were washed and then incubated in the presence (Desensitized) or absence (Control) of 200 μM ANP for 1 h. Crude membranes were prepared and assayed for guanylyl cyclase activity in the presence of ANP, ATP, and Mg2+-GTP (A) or Mn2+-GTP and 1% Triton X-100 (B). The vertical bars centered above the columns represent the range of values obtained from two separate determinations, which were determined in duplicate. When the error is <3%, the error bars are not shown.
DISCUSSION
In this report, we have described serine and threonine to glutamate mutations within the KHD of NPR-A, which functionally substitute for the phosphorylated versions of these amino acids in facilitating hormone responsiveness. These observations are consistent with our previous data, which suggested that the phosphorylation of NPR-A is required for ANP-dependent activation (Potter and Hunter, 1998b). Although not as hormonally responsive as the wild-type receptors, these glutamate-substituted mutants proved to be extremely useful tools because they allowed us to test directly the role of dephosphorylation in the desensitization of NPR-A. We observed that desensitization of the NPR-A-6E mutant was markedly diminished compared with the wild-type receptor both in vitro and in vivo. Taken together, the data strongly suggest that receptor dephosphorylation is required for normal desensitization.
Whether the hormone-dependent dephosphorylation of NPR-A is mediated by the activation of a PP or the inactivation of a protein kinase it not clear, but the blocking effect of microcystin in the membrane preparations suggests that it may be the former. The identity of the responsible PP is not currently known, but our results and those of Foster and Garbers (1998) suggest that it is likely to be a membrane-associated, microcystin- and okadaic acid-sensitive phosphatase species, which currently include PP1, PP2A, PP4, and PP5. We have tested the ability of purified preparations of PP1, PP2A, and PP5, a phosphatase that was recently shown to associate with the KHD of NPR-A in a two-hybrid screen (Chinkers, 1994), to dephosphorylate immunopurified NPR-A bound to protein A-agarose (Cohen and Potter, unpublished observations). We found that all three phosphatases were able to dephosphorylate NPR-A but that none of these enzymes was able to remove all of the phosphate from the receptor, even at relatively high concentrations. However, we have not tested whether the presence of ANP and ATP may make NPR-A a better substrate for these phosphatases. Finally, it is important to note that we have not been able to block either the homologous or heterologous desensitization of NPR-A in whole 293 cells with micromolar concentrations of okadaic acid, which should be sufficient to inhibit PP1, PP2A, PP4, and PP5 completely. The reason for this is not known, but it may be that the intracellular concentration of okadaic acid is less than is necessary to inhibit the relevant PP over the period assayed, or else the physiological PP may be okadaic acid insensitive e.g., PP2B or PP2C. The discrepancy between the in vitro and in vivo sensitivity also could be explained if cell lysis allows a nonphysiological membrane-associated, microcystin-sensitive PP access to NPR-A in the in vitro assays.
It is important to point out that the initial phase of the desensitization of NPR-A-6E was not completely abolished. This indicates that some other, as yet unidentified, mechanism(s) is involved in this process. The nature of the dephosphorylation-independent process is currently unknown. One possibility is that a specific regulatory protein is required for the activation of this receptor and that this protein is degraded or inactivated in response to ANP. Another possibility is that NPR-A could be internalized in endosomes and multivesicular bodies and therefore be excluded from activators or substrate. Alternatively, it may be that NPR-A or its putative regulatory protein is inhibited in a phosphorylation-independent posttranslational modification process, such as fatty acid acylation or methylation. Finally, we note that in contrast to the wild-type receptor, the detergent-dependent guanylyl cyclase activity of NPR-A-6E (i.e., total receptor level) was not reduced by up to 4 h of ANP exposure (Figure 6A). This suggests that receptor dephosphorylation may also be involved in the down-regulation of NPR-A. The mechanism of down-regulation is not known, but because there is a corresponding decrease in receptor protein levels, it presumably results from degradation. Whether internalization, degradation, or both processes are affected by dephosphorylation is currently not known.
There are many similarities between the structure and regulation of mammalian natriuretic peptide and sea urchin egg peptide guanylyl cyclase receptors (Chinkers and Garbers, 1991). This is particularly apparent regarding their common mechanism of desensitization. Recently, Furuya et al. (1998) used mass spectrometric analysis to identify 14 of a predicted 26 phosphorylation sites within the H. pulcherrimus sea urchin guanylyl cyclase. Unlike NPR-A and -B, this sea urchin cyclase contained several sites (894, 918, 927, and 930) located within the guanylyl cyclase catalytic domain. However, two of the identified sites (561 and 565) are located in the same general region as the N-terminal sites found in NPR-A and NPR-B. What role, if any, these sites play in the regulation of the egg peptide-sensitive guanylyl cyclase activity of this receptor has not been reported.
In conclusion, cell surface guanylyl cyclases are, to our knowledge, the only receptors that are desensitized by dephosphorylation. This mechanism is the complete opposite of that used by G-protein–coupled receptors, which are attenuated by direct receptor phosphorylation. These serpentine receptors have evolved a highly specialized network of proteins that are required for this process, including specific G-protein receptor kinases, phosphatases, and molecules known as arrestins that associate with specific receptors in a phosphorylation-dependent manner (Krupnick and Benovic, 1998). Whether a family of regulatory proteins has specifically evolved to regulate guanylyl cyclase receptors or whether these receptors use more general cellular machinery for these purposes remains to be determined. However, it is now clear that protein kinases and phosphatases are crucial regulators of some members of this expanding family of enzymes.
ACKNOWLEDGMENTS
This work was supported by US Public Health Service grants CA-14195 and CA-39780 (to T.H.). L.R.P. was supported by National Research Service Award CA-67452 from the National Institutes of Health. T.H. is a Frank and Else Schilling American Cancer Society Research Professor.
Abbreviations used:
- AMPPNP
adenosine 5′-(β,γ-imino)triphosphate
- ANP
atrial natriuretic peptide
- CMV
cytomegalovirus
- CNP
C-type natriuretic peptide
- DMEM
Dulbecco’s modified Eagle’s medium
- GC-A
guanylyl cyclase-A
- KHD
kinase homology domain
- NPR-A
natriuretic peptide receptor A
- NPR-B
natriuretic peptide receptor B
- PP
protein phosphatase
REFERENCES
- Bentley JK, Tubb DJ, Garbers DL. Receptor-mediated activation of spermatozoan guanylate cyclase. J Biol Chem. 1986;261:14859–14862. [PubMed] [Google Scholar]
- Chinkers M. Targeting of a distinctive protein-serine phosphatase to the protein kinase-like domain of the atrial natriuretic peptide receptor. Proc Natl Acad Sci USA. 1994;91:11075–11079. doi: 10.1073/pnas.91.23.11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinkers M, Garbers DL. The protein kinase domain of the ANP receptor is required for signaling. Science. 1989;245:1392–1394. doi: 10.1126/science.2571188. [DOI] [PubMed] [Google Scholar]
- Chinkers M, Garbers DL. Signal transduction by guanylyl cyclases. Annu Rev Biochem. 1991;60:553–575. doi: 10.1146/annurev.bi.60.070191.003005. [DOI] [PubMed] [Google Scholar]
- Chinkers M, Garbers DL, Chang MS, Lowe DG, Chin HM, Goeddel DV, Schulz S. A membrane form of guanylate cyclase is an atrial natriuretic peptide receptor. Nature. 1989;338:78–83. doi: 10.1038/338078a0. [DOI] [PubMed] [Google Scholar]
- Domino SE, Tubb DJ, Garbers DL. Assay of guanylyl cyclase catalytic activity. Methods Enzymol. 1991;195:345–355. doi: 10.1016/0076-6879(91)95179-n. [DOI] [PubMed] [Google Scholar]
- Drewett JG, Garbers DL. The family of guanylyl cyclase receptors and their ligands. Endocr Rev. 1994;15:135–162. doi: 10.1210/edrv-15-2-135. [DOI] [PubMed] [Google Scholar]
- Foster DC, Garbers DL. Dual role for adenine nucleotides in the regulation of the atrial natriuretic peptide receptor, guanylyl cyclase-A. J Biol Chem. 1998;273:16311–16318. doi: 10.1074/jbc.273.26.16311. [DOI] [PubMed] [Google Scholar]
- Furuya H, Yoshino K, Shimizu T, Mantoku T, Takeda T, Nomura K, Suzuki N. Mass spectrometric analysis of phosphoserine residues conserved in the catalytic domain of membrane-bound guanylyl cyclase from sea urchin spermatozoa. Zool Sci. 1998;15:507–516. doi: 10.2108/0289-0003(1998)15[507:MSAOPR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Garbers DL. Molecular basis of fertilization. Annu Rev Biochem. 1989;58:719–742. doi: 10.1146/annurev.bi.58.070189.003443. [DOI] [PubMed] [Google Scholar]
- Garbers DL, Lowe DG. Guanylyl cyclase receptors. J Biol Chem. 1994;269:30741–3074. [PubMed] [Google Scholar]
- Katafuchi T, Takashima A, Kashiwagi M, Hagiwara H, Takei Y, Hirose S. Cloning and expression of eel natriuretic-peptide receptor B and comparison with its mammalian counterparts. Eur J Biochem. 1994;222:835–842. doi: 10.1111/j.1432-1033.1994.tb18930.x. [DOI] [PubMed] [Google Scholar]
- Koller KJ, Lipari MT, Goeddel DV. Proper glycosylation and phosphorylation of the type A natriuretic peptide receptor are required for hormone-stimulated guanylyl cyclase activity. J Biol Chem. 1993;268:5997–6003. [PubMed] [Google Scholar]
- Krupnick JG, Benovic JL. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- Liu W, Yoon J, Burg M, Chen L, Pak WL. Molecular characterization of two Drosophila guanylate cyclases expressed in the nervous system. J Biol Chem. 1995;270:12418–12427. doi: 10.1074/jbc.270.21.12418. [DOI] [PubMed] [Google Scholar]
- Lowe DG, Chang MS, Hellmiss R, Chen E, Singh S, Garbers DL, Goeddel DV. Human atrial natriuretic peptide receptor defines a new paradigm for second messenger signal transduction. EMBO J. 1989;8:1377–1384. doi: 10.1002/j.1460-2075.1989.tb03518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter LR. Phosphorylation-dependent regulation of the guanylyl cyclase-linked natriuretic peptide receptor B: dephosphorylation is a mechanism of desensitization. Biochemistry. 1998;37:2422–2429. doi: 10.1021/bi972303k. [DOI] [PubMed] [Google Scholar]
- Potter LR, Garbers DL. Dephosphorylation of the guanylyl cyclase-A receptor causes desensitization. J Biol Chem. 1992;267:14531–14534. [PubMed] [Google Scholar]
- Potter LR, Garbers DL. Protein kinase C-dependent desensitization of the atrial natriuretic peptide receptor is mediated by dephosphorylation. J Biol Chem. 1994;269:14636–14642. [PubMed] [Google Scholar]
- Potter LR, Hunter T. Identification and characterization of the major phosphorylation sites of the B-type natriuretic peptide receptor. J Biol Chem. 1998a;273:15533–15539. doi: 10.1074/jbc.273.25.15533. [DOI] [PubMed] [Google Scholar]
- Potter LR, Hunter T. Phosphorylation of the kinase homology domain is essential for activation of the A-type natriuretic peptide receptor. Mol Cell Biol. 1998b;18:2164–2172. doi: 10.1128/mcb.18.4.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramarao CS, Garbers DL. Purification and properties of the phosphorylated form of guanylate cyclase. J Biol Chem. 1988;263:1524–1529. [PubMed] [Google Scholar]
- Ruskoaho H. Atrial natriuretic peptide: synthesis, release, and metabolism. Pharmacol Rev. 1992;44:479–602. [PubMed] [Google Scholar]
- Sibley DR, Lefkowitz RJ. Molecular mechanisms of receptor desensitization using the β-adrenergic receptor-coupled adenylate cyclase system as a model. Nature. 1985;317:124–129. doi: 10.1038/317124a0. [DOI] [PubMed] [Google Scholar]
- Thorpe DS, Garbers DL. The membrane form of guanylate cyclase. Homology with a subunit of the cytoplasmic form of the enzyme. J Biol Chem. 1989;264:6545–6549. [PubMed] [Google Scholar]
- Thorsness PE, Koshland DE., Jr Inactivation of isocitrate dehydrogenase by phosphorylation is mediated by the negative charge of the phosphate. J Biol Chem. 1987;262:10422–10425. [PubMed] [Google Scholar]
- Vacquier VD, Moy GW. Stoichiometry of phosphate loss from sea urchin sperm guanylate cyclase during fertilization. Biochem Biophys Res Commun. 1986;137:1148–1152. doi: 10.1016/0006-291x(86)90345-1. [DOI] [PubMed] [Google Scholar]
- Ward GE, Moy GW, Vacquier VD. Dephosphorylation of sea urchin sperm guanylate cyclase during fertilization. Adv Exp Med Biol. 1986;207:359–382. doi: 10.1007/978-1-4613-2255-9_19. [DOI] [PubMed] [Google Scholar]
- Woods M, Houslay MD. Desensitization of atriopeptin stimulated accumulation and extrusion of cyclic GMP from a kidney epithelial cell line (MDCK) Biochem Pharmacol. 1991;41:385–394. doi: 10.1016/0006-2952(91)90535-d. [DOI] [PubMed] [Google Scholar]
- Yan M, Templeton DJ. Identification of 2 serine residues of MEK-1 that are differentially phosphorylated during activation by raf and MEK kinase. J Biol Chem. 1994;269:19067–19073. [PubMed] [Google Scholar]
- Yasunari K, Kohno M, Murakawa K, Yokokawa K, Horio T, Takeda T. Phorbol ester and atrial natriuretic peptide receptor response on vascular smooth muscle. Hypertension. 1992;19:314–319. doi: 10.1161/01.hyp.19.4.314. [DOI] [PubMed] [Google Scholar]
- Yu S, Avery L, Baude E, Garbers DL. Guanylyl cyclase expression in specific sensory neurons: a new family of chemosensory receptors. Proc Natl Acad Sci USA. 1997;94:3384–3387. doi: 10.1073/pnas.94.7.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]