Abstract
Although intermittent episodes of low-level viremia are often observed in well-suppressed highly active antiretroviral therapy (HAART)-treated patients, the timing and amplitude of viral blips have never been examined in detail. We analyze here the dynamics of viral blips, i.e., plasma VL measurements of >50 copies/ml, in 123 HAART-treated patients monitored for a mean of 2.6 years (range, 5 months to 5.3 years). The mean (± the standard deviation) blip frequency was 0.09 ± 0.11/sample, with about one-third of patients showing no viral blips. The mean viral blip amplitude was 158 ± 132 human immunodeficiency virus type 1 (HIV-1) RNA copies/ml. Analysis of the blip frequency and amplitude distributions suggest that two blips less than 22 days apart have a significant chance of being part of the same episode of viremia. The data are consistent with a hypothetical model in which each episode of viremia consists of a phase of VL rise, followed by two-phase exponential decay. Thus, the term “viral blip” may be a misnomer, since viral replication appears to be occurring over an extended period. Neither the frequency nor the amplitude of viral blips increases with longer periods of observation, but the frequency is inversely correlated with the CD4+-T-cell count at the start of therapy, suggesting that host-specific factors but not treatment fatigue are determinants of blip frequency.
Since the introduction of highly active antiretroviral therapy (HAART), there has been a dramatic decrease in human immunodeficiency virus (HIV)-related mortality. In diverse cohorts, most HAART-treated patients attain “undetectable” levels of plasma viral RNA (<50 copies/ml) within months of starting antiviral therapy. However, levels of HIV type 1 (HIV-1) RNA in plasma of <50 copies/ml do not imply that viral replication has stopped (2, 4, 5, 9, 21, 23, 24), and most patients have intermittent positive plasma HIV-1 RNA determinations (viral blips) (2, 18, 23). The source and meaning of viral blips in the setting of seemingly effective HAART remain unclear. Viral blips might result from release of drug-sensitive virions from the latent reservoir (7) or might signal viral replication that occurs as a result of lack of adherence to drug treatment or increases in target cells secondary to infection or vaccination (10) that allow greater opportunities for viral replication when drug therapy is not completely suppressive. It has been reported that viral blips are correlated with the emergence of drug-resistant virions (1), a finding suggestive of ongoing replication driving blips. In addition, we previously showed that an increased frequency of blips correlates with a slower decay of latently infected cells (18), again suggesting ongoing replication during blips, which in this case refills viral reservoirs (8). Easterbrook et al. (3) found that HAART-treated patients exhibiting intermittent viremia of >400 copies/ml were three times more likely to experience sustained viral rebound and to have impaired CD4 cell rises relative to those that maintained undetectable viral loads (VLs; <400 copies/ml). In contrast, Havlir et al. (6) found that blips in patients treated with protease inhibitor-based HAART regimens were not associated with virologic failure over 4.5 years of observation, and Sklar et al. (22) found that the occurrence of transient viremia was independent of whether the patient was HAART naive or experienced or currently taking protease inhibitors or not. Also, such viremia did not appear to affect the risk of developing sustained viremia.
Whether blips represent random events or follow some pattern that could provide information about ongoing events in patients with “undetectable” VLs is the focus of the present study. Here we provide a comprehensive analysis of the dynamics of viral blips by analyzing plasma VL data from eight prospective studies of combinations of antiviral therapy in heterogeneous cohorts of HIV type 1 (HIV-1)-infected individuals (Table 1).
TABLE 1.
Treatment and baseline characteristics at the time of antiretroviral treatment initiation, as well as the time of observation during the period of viral load suppression, the number of viral load measurements taken during this period, and the frequency of viral blips during this period
| Treatmenta | No. of patients | Mean ± SD
|
||||
|---|---|---|---|---|---|---|
| CD4+ T cells/μl | HIV-1 RNA (log copies/ml) | Time of observation (days) | No. of VL measurements | Frequency (blips/sample) | ||
| LOP/RIT/TDF/EFV/3TC | 13 | 383 ± 128 | 4.82 ± 0.80 | 187 ± 81 | 8 ± 3 | 0.05 ± 0.08 |
| RIT/SAQ/ZDV/3TC | 21 | 454 ± 283 | 4.86 ± 0.97 | 1,156 ± 372 | 39 ± 16 | 0.13 ± 0.09 |
| ABC/APV/3TC | 29 | 564 ± 237 | 5.15 ± 0.97 | 473 ± 199 | 17 ± 8 | 0.06 ± 0.08 |
| ABC/APV/ZDV/3TC | 26 | 515 ± 322 | 4.84 ± 0.70 | 919 ± 320 | 29 ± 11 | 0.08 ± 0.08 |
| NLF/RIT/DDI/D4T | 11 | 478 ± 177 | 4.37 ± 0.74 | 720 ± 338 | 23 ± 10 | 0.14 ± 0.15 |
| IND/ZDV/3TC | 8 | 423 ± 102 | 5.13 ± 0.83 | 961 ± 204 | 24 ± 7 | 0.06 ± 0.10 |
| NLF/ZDV/3TC | 10 | 288 ± 164 | 4.99 ± 0.51 | 1,379 ± 490 | 40 ± 17 | 0.17 ± 0.15 |
| RIT/ZDV/3TC | 5 | 591 ± 194 | 4.21 ± 0.93 | 1,194 ± 388 | 24 ± 12 | 0.07 ± 0.13 |
| All | 123 | 474 ± 254 | 4.88 ± 0.88 | 810 ± 468 | 26 ± 15 | 0.09 ± 0.11 |
Antiretroviral agents are as defined in Materials and Methods.
MATERIALS AND METHODS
VL data and substudy treatments.
VL data were obtained with a reverse transcriptase PCR (RT-PCR) assay (Amplicor; Roche Diagnostics Systems, Alameda, Calif.) with a lower threshold of 50 copies/ml of plasma. The patients were tested on average once a month and on average had 26 VL measurements, taken during their period of sustained VL suppression (Table 1). Given the density of sampling and the mean follow-up period, this set of patients represents a relatively rich data set from which knowledge on blip dynamics can be extracted.
Study subjects were treated with daily dosages of selected antiviral medications in one of eight combinations: (i) lopinavir (LOP), 1,066 mg; ritonavir (RIT), 266 mg; tenofovir DF (TDF), 300 mg; efavirenz (EFV), 600 mg; and lamivudine (3TC), 300 mg; (ii) RIT, 800 mg; saquinavir (SAQ), 800 mg; zidovudine (ZDV), 600 mg; and 3TC, 300 mg; (iii) abacavir (ABC), 600 mg; amprenavir (APV), 2,400 mg; and 3TC, 300 mg; (iv) ABC, 600 mg; APV, 2,400 mg; ZDV, 600 mg; and 3TC, 300 mg; (v) nelfinavir (NLF), 1,500 mg; RIT, 800 mg; didanosine (DDI), 400 mg; and stavudine (D4T), 80 mg; (vi) indinavir (IND), 2,400 mg; ZDV, 600 mg; and 3TC, 300 mg; (vii) NLF, 2,500 mg; ZDV, 600 mg; and 3TC, 300 mg; and (viii) RIT, 2,400 mg; ZDV, 600 mg; and 3TC, 300 mg (Table 1).
Statistical analysis.
The distribution of the number of patients versus the viral blip frequency (see Fig. 2A) was obtained by subdividing the range of observed viral blip frequencies per patient into four subintervals and by counting the number of patients (y axis) exhibiting a viral blip frequency within a given subinterval (the extremes of each subinterval are shown on the x axis). Analogously, the distribution of number of blips versus viral blip amplitude (see Fig. 4a) was obtained by counting the number of blips (y axis) with amplitude in each 50-copy-width subinterval (the extremes of each subinterval are shown on the x axis). The relative risk (RR) of observing a blip at the VL measurement after a blip if the VL measurement is made <Δt days later versus the situation in which the VL measurement is made ≥Δt days later was calculated as follows: RR = (N1/T1)/(N2/T2). For each Δt ≥ 8 days, we first counted the number of pairs of consecutive VL measurements with the first being a blip and the time between the measurements being <Δt days (T1); we then counted the number of pairs among T1 that had a blip at the second VL measurement (N1). T2 and N2 are defined analogously with <Δt replaced by ≥Δt days. The RR value obtained from the patient database was then compared for each Δt with RRrnd, defined as the mean relative risk computed in 1,000 randomly reordered databases in which the data were randomly reordered within each patient from the third to the penultimate VL measurement.
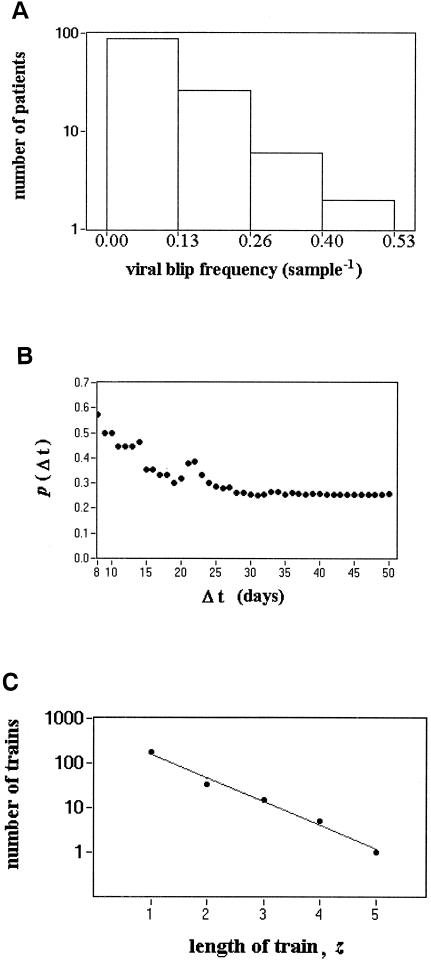
FIG. 2.
Viral blip frequency. (A) Distribution of viral blip frequencies. The histogram was obtained by subdividing the range of observed blip frequencies per patient into four subintervals and counting the number of patients with a blip frequency within each subinterval (the extremes of each subinterval are shown on the x axis). Occurrence of consecutive blips. (B) The probability, p(Δt), of observing a blip at the VL measurement after the observation of a viral blip and made at a time <Δt days later. p(Δt) decreases with the time between the two VL measurements and after ∼30 days stabilizes at a value that is independent of the sampling time. (C) Number of trains versus the length of trains.
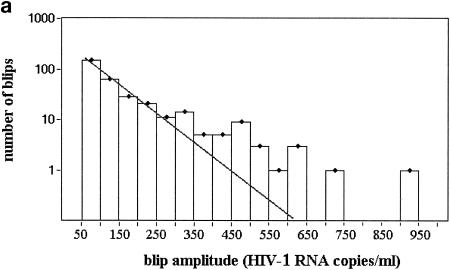
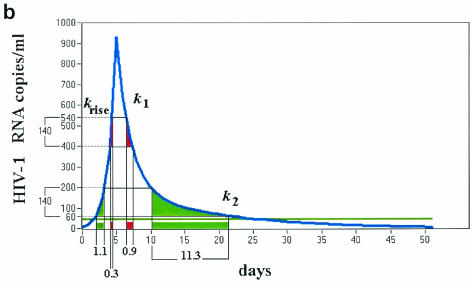
FIG. 4.
Viral blip amplitudes, blip shape, and correlation between CD4+-T-cell count at the start of therapy and viral blip frequency. (a) Distribution of number of blips versus viral blip amplitude. The histogram was obtained by counting the number of blips (y axis) with amplitude in each subinterval of 50 HIV-1 RNA copies/ml of width (the extremes of each subinterval are shown on the x axis). (b) Hypothetical blip with a rapidly rising and then two-phase exponential decline. If one randomly samples this profile, the number of blips with amplitude in the low range (60 to 200) would be ∼10-fold higher than the number of blips in the high range (400 to 540). This requires the ratio between k1 and k2 to be ∼10, and the rising phase must be sharp enough to be neglected. The same ratio was observed in the distribution of blip amplitudes collected from the entire database of patients and is sensitive to the ratio between the two decay constants k1 and k2 but not to the duration of the intermittent episode over the threshold. Values of k1 = 0.6 day−1 and k2 = 0.06 day−1 are consistent with a duration over threshold of approximately 20 to 30 days. (c) CD4+-T-cell count at the start of therapy versus viral blip frequency. Spearman rank correlation, ρ = −0.35 (P = 3.9 × 10−5). The regression line and the 95% confidence interval are shown.
The change in viral blip frequency over time was analyzed by subdividing the period of VL suppression in nonoverlapping and consecutive subperiods. The analysis was restricted to patients that were observed for >2 years (during the period of sustained VL suppression), 3 years, or 4 years. Each subinterval was fixed to 180 days (6 months), 270 days (9 months), or 360 days (1 year), and the analysis was limited to the first 2, 3, or 4 years, respectively. Given the definition of the period of VL suppression, the left extreme of the first time window was fixed at the third VL measurement of the period of suppression. The difference between the means of the blip frequency within each subperiod was tested for significance by the nonparametric repeated-measurement analysis of variance (Friedman test) (13). The difference between the means of the viral blip amplitudes occurring within each subperiod was tested for significance by the Kruskal-Wallis test. The correlation between frequency of viral blips during the period of VL suppression and VL (log number of copies/milliliter) or CD4+-T-cell counts (cells/microliter) at day zero (start of therapy) was tested for significance with the Spearman rank correlation test.
For some analyses, data were randomly reordered within each patient maintaining the same viral blip frequency. To comply with the definition of the period of VL suppression, the data randomized for each patient consisted of the VL measurements from the third to the penultimate VL measurement. Thus, if a patient i had ni VL measurements, then each VL measurement was assigned to a new rank, randomly extracted without replacement from the set of integers: 3,…, ni − 1.
RESULTS
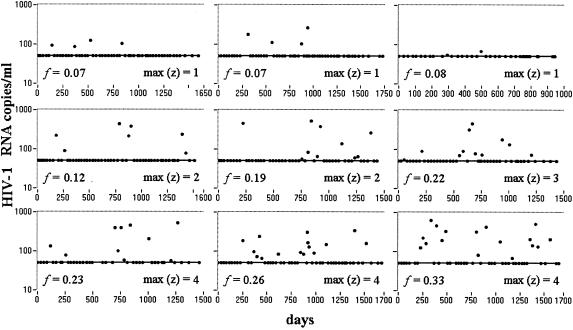
The general pattern of VL decay observed consisted of a fast first phase, followed by a slower second phase, a finding consistent with previous observations (16). After 2 to 6 months of therapy, VLs in most patients fell below the threshold value of the RT-PCR assay used in these trials, i.e., 50 copies/ml. After this period, patients usually had VLs below threshold with occasional blips. We define the period of sustained VL suppression as starting when two consecutive VL measurements below threshold occur and ending at the last VL measurement below threshold. Figure 1 shows the periods of sustained VL suppression in nine different patients showing different viral blip frequencies.
FIG. 1.
Occurrence of blips during the period of VL suppression in nine representative patients. The period starts when two consecutive VL measurements at below the threshold value occur and terminates at the last VL measurement that is below the threshold value. Longer sequences of consecutive blips are usually observed in patients that show higher viral blip frequencies. max(z), maximum number of consecutive blips; f, viral blip frequency.
The eight clinical trials included 175 patients. Patients were eliminated from this analysis if they did not show a period of sustained VL suppression or if this period was too poorly sampled (<4 VL measurements). Using these criteria, a subgroup of 123 patients was available for viral blip analysis. In 121 of these patients this period lasted for the entire period of observation. The remaining two patients showed a sustained rebound of viremia, and the period of analysis was terminated at the last VL measurement below threshold preceding the rebound. If the patient abandoned the study or the follow-up ended, the period of VL suppression was terminated at the last VL measurement below threshold. Overall, the 123 selected periods of suppression consisted, on average, of a VL of 26 ± 15 (mean ± standard deviation) measurements and covered a time period of 810 ± 468 days. The baseline characteristics of the patients at the time of antiretroviral treatment initiation are shown in Table 1.
Viral blip frequency.
The mean and median frequencies of viral blips in the 123 patients were 0.09 and 0.06/sample, respectively (Table 1). The distribution of viral blip frequencies, shown in Fig. 2A, was wide, with 41 patients showing no blips and 1 patient showing as many as one blip for every two samples. This distribution suggests that patients have different tendencies to show viral blips and argues against the hypothesis that viral blips simply represent assay variation. This can be addressed more formally by examining the distribution of the number of patients showing s blips. If patients have the same a priori probability to have a blip, then the probability of having s VLs over a given threshold, T, in n VL measurements is given by the binomial distribution. In our study, VLs were not measured the same number of times for each patient, invalidating the use of the simple binomial probability distribution. However, one can generalize the binomial distribution to the case in which patients are observed a different number of times and, using this distribution, we have shown that in general patients have different tendencies to show blips, arguing against assay variations being the major cause of blips (14).
If blips do not represent assay variations, then we need to explore the physiological factors that differ among patients, which might explain the observed variability, and determine whether the occurrence of blips is of clinical consequence for the patient. To do this, we first asked how much virus is associated with a blip. To be precise, we distinguish the terms viral blip and an intermittent episode of viremia (IEV), which we define as starting when the VL increases over the threshold and ending when the VL returns below the threshold. Thus, two VL measurements above threshold, i.e., blips, may belong to the same IEV or may belong to two independent intermittent episodes of viremia. In order to estimate the length of an episode of viremia, we sought to determine whether, if we observed one blip at time t, the probability of observing another blip at times up to t + Δt was higher for small values of Δt than for large values. The idea here was that over short time intervals we should be sampling the same episode of viremia and, as the time interval gets longer, we should begin sampling different episodes of viremia.
Figure 2B shows p(Δt), the probability of observing two consecutive blips at two consecutive VL measurements, given that the first measurement is a blip and the subsequent VL measurement is made at any time up to Δt time units later. We calculated p(Δt) by first determining all pairs of VL measurements in which the first is a blip and the subsequent VL measurement is made <Δt time units later. The fraction of these pairs in which the second VL measurement was also a blip is p(Δt). When the sampling time is >4 weeks the probability stabilizes to a value that is independent of Δt, suggesting that episodes of viremia may last as long as 4 weeks. To obtain a more accurate estimate, we also computed the relative risk of observing a blip when the second VL measurement is made <Δt days later versus when the second VL measurement is made ≥Δt days later. The relative risk of showing a blip at the VL measurement following a blip becomes statistically indistinguishable (P > 0.05) at Δt = 22 days from the relative risk computed from a data set in which the VL data for each patient were randomly reordered. Note that the number of blips in VL measurements scheduled <22 days from the previous blip accounts for only 5.5% of the total number of blips, but nevertheless this small fraction turned out to be sufficient to highlight a statistically significant effect in the relative risk analysis.
Consecutive blips in VL measurements scheduled less than 22 days apart occur in patients showing a mean blip frequency of 0.25 ± 0.15. This is not significantly different from the mean blip frequency (0.23 ± 0.11; Student t test, P = 0.62) of patients in which consecutive blips were observed in VL measurements taken more than 22 days apart (i.e., the complementary group in the relative risk analysis). The latter evidence excludes the possibility that patients that had a VL measurement shortly after the occurrence of a blip were also the ones who showed a general higher tendency to show blips during the entire period of suppression. The elevated probability of showing a blip soon after a blip is additional evidence that blips do not represent assay variations.
Viral blips occur substantially at random.
We next sought to determine whether blips occur randomly or do they tend to follow some pattern in time. In Fig. 1, showing the blips in 9 patients, 2, 3 or 4 consecutive blips sometimes occur. Does this imply blips are not random or can such “trains” or sequences of consecutive blips occur by chance? To address this, we sought to determine whether the distribution of sequences of z consecutive blips differs from the distribution obtained from a “random data set” created by randomizing the data within each patient and thus destroying any order, if present, of blip arrival. Because blips that occur less than 22 days apart may be part of the same episode of viremia and hence not random, we analyzed all of the blip data, as well as a “filtered” set of data in which blips following a blip by less than 22 days were eliminated. Both analyses gave similar results. This is not surprising because blips that occur less than 22 days apart account for only 5.5% of all blips. Figure 2C shows that the number of trains of z consecutive blips in our full data set falls exponentially with z (slope, m = −1.22; 95% confidence interval, −1.42 to −1.02; test of significance m ≠ 0, P = 3.5 × 10−4). By randomizing the data within each patient we observed a similar exponentially decaying train distribution with a mean decay constant that is not different from the one observed in the original data (P = 0.32; mean ± the standard deviation slope among randomly reordered data, m = −1.27 ± 0.10; test of significance, m ≠ 0; mean value of P = 7.3 × 10−3). Thus, trains of consecutive of blips, even as long as five, can be reproduced by the casual occurrence of consecutive and independent blips and do not necessarily represent a sustained episode of viremia lasting for the period covered by the train, nor do they necessarily indicate treatment failure.
Long trains tend to occur in patients that have a high frequency of blips as evidenced by a positive correlation between the maximum number of consecutive blips per patient and blip frequency (Spearman rank correlation between viral blip frequency and maximum length of trains per patient, ρ = 0.66 [P = 8.0 × 10−12]. Since this analysis is based on estimates of viral blip frequency in each patient, i.e., on the observed number of blips divided by the total number of observations, which have different reliabilities depending on the total number of observations, the results could be skewed. For example, if patient A showed 1 blip out of 5 VLs, and patient B showed 10 blips out of 50 VLs, both patients will have viral blip frequency 0.2/sample but the estimate of viral blip frequency is more “reliable” for patient B than for patient A. However, when we only consider patients that were observed for more than 35 times during the period of suppression, the correlation between viral blip frequency and maximum length of trains remains and even improves [Spearman rank correlation, n = 32, ρ = 0.78, P = 4.6 × 10−9]. In addition, the same Spearman rank correlation between the maximum length of trains and the frequency of viral blips per patient was observed in the randomized data [ρ = 0.66 ± 0.04]). This can be qualitatively observed in Fig. 1, where patients with isolated blips are the ones with lower viral blip frequencies, whereas patients with longer trains are the ones who have higher tendencies to show blips. Due to this correlation, it is not common to observe a relatively long sequence of consecutive blips and no other blips during the period of suppression. Such an occurrence should raise concerns about adherence or other causes of incomplete suppression of viral replication even when the VL amplitudes of the blips are not increasing.
Viral blip frequency does not increase with longer observation periods.
In Fig. 3 the mean viral blip frequency observed in nonoverlapping subperiods of 6 months, 9 months, or 1 year is shown. The analysis was restricted to patients that were observed more than 2 years (Fig. 3a), 3 years (Fig. 3b), or 4 years (Fig. 3c). A repeated-measures test, as well as the Friedman test to account for the lack of a normal distribution of viral blip frequency, did not detect statistically significant changes in viral blip frequencies between subintervals. Thus, the viral blip frequency does not increase with longer periods of observation. This result, together with the previous analysis showing that blips appear to be distributed continuously over the period of suppression, suggests that viral blip frequency is constant over time. By using linear regression to estimate the slope of the viral blip frequency versus the subinterval, in each patient, we identified two patients in the 2-year group and one patient each in the 3-year and 4-year groups with a negative slope significantly different from zero with 0.01 < P < 0.05 (data not shown). Thus, four patients were outliers and showed evidence of viral blip frequency changing with time. However, the frequency in these four patients decreased rather than increased as one would have expected if blips predicted treatment failure.
FIG. 3.
Mean viral blip frequency (▪) and mean viral blip amplitude (•) ± the standard error in nonoverlapping subperiods of observations. The mean viral blip frequency and the mean viral blip amplitude do not vary significantly with the time of observation. The change in mean viral blip frequency was tested for significance (Pf) with nonparametric repeated-measures analysis of variance (Friedman test). The change in mean viral blip amplitude was tested for significance (PA) with the Kruskal-Wallis test. (a) Analysis restricted to patients that were observed more than 2 years from the third visit during the period of VL suppression (n = 63). Subintervals were fixed at 180 days (6 months), and the analysis was limited to the first 2 years (Pf = 0.17 and PA = 0.44). (b) Analysis restricted to patients that were observed more than 3 years from the third visit during the period of VL suppression (n = 30). Subintervals were fixed at 270 days (9 months), and the analysis was limited to the first three years (Pf = 0.44 and PA = 0.84). (c) Analysis restricted to patients observed more than 4 years from the third visit during the period of VL suppression (n = 10). Subintervals were fixed at 360 days (1 year), and the analysis was limited to the first four years (Pf = 0.08 and PA = 0.35).
Viral blip amplitudes.
The mean ± the standard deviation and median viral blip amplitude were 158 ± 132 and 104 HIV-1 RNA copies/ml, respectively. The mean viral blip amplitudes observed in nonoverlapping subperiods of 6 months, 9 months, or 1 year did not show statistically significance changes over time (Fig. 3), as well as in sequences of consecutive blips (data not shown). In addition, the mean viral blip amplitude in sequences of consecutive blips does not increase within each sequence (data not shown). Thus, one blip does not drive a following blip of higher amplitude.
The distribution of blip amplitudes has an interesting pattern, with most blips having low amplitude and a few blips having large amplitude. The distribution of viral blip amplitudes, shown in Fig. 4a, appears to be exponential at low amplitudes (i.e., for blips up to ∼400 copies/ml) but decreases more slowly than exponentially at high amplitudes. We investigated whether particular profiles of intermittent episodes of viremia might generate the peculiar pattern of the observed distribution.
VL profile during an IEV (blip shape).
If we assume that the time a VL is measured is random with respect to the VL changes occurring during an IEV, then one is equally likely to sample an IEV at any point in its history. Although, to our knowledge, no one has ever sampled a patient frequently enough during an IEV to know how the VL changes during the episode, we do know that after a patient is put on HAART the VL decays exponentially in a rapid first phase, followed by a slower second exponential decay (16). Also, after a therapy interruption, VLs tend to rise rapidly (19, 20). Thus, we investigated different shapes or set of shapes for an IEV that are compatible with the measured amplitude distribution. In mathematical terms we are solving what has been called an “inverse problem,” i.e., computing from the amplitude distribution an underlying distribution of “blip shapes” that generated the given amplitude distribution. In general, such problems do not have unique solutions.
We can show that a rapidly rising function followed by a two-phase exponential decay can generate the observed distribution of viral blip amplitudes, even when the peak VL in each IEV is different (Fig. 4b). Although we cannot estimate the two decay constants separately, we can estimate that their ratio must be about 10 (Fig. 4b). If we use the additional information of a mean duration over a threshold of approximately 20 to 30 days, then the two decay constants approach the values of 0.6 and 0.06 day−1, values which are similar to the decay constants estimated in previous studies and reflect the death rates of short and long-lived subpopulations of infected cells (15).
Viral blip frequency correlates with factors that precede the period of treatment.
Finally, we observed a statistically significant inverse correlation between the CD4+-T-cell count at the start of therapy, CD40, and the frequency of viral blips during the period of suppression (ρ = −0.35, P = 3.7 × 10−5) (Fig. 4c). As expected, a positive correlation (ρ = 0.28, P = 8.7 × 10−4) was observed between the VL at the start of therapy, V0, and the blip frequency. A multivariate correlation analysis in which both the variables V0 and CD40 were considered did not show an increase in the prediction of blip frequency.
Taken together, these observations suggest that viral blip frequency is independent of the period of treatment but does appear to be, at least partially, determined by factors established preceding treatment, which affect the probability of showing blips during treatment.
DISCUSSION
Studies in which patients on potent antiretroviral therapy were periodically tested for plasma HIV-1 RNA by using assays with a detection limit of 50 copies/ml have shown that most well-suppressed patients demonstrate intermittent positive plasma HIV-1 RNA determinations (viral blips) (2, 18, 23). The source and the meaning of these episodic low-level viremias in the setting of seemingly effective HAART remain unclear. Nevertheless, achieving low levels of viremia during antiretroviral treatment predicts a sustained virological response. Kempf et al. reported a strong association between the nadir plasma HIV-1 RNA and the durability of response to treatment (11). Using a more sensitive PCR assay, Raboud et al. observed that patients whose viremia fell to <20 copies/ml are less prone to virological failure than those who stayed at levels above this threshold (17). Havlir et al. found an association between viral blips and a higher steady state of viral replication, but not virological failure over 4.5 years of observation, with virological failure defined as two consecutive plasma VLs of >200 copies/ml (6). Whether the emergence of drug-resistant virions is associated with viral blips during treatment is still controversial (1, 7) and deserves further and prompt investigation. In principle, blips might arise also as a result of lack in adherence to therapy. Thus, we have investigated the occurrence of viral blips in a group of 123 patients treated with eight different protease inhibitor-containing treatment regimens, with the objective of characterizing blip arrival.
In examining the distribution of viral blip frequencies in the entire population, we showed that the variability in the observed number of blips per patient could not simply be explained by chance, thus supporting the conclusion that patients have different tendencies to show blips. This conclusion also supports the notion that the majority of blips are not simply assay measurement error. Then, by asking whether the observation of a blip predicts the arrival of other blips at subsequent VL measurements, we showed that blips arrive “substantially” at random. The adjective “substantial” is used to highlight that some degree of structure is present in blip arrivals, as confirmed by a risk analysis showing that, when a VL measurement is taken within 22 days of a blip, there is a significantly higher probability that the new VL measurement is still a blip. This structure in blip arrivals can be reproduced by assuming that intermittent episodes of viremia start at random and have a common duration of ca. 20 to 30 days. In this case, two consecutive VL measurements, if scheduled too close to each other, might capture the same IEV, which ultimately explains the observed degree of structure.
In addition, we found patients showing longer sequences of consecutive blips are also the ones who have higher viral blip frequencies during the period of VL suppression, whereas patients showing only isolated blips are the ones who have lower viral blip frequencies. Thus, a sequence of consecutive blips, even when they are as long as five, can be reproduced by the casual occurrence of independent blips and do not necessarily represent sustained VL over the threshold for the period covered by the sequence of blips.
In the patients studied it appears that blip frequency and amplitude do not increase with time on therapy, suggesting that reduced adherence as a result of treatment fatigue with prolonged therapy is not a major factor in generating the observed blips (Fig. 3). Finally, the observed inverse correlation between CD4+-T-cell count at the start of therapy and viral blip frequency suggests that the frequency of these randomly arriving intermittent episodes of viremia, is, at least partially, determined by factors that precede the period of treatment. The evidence that an IEV may last over the threshold of 50 copies/ml for 20 days or more also implies that ongoing replication and not simply release of virus from activation of latently infected cells underlies the appearance of a blip. The exact cause of blips remains unknown, although one can speculate that incomplete drug penetration into certain compartments is allowing viral replication to occur (12) and random events, such as intercurrent illnesses, increase the number of activated CD4+ target cells and consequently the amount of residual viral replication (10). Because our work suggests that blips are typically part of a 20- to 30-day episode of elevated viremia, it is unlikely that a blip simply corresponds to one or a few missed drug dosages. If ongoing replication is occurring, therapy is not fully potent or the drugs being used cannot fully penetrate all compartments. In such cases, therapy changes or the addition of newer, more potent agents to a drug regime may suppress blips. Whether this will lead to clinical benefit remains to be determined in larger prospective trials but, as reported here, isolated blips and even sequences of successive blips were followed in all but two cases by a return to a level of suppression of <50 copies/ml and not to virological failure.
Acknowledgments
This study was performed under the auspices of the U.S. Department of Energy and was supported by NIH grants RR06555, AI28433, 1A141387, and AI41534; the General Clinical Research Center of Rockefeller University (MO1-RR00102); and the Columbia-Rockefeller Center for AIDS Research (A142848).
We acknowledge the clinical assistance of Rhonda Kost, Andrew Talal, and Bharat Ramratnam of the Rockefeller University Hospital Clinic and Roche Diagnostics for providing HIV-1 testing materials. We also thank Carla Wofsy, Jerome Percus, and Ora Percus for helpful discussions on the mathematical and statistical techniques used to analyze the data.
REFERENCES
- 1.Cohen-Stuart, J. W., A. M. Wensing, C. Kovacs, M. Righart, D. de Jong, S. Kaye, R. Schuurman, C. J. Visser, and C. A. Boucher. 2001. Transient relapses (“blips”) of plasma HIV RNA levels during HAART are associated with drug resistance. J. Acquir. Immune Defic. Syndr. 28:105-113. [DOI] [PubMed] [Google Scholar]
- 2.Dornadula, G., H. Zhang, B. VanUitert, J. Stern, L. Livornese, Jr., M. J. Ingerman, J. Witek, R. J. Kedanis, J. Natkin, J. DeSimone, and R. J. Pomerantz. 1999. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. JAMA 282:1627-1632. [DOI] [PubMed] [Google Scholar]
- 3.Easterbrook, P. J., N. Ives, A. Waters, J. Mullen, S. O'Shea, B. Peters, and B. G. Gazzard. 2002. The natural history and clinical significance of intermittent viraemia in patients with initial viral suppression to <400 copies/ml. AIDS 16:1521-1527. [DOI] [PubMed] [Google Scholar]
- 4.Furtado, M. R., D. S. Callaway, J. P. Phair, K. J. Kunstman, J. L. Stanton, C. A. Macken, A. S. Perelson, and S. M. Wolinsky. 1999. Persistence of HIV-1 transcription in peripheral-blood mononuclear cells in patients receiving potent antiretroviral therapy. N. Engl. J. Med. 340:1614-1622. [DOI] [PubMed] [Google Scholar]
- 5.Gunthard, H. F., S. D. Frost, A. J. Leigh-Brown, C. C. Ignacio, K. Kee, A. S. Perelson, C. A. Spina, D. V. Havlir, M. Hezareh, D. J. Looney, D. D. Richman, and J. K. Wong. 1999. Evolution of envelope sequences of human immunodeficiency virus type 1 in cellular reservoirs in the setting of potent antiviral therapy. J. Virol. 73:9404-9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Havlir, D. V., R. Bassett, D. Levitan, P. Gilbert, P. Tebas, A. C. Collier, M. S. Hirsch, C. Ignacio, J. Condra, H. F. Gunthard, D. D. Richman, and J. K. Wong. 2001. Prevalence and predictive value of intermittent viremia with combination hiv therapy. JAMA 286:171-179. [DOI] [PubMed] [Google Scholar]
- 7.Hermankova, M., S. C. Ray, C. Ruff, M. Powell-Davis, R. Ingersoll, R. T. D'Aquila, T. C. Quinn, J. D. Siliciano, R. F. Siliciano, and D. Persaud. 2001. HIV-1 drug resistance profiles in children and adults with viral load of <50 copies/ml receiving combination therapy. JAMA 286:196-207. [DOI] [PubMed] [Google Scholar]
- 8.Ho, D. D. 1998. Toward HIV eradication or remission: the tasks ahead. Science 280:1866-1867. [DOI] [PubMed] [Google Scholar]
- 9.Hockett, R. D., J. M. Kilby, C. A. Derdeyn, M. S. Saag, M. Sillers, K. Squires, S. Chiz, M. A. Nowak, G. M. Shaw, and R. P. Bucy. 1999. Constant mean viral copy number per infected cell in tissues regardless of high, low, or undetectable plasma HIV RNA. J. Exp. Med. 189:1545-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones, L. E., and A. S. Perelson. 2002. Modeling the effects of vaccination on chronically infected HIV-positive patients. J. Acquir. Immune Defic. Syndr. 31:369-377. [DOI] [PubMed] [Google Scholar]
- 11.Kempf, D. J., R. A. Rode, Y. Xu, E. Sun, M. E. Heath-Chiozzi, J. Valdes, A. J. Japour, S. Danner, C. Boucher, A. Molla, and J. M. Leonard. 1998. The duration of viral suppression during protease inhibitor therapy for HIV-1 infection is predicted by plasma HIV-1 RNA at the nadir. AIDS 12:F9-F14. [DOI] [PubMed] [Google Scholar]
- 12.Kepler, T. B., and A. S. Perelson. 1998. Drug concentration heterogeneity facilitates the evolution of drug resistance. Proc. Natl. Acad. Sci. USA 95:11514-11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motulsky, H. 1995. Intuitive biostatistics, p. 250-254. Oxford University Press, New York, N.Y.
- 14.Percus, J. K., O. E. Percus, M. Markowitz, D. D. Ho, M. Di Mascio, and A. S. Perelson. 2003. The distribution of viral blips observed in HIV-1-infected patients treated with combination antiretroviral therapy. Bull. Math Biol. 65:263-277. [DOI] [PubMed] [Google Scholar]
- 15.Perelson, A. S., P. Essunger, Y. Cao, M. Vesanen, A. Hurley, K. Saksela, M. Markowitz, and D. D. Ho. 1997. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 387:188-191. [DOI] [PubMed] [Google Scholar]
- 16.Perelson, A. S., P. Essunger, and D. D. Ho. 1997. Dynamics of HIV-1 and CD4+ lymphocytes in vivo. AIDS 11:S17-S24. [PubMed] [Google Scholar]
- 17.Raboud, J. M., J. S. Montaner, B. Conway, S. Rae, P. Reiss, S. Vella, D. Cooper, J. Lange, M. Harris, M. A. Wainberg, P. Robinson, M. Myers, and D. Hall. 1998. Suppression of plasma viral load below 20 copies/ml is required to achieve a long-term response to therapy. AIDS 12:1619-1624. [DOI] [PubMed] [Google Scholar]
- 18.Ramratnam, B., J. E. Mittler, L. Zhang, D. Boden, A. Hurley, F. Fang, C. A. Macken, A. S. Perelson, M. Markowitz, and D. D. Ho. 2000. The decay of the latent reservoir of replication-competent HIV-1 is inversely correlated with the extent of residual viral replication during prolonged anti-retroviral therapy. Nat. Med. 6:82-85. [DOI] [PubMed] [Google Scholar]
- 19.Ruiz, L., G. Carcelain, J. Martinez-Picado, S. Frost, S. Marfil, R. Paredes, J. Romeu, E. Ferrer, K. Morales-Lopetegi, B. Autran, and B. Clotet. 2001. HIV dynamics and T-cell immunity after three structured treatment interruptions in chronic HIV-1 infection. AIDS 15:F19-F27. [DOI] [PubMed] [Google Scholar]
- 20.Ruiz, L., J. Martinez-Picado, J. Romeu, R. Paredes, M. K. Zayat, S. Marfil, E. Negredo, G. Sirera, C. Tural, and B. Clotet. 2000. Structured treatment interruption in chronically HIV-1-infected patients after long-term viral suppression. AIDS 14:397-403. [DOI] [PubMed] [Google Scholar]
- 21.Sharkey, M. E., I. Teo, T. Greenough, N. Sharova, K. Luzuriaga, J. L. Sullivan, R. P. Bucy, L. G. Kostrikis, A. Haase, C. Veryard, R. E. Davaro, S. H. Cheeseman, J. S. Daly, C. Bova, R. T. Ellison III, B. Mady, K. K. Lai, G. Moyle, M. Nelson, B. Gazzard, S. Shaunak, and M. Stevenson. 2000. Persistence of episomal HIV-1 infection intermediates in patients on highly active anti-retroviral therapy. Nat. Med. 6:76-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sklar, P. A., D. J. Ward, R. K. Baker, K. C. Wood, Z. Gafoor, C. F. Alzola, A. C. Moorman, and S. D. Holmberg. 2002. Prevalence and clinical correlates of HIV viremia (“blips”) in patients with previous suppression below the limits of quantification. AIDS 16:2035-2041. [DOI] [PubMed] [Google Scholar]
- 23.Yerly, S., T. V. Perneger, S. Vora, B. Hirschel, and L. Perrin. 2000. Decay of cell-associated HIV-1 DNA correlates with residual replication in patients treated during acute HIV-1 infection. AIDS 14:2805-2812. [DOI] [PubMed] [Google Scholar]
- 24.Zhang, L., B. Ramratnam, K. Tenner-Racz, Y. He, M. Vesanen, S. Lewin, A. Talal, P. Racz, A. S. Perelson, B. T. Korber, M. Markowitz, and D. D. Ho. 1999. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N. Engl. J. Med. 340:1605-1613. [DOI] [PubMed] [Google Scholar]