Abstract
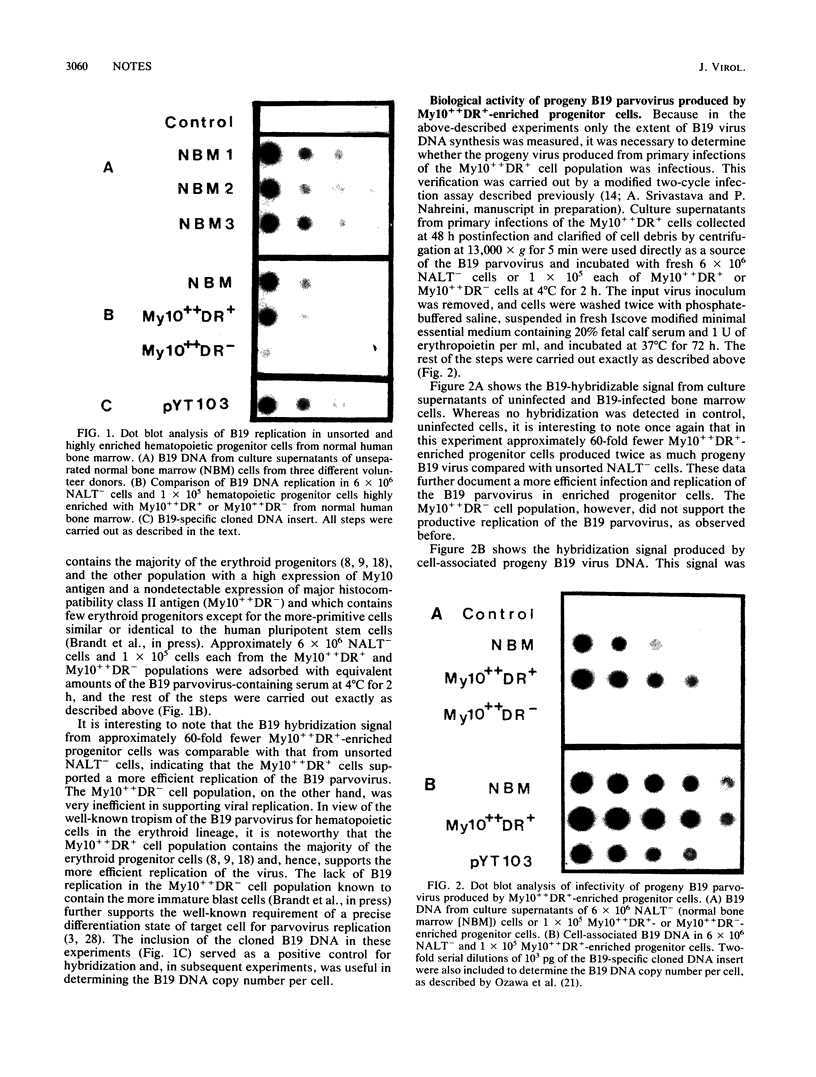
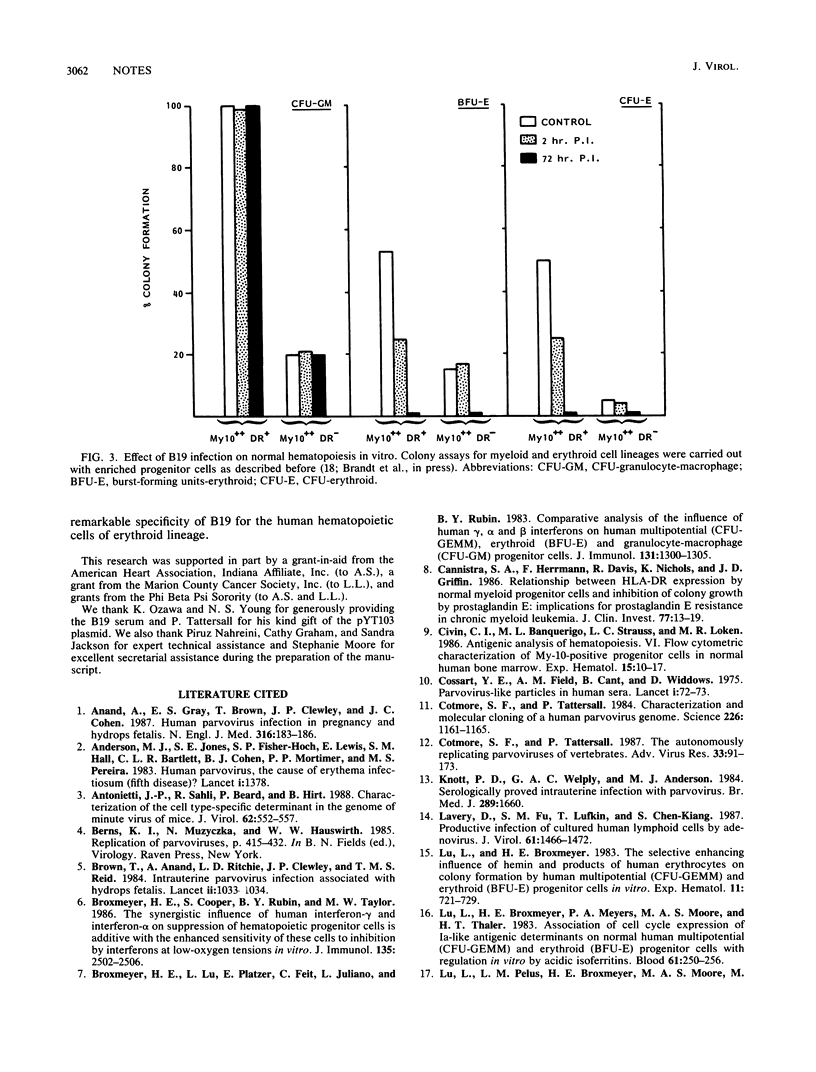
The target cell specificity of the B19 parvovirus infection was examined by isolating highly enriched hematopoietic progenitor and stem cells from normal human bone marrow. The efficiency of the B19 parvovirus replication in enriched erythroid progenitor cells was approximately 100-fold greater than that in unseparated bone marrow cells. The more-primitive progenitor cells identical to or closely related to the human pluripotent hematopoietic stem cells, on the other hand, did not support viral replication. The B19 progeny virus produced by the enriched erythroid progenitor cells was infectious and strongly suppressed erythropoiesis in vitro. The susceptibility of both the more-primitive erythroid progenitors (burst-forming units-erythroid) and the more-mature erythroid progenitors (CFU-erythroid) to the cytolytic response of the virus and the lack of effect on the myeloid progenitors (CFU-granulocyte-macrophage) further give evidence to the remarkable tropism of the B19 parvovirus for human hematopoietic cells of erythroid lineage.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anand A., Gray E. S., Brown T., Clewley J. P., Cohen B. J. Human parvovirus infection in pregnancy and hydrops fetalis. N Engl J Med. 1987 Jan 22;316(4):183–186. doi: 10.1056/NEJM198701223160403. [DOI] [PubMed] [Google Scholar]
- Anderson M. J., Jones S. E., Fisher-Hoch S. P., Lewis E., Hall S. M., Bartlett C. L., Cohen B. J., Mortimer P. P., Pereira M. S. Human parvovirus, the cause of erythema infectiosum (fifth disease)? Lancet. 1983 Jun 18;1(8338):1378–1378. doi: 10.1016/s0140-6736(83)92152-9. [DOI] [PubMed] [Google Scholar]
- Antonietti J. P., Sahli R., Beard P., Hirt B. Characterization of the cell type-specific determinant in the genome of minute virus of mice. J Virol. 1988 Feb;62(2):552–557. doi: 10.1128/jvi.62.2.552-557.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T., Anand A., Ritchie L. D., Clewley J. P., Reid T. M. Intrauterine parvovirus infection associated with hydrops fetalis. Lancet. 1984 Nov 3;2(8410):1033–1034. doi: 10.1016/s0140-6736(84)91126-7. [DOI] [PubMed] [Google Scholar]
- Broxmeyer H. E., Cooper S., Rubin B. Y., Taylor M. W. The synergistic influence of human interferon-gamma and interferon-alpha on suppression of hematopoietic progenitor cells is additive with the enhanced sensitivity of these cells to inhibition by interferons at low oxygen tension in vitro. J Immunol. 1985 Oct;135(4):2502–2506. [PubMed] [Google Scholar]
- Broxmeyer H. E., Lu L., Platzer E., Feit C., Juliano L., Rubin B. Y. Comparative analysis of the influences of human gamma, alpha and beta interferons on human multipotential (CFU-GEMM), erythroid (BFU-E) and granulocyte-macrophage (CFU-GM) progenitor cells. J Immunol. 1983 Sep;131(3):1300–1305. [PubMed] [Google Scholar]
- Cannistra S. A., Herrmann F., Davis R., Nichols K., Griffin J. D. Relationship between HLA-DR expression by normal myeloid progenitor cells and inhibition of colony growth by prostaglandin E. Implications for prostaglandin E resistance in chronic myeloid leukemia. J Clin Invest. 1986 Jan;77(1):13–20. doi: 10.1172/JCI112267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civin C. I., Banquerigo M. L., Strauss L. C., Loken M. R. Antigenic analysis of hematopoiesis. VI. Flow cytometric characterization of My-10-positive progenitor cells in normal human bone marrow. Exp Hematol. 1987 Jan;15(1):10–17. [PubMed] [Google Scholar]
- Cossart Y. E., Field A. M., Cant B., Widdows D. Parvovirus-like particles in human sera. Lancet. 1975 Jan 11;1(7898):72–73. doi: 10.1016/s0140-6736(75)91074-0. [DOI] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. Characterization and molecular cloning of a human parvovirus genome. Science. 1984 Dec 7;226(4679):1161–1165. doi: 10.1126/science.6095448. [DOI] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- Knott P. D., Welply G. A., Anderson M. J. Serologically proved intrauterine infection with parvovirus. Br Med J (Clin Res Ed) 1984 Dec 15;289(6459):1660–1660. doi: 10.1136/bmj.289.6459.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavery D., Fu S. M., Lufkin T., Chen-Kiang S. Productive infection of cultured human lymphoid cells by adenovirus. J Virol. 1987 May;61(5):1466–1472. doi: 10.1128/jvi.61.5.1466-1472.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Broxmeyer H. E., Meyers P. A., Moore M. A., Thaler H. T. Association of cell cycle expression of Ia-like antigenic determinations on normal human multipotential (CFU-GEMM) and erythroid (BFU-E) progenitor cells with regulation in vitro by acidic isoferritins. Blood. 1983 Feb;61(2):250–256. [PubMed] [Google Scholar]
- Lu L., Broxmeyer H. E. The selective enhancing influence of hemin and products of human erythrocytes on colony formation by human multipotential (CFUGEMM) and erythroid (BFUE) progenitor cells in vitro. Exp Hematol. 1983 Sep;11(8):721–729. [PubMed] [Google Scholar]
- Lu L., Pelus L. M., Broxmeyer H. E., Moore M. A., Wachter M., Walker D., Platzer E. Enhancement of the proliferation of human marrow erythroid (BFU-E) progenitor cells by prostaglandin E requires the participation of OKT8-positive T lymphocytes and is associated with the density expression of major histocompatibility complex class II antigens on BFU-E. Blood. 1986 Jul;68(1):126–133. [PubMed] [Google Scholar]
- Lu L., Walker D., Broxmeyer H. E., Hoffman R., Hu W., Walker E. Characterization of adult human marrow hematopoietic progenitors highly enriched by two-color cell sorting with My10 and major histocompatibility class II monoclonal antibodies. J Immunol. 1987 Sep 15;139(6):1823–1829. [PubMed] [Google Scholar]
- Lu L., Welte K., Gabrilove J. L., Hangoc G., Bruno E., Hoffman R., Broxmeyer H. E. Effects of recombinant human tumor necrosis factor alpha, recombinant human gamma-interferon, and prostaglandin E on colony formation of human hematopoietic progenitor cells stimulated by natural human pluripotent colony-stimulating factor, pluripoietin alpha, and recombinant erythropoietin in serum-free cultures. Cancer Res. 1986 Sep;46(9):4357–4361. [PubMed] [Google Scholar]
- Mortimer P. P., Humphries R. K., Moore J. G., Purcell R. H., Young N. S. A human parvovirus-like virus inhibits haematopoietic colony formation in vitro. 1983 Mar 31-Apr 6Nature. 302(5907):426–429. doi: 10.1038/302426a0. [DOI] [PubMed] [Google Scholar]
- Ozawa K., Kurtzman G., Young N. Productive infection by B19 parvovirus of human erythroid bone marrow cells in vitro. Blood. 1987 Aug;70(2):384–391. [PubMed] [Google Scholar]
- Ozawa K., Kurtzman G., Young N. Replication of the B19 parvovirus in human bone marrow cell cultures. Science. 1986 Aug 22;233(4766):883–886. doi: 10.1126/science.3738514. [DOI] [PubMed] [Google Scholar]
- Pattison J. R., Jones S. E., Hodgson J., Davis L. R., White J. M., Stroud C. E., Murtaza L. Parvovirus infections and hypoplastic crisis in sickle-cell anaemia. Lancet. 1981 Mar 21;1(8221):664–665. doi: 10.1016/s0140-6736(81)91579-8. [DOI] [PubMed] [Google Scholar]
- Reid D. M., Reid T. M., Brown T., Rennie J. A., Eastmond C. J. Human parvovirus-associated arthritis: a clinical and laboratory description. Lancet. 1985 Feb 23;1(8426):422–425. doi: 10.1016/s0140-6736(85)91146-8. [DOI] [PubMed] [Google Scholar]
- Saarinen U. M., Chorba T. L., Tattersall P., Young N. S., Anderson L. J., Palmer E., Coccia P. F. Human parvovirus B19-induced epidemic acute red cell aplasia in patients with hereditary hemolytic anemia. Blood. 1986 May;67(5):1411–1417. [PubMed] [Google Scholar]
- Serjeant G. R., Topley J. M., Mason K., Serjeant B. E., Pattison J. R., Jones S. E., Mohamed R. Outbreak of aplastic crises in sickle cell anaemia associated with parvovirus-like agent. Lancet. 1981 Sep 19;2(8247):595–597. doi: 10.1016/s0140-6736(81)92739-2. [DOI] [PubMed] [Google Scholar]
- Siegl G., Bates R. C., Berns K. I., Carter B. J., Kelly D. C., Kurstak E., Tattersall P. Characteristics and taxonomy of Parvoviridae. Intervirology. 1985;23(2):61–73. doi: 10.1159/000149587. [DOI] [PubMed] [Google Scholar]
- Spalholz B. A., Tattersall P. Interaction of minute virus of mice with differentiated cells: strain-dependent target cell specificity is mediated by intracellular factors. J Virol. 1983 Jun;46(3):937–943. doi: 10.1128/jvi.46.3.937-943.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. G., Woolf A. D., Mortimer P. P., Cohen B. J., Blake D. R., Bacon P. A. Human parvovirus arthropathy. Lancet. 1985 Feb 23;1(8426):419–421. doi: 10.1016/s0140-6736(85)91145-6. [DOI] [PubMed] [Google Scholar]
- Young N. S., Mortimer P. P., Moore J. G., Humphries R. K. Characterization of a virus that causes transient aplastic crisis. J Clin Invest. 1984 Jan;73(1):224–230. doi: 10.1172/JCI111195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young N., Harrison M., Moore J., Mortimer P., Humphries R. K. Direct demonstration of the human parvovirus in erythroid progenitor cells infected in vitro. J Clin Invest. 1984 Dec;74(6):2024–2032. doi: 10.1172/JCI111625. [DOI] [PMC free article] [PubMed] [Google Scholar]





