Abstract
Staphylococci often form biofilms, sessile communities of microcolonies encased in an extracellular matrix that adhere to biomedical implants or damaged tissue. Infections associated with biofilms are difficult to treat, and it is estimated that sessile bacteria in biofilms are 1,000 to 1,500 times more resistant to antibiotics than their planktonic counterparts. This antibiotic resistance of biofilms often leads to the failure of conventional antibiotic therapy and necessitates the removal of infected devices. Lysostaphin is a glycylglycine endopeptidase which specifically cleaves the pentaglycine cross bridges found in the staphylococcal peptidoglycan. Lysostaphin kills Staphylococcus aureus within minutes (MIC at which 90% of the strains are inhibited [MIC90], 0.001 to 0.064 μg/ml) and is also effective against Staphylococcus epidermidis at higher concentrations (MIC90, 12.5 to 64 μg/ml). The activity of lysostaphin against staphylococci present in biofilms compared to those of other antibiotics was, however, never explored. Surprisingly, lysostaphin not only killed S. aureus in biofilms but also disrupted the extracellular matrix of S. aureus biofilms in vitro on plastic and glass surfaces at concentrations as low as 1 μg/ml. Scanning electron microscopy confirmed that lysostaphin eradicated both the sessile cells and the extracellular matrix of the biofilm. This disruption of S. aureus biofilms was specific for lysostaphin-sensitive S. aureus, as biofilms of lysostaphin-resistant S. aureus were not affected. High concentrations of oxacillin (400 μg/ml), vancomycin (800 μg/ml), and clindamycin (800 μg/ml) had no effect on the established S. aureus biofilms in this system, even after 24 h. Higher concentrations of lysostaphin also disrupted S. epidermidis biofilms.
Staphylococcal infections of both Staphylococcus aureus and Staphylococcus epidermidis continue to be a major problem in hospital settings, especially among immunocompromised and immunosuppressed patients, particularly those with indwelling devices (29). In the Centers for Disease Control and Prevention's national emerging infectious disease plan entitled Preventing Emerging Infectious Diseases: a Strategy for the 21st Century (4), one of the specified goals is to determine how to control biofilms on medical devices and tubing in medical settings. In 1998, an estimated 200 million catheters, including urinary catheters and central venous catheters, were implanted in North American patients alone (14). Staphylococci cause a large percentage of catheter infections, and like many other pathogens, rather than living as free planktonic cells within the host, they tend to form a multilayered community of sessile bacteria cells known as a biofilm on medical implants or damaged tissue (9, 10, 15, 17). Biofilm infections are difficult to treat due to their inherent antibiotic resistance (9, 15, 17).
Once a staphylococcal biofilm has formed on an implanted medical device or damaged tissue, it is difficult to disrupt. A biofilm-infected implant often must be removed and replaced, placing the patient at increased risk for complications due to these additional procedures (5, 32). Current antimicrobial therapies for biofilms have largely proven unsuccessful (15), and the exact explanation for these treatment failures is still unclear (9). Additional strategies for the clearance of biofilm-associated infections are needed.
Lysostaphin is an antibacterial enzyme which is specifically capable of cleaving the cross-linking pentaglycine bridges in the cell walls of staphylococci (38). S. aureus cell walls contain high proportions of pentaglycine, making lysostaphin a highly effective agent against both actively growing and quiescent bacteria. Lysostaphin has also been shown to be effective against S. epidermidis, albeit at higher concentrations of the enzyme (25, 42). Lysostaphin has gained renewed interest as an antistaphylococcal therapeutic agent (12, 24, 30) because of the growing emergence of antibiotic-resistant S. aureus. Antibiotic-resistant S. aureus organisms (including both methicillin-resistant S. aureus [MRSA] and intermediately vancomycin-susceptible S. aureus) are susceptible to lysostaphin action (21). Despite the effectiveness of lysostaphin against S. aureus both in vitro and in various animal models, it has yet to be determined whether lysostaphin would prove any more effective than any other antibiotic against staphylococci in biofilms. In this paper, we demonstrate the efficacy of lysostaphin against S. aureus and S. epidermidis biofilms in vitro by use of a modification of previous biofilm assays (6, 13, 19).
MATERIALS AND METHODS
Bacterial strains.
Six S. aureus and three S. epidermidis strains were used in these studies and are listed in Table 1. Stocks were maintained at −70°C in tryptic soy broth (TSB) before their use in the biofilm assays. Strain ATCC 35556 (SA113) was selected because it is considered a benchmark strain among the biofilm-producing S. aureus species (11, 19), and S. epidermidis strain ATCC 35984 was used for the same reason (34). Pseudomonas aeruginosa strain ATCC 15692 was also examined in some experiments.
TABLE 1.
Strains of staphylococci used in these studies
| Strain | Description and antibiotic resistancea | Lysostaphin MIC (μg/ml) | Origin or referencec |
|---|---|---|---|
| S. aureus | |||
| ATCC 49521 | MSSA, capsule type 5 | 0.008 | Direct from ATCC |
| ATCC 35556 (SA113) | MSSA | 0.004 | 19 |
| ATCC 35556 dltA-KO | MSSA, dltA knockout | 0.002 | 19 |
| Col | MRSA | 0.008 | NARSA NRS100 |
| MBT 5040b | MRSA, streptomycin resistant | 0.004 | Fresh clinical isolate obtained from WRAMC |
| MBT 5040 LysoR | Lysostaphin-resistant variant | >>32 | Isolated in vitro from MBT 5040 |
| SA5 LysoR | Lysostaphin-resistant variant | >>32 | Isolated in vitro |
| S. epidermidis | |||
| ATCC 35984 | High slime producerd | 64 | Direct from ATCC |
| SE1175 | Moderate slime producerd | 2 | Clinical isolateb |
| Hay | Low slime producerd | 32 | ATCC 55133, direct from ATCC |
Confirmed by disk diffusion assay. MSSA, methicillin-susceptible S. aureus.
Identity confirmed by the API STAPH identification test (bioMerieux, Lombard, Ill).
WRAMC, Walter Reed Army Medical Center; ATCC, American Type Culture Collection.
As determined by the method described in reference 6; data not shown.
Antimicrobial agents.
Recombinant homogenous lysostaphin was purchased from Nutrition 21, Inc. (Purchase, N.Y.), or produced by Biosynexus Incorporated. Oxacillin, vancomycin, and clindamycin were purchased from Sigma Chemical Co. (St. Louis, Mo.).
Biofilm plate assays with lysostaphin treatment.
The biofilm plate assay used was a modification of several previous biofilm plate assays (6, 13, 19). Briefly, S. aureus from a blood agar plate (Remel, Lenexa, Kans.) was grown in 5 ml of TSB (BBL, Sparks, Md.) supplemented with 0.25% d-(+)-glucose (Sigma). The overnight bacterial culture was diluted 1:50 in TSB plus 0.25% glucose to a final volume of 200 μl in each well of a 96-well tissue culture plate (Costar Inc., Corning, N.Y.), a final volume of 500 μl in each chamber of a Lab-Tek II chamber slide (Nalge-Nunc International, Naperville, Ill.), or a final volume of 1 ml in polycarbonate transwell tissue culture inserts (10-mm-diameter tissue culture inserts, 0.02-μm-pore-size Anapore membrane; Nalge-Nunc International) placed in a 24-well tissue culture plate (Nunclon surface multidish with 24 wells; Nalge-Nunc International).
The various plates were incubated at 37°C with shaking at 100 rpm for 24 h; in some experiments, plates were removed from the shaker and supplemented with either 50 μl (96-well plates) or 200 μl (tissue culture inserts) of additional fresh TSB plus 0.25% glucose to compensate for medium evaporation. These plates were placed in the stationary incubator at 37°C, and growth was allowed to continue for an additional 24 h.
After 24 to 48 h of growth, depending on the experiment, the various wells were washed twice with 200 μl of phosphate-buffered saline (PBS) per well (96-well plates), 500 μl of PBS per chamber (chamber slides), or 1 ml of double-distilled water (ddH2O) per insert (transwells) to remove nonadherent bacteria. The biofilm-containing wells were treated either with various concentrations of lysostaphin, namely, 0.8 to 200 μg/ml (96-well plates), 25 to 200 μg/ml (chamber slides), or 50 to 100 μg/ml (tissue culture inserts), diluted in PBS or TSB plus 0.25% glucose, or with PBS or TSB plus 0.25% glucose alone (control wells) for up to 24 h with gentle shaking at ambient temperature. The treatment buffer was gently aspirated at the end of treatment, and the wells were washed once with volumes of PBS equivalent to the treatment volume. The assay plates were inverted and allowed to dry at 37°C for approximately 2 h. The wells of the 96-well plate and the tissue culture inserts were stained with 200 μl of enhanced Gram safranin (Remel) for 1 min. The stain was removed, and the wells were gently washed twice with 200 μl of PBS (microtiter plates) or 500 μl of ddH2O (transwells). The assay plates were observed for the degree and intensity of staining in the wells. Following incubations and washing, partitions on chamber slides were removed, and the slides were heat fixed and Gram stained (Remel).
A biofilm of P. aeruginosa was formed by a method similar to that used for staphylococci. A plastic chamber slide containing TSB plus 0.25% glucose was inoculated with an overnight culture of P. aeruginosa, and a biofilm was allowed to form for 48 h. The established P. aeruginosa biofilm was treated with 200 μg of lysostaphin/ml for 5 h in PBS. Following treatment, the P. aeruginosa biofilm was washed with PBS and Gram stained.
To prepare samples for scanning electron microscopy (SEM), the procedures of the biofilm plate assay in transwell inserts (described above) were followed. After the inserts were air dried, they were fixed in a 4% glutaraldehyde solution (16, 39) in ddH2O (pH 7.0). SEM was performed at the Carnegie Institute of Washington (Department of Embryology, Baltimore, Md.).
The SEM samples were stored at 4°C in TSB for several days prior to being processed. The tissue culture inserts were dehydrated in the following ethanol series: 35% ethanol (three times for 10 min), 50% ethanol (10 min), 75% ethanol (10 min), 95% ethanol (10 min), and 100% ethanol (three times for 10 min). The samples were placed in hexamethyldisilazane twice for 30 min each time and then were dried and sputter coated.
Comparison of lysostaphin to antibiotics in a 96-well plate assay.
To directly compare the kinetics of lysostaphin's effect on S. aureus biofilms to those of other antibiotics, biofilms were prepared in 96-well plate assays as described above. The plates were washed twice with PBS, and serial dilutions of lysostaphin or antibiotics, as noted in the legend to Fig. 5 and Results, were added to each well in either PBS or TSB plus 0.25% glucose in a total volume of 200 μl per well. The wells were agitated at room temperature for 24 h. The absorbance at 650 nm of bacteria in biofilm was measured every 20 min for the first 3 h after treatment and then again at 24 h using a microtiter plate reader. After treatment was complete, the wells were washed with PBS and then dried. Wells were stained with safranin and then washed twice with PBS to remove excess stain.
FIG. 5.
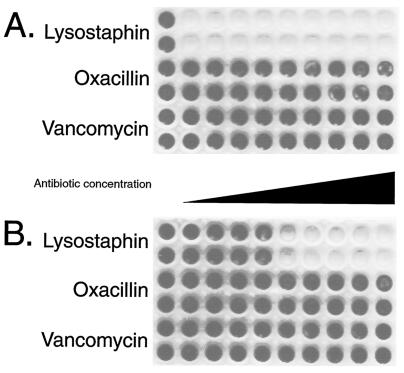
Oxacillin or vancomycin had no visible effect on S. aureus biofilms in PBS or bacterial media after incubation for 24 h. Polystyrene 96-well tissue culture wells were inoculated with, per ml, ∼108 CFU of S. aureus ATCC 35556 (MIC of lysostaphin, 0.004 μg/ml; MIC of oxacillin, 0.125 μg/ml; and MIC of vancomycin, 1 μg/ml). Following 24 h of biofilm formation, wells were washed twice with PBS and then treated for 24 h in either PBS (A) (this panel shows one of the plates from the experiment reported in Fig. 4) or TSB plus 0.25% glucose bacterial medium (B) with either no added antibiotic (first column of wells in each plate) or serial twofold dilutions of lysostaphin (first two rows; 0.8 to 200 μg/ml), oxacillin (third and fourth rows; 1.6 μg/ml to 400 μg/ml), or vancomycin (bottom two rows; 3.2 to 800 μg/ml) as indicated on the figure. Following treatment, the wells were washed with PBS and then stained with safranin. The darkly staining wells indicate the presence of biofilm following treatment. Lysostaphin in PBS cleared the biofilm at 0.8 μg/ml (A), while lysostaphin in TSB plus 0.25% glucose cleared the biofilm at 12.5 μg/ml (B).
RESULTS
Lysostaphin specifically disrupts S. aureus biofilms on abiotic surfaces.
The biofilm-forming capacities of the various S. aureus strains were observed by cultivating the biofilms on polycarbonate, polystyrene, or glass surfaces. The six S. aureus strains (Table 1) were inoculated in the wells of tissue culture-treated microtiter plates, polycarbonate transwells, or chambers of chamber slides and allowed to form biofilms over a 24- to 48-h period. The resulting S. aureus biofilms in tissue culture wells (Fig. 1 and 2) and inserts (data not shown) were stained with safranin, while the chamber slides (data not shown and see Fig. 6) were Gram stained to examine the biofilms on the surface of the slide. As previously reported (13, 19), the dltA-negative mutant of strain ATCC 35556 did not form a biofilm in our system (data not shown); however, all other strains of S. aureus examined formed biofilms on the various surfaces.
FIG. 1.
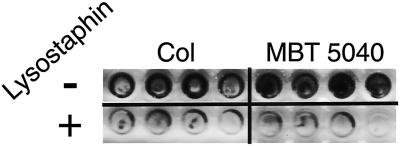
Lysostaphin-disrupted biofilms of MRSA strains Col and MBT 5040. Ninety-six-well polystyrene tissue culture wells (four for each sample) were inoculated with ∼108 CFU of S. aureus strain Col or MBT 5040 (as indicated). After we allowed 48 h for biofilm formation, the wells were washed twice and then incubated with (+) or without (−) 50 μg of lysostaphin/ml in PBS for 3 h. Following incubation, the wells were washed again and stained with safranin to visualize biofilms.
FIG. 2.
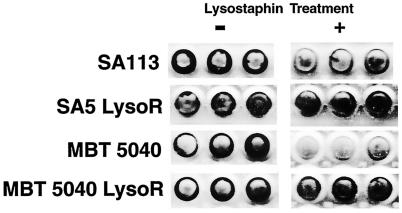
Lysostaphin did not disrupt biofilms formed by lysostaphin-resistant S. aureus variants. Tissue culture wells (three for each sample) of a microtiter plate were inoculated with ∼108 CFU of S. aureus strain SA113, SA5 LysoR, MBT 5040, or MBT 5040 LysoR (as indicated). After we allowed 48 h for biofilm formation, the wells were washed twice and then incubated with (+) or without (−) 50 μg of lysostaphin/ml in PBS for 3 h. Following incubation, the wells were washed again and stained with safranin to visualize biofilms.
FIG. 6.
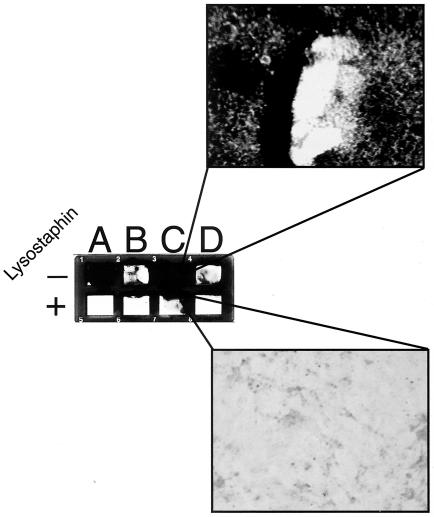
Lysostaphin-disrupted S. epidermidis biofilms. Glass chamber slide wells were inoculated with ∼5 × 107 CFU of either S. aureus strain SA113 as a control (A), S. epidermidis strain Hay (B), S. epidermidis strain ATCC 35984 (C), or S. epidermidis strain SE1175 (D) per ml. Biofilms were allowed to form for 24 h, and then wells were washed twice with PBS. Established biofilms were treated with PBS alone or with lysostaphin in PBS (200 μg/ml) for 3 h. Following treatment, the wells were washed with PBS followed by ddH2O and then Gram stained. The biofilms in the PBS-treated row (−) stained gram positive, while no gram-positive cells were seen in lysostaphin-treated (+) S. epidermidis wells. Only the residual extracellular glycocalyx in the corners of the wells stained gram negative in the lysostaphin-treated wells. The two enlarged sections reveal the multilayered biofilm of S. epidermidis strain ATCC 35984 (top) and the residual glycocalyx of the same strain with no intact staphylococci following lysostaphin treatment (bottom).
The capacity of lysostaphin to disrupt the biofilms of the various S. aureus strains was determined by visually comparing the differences of safranin stain or Gram stain intensities between lysostaphin-treated and untreated biofilms. Figure 1 shows 48-h biofilms of MRSA strains Col and MBT 5040 treated for 3 h with lysostaphin (50 μg/ml) or PBS. The darkly stained, PBS-treated Col and MBT 5040 biofilms contrasted with the lysostaphin-treated wells that had light or no staining, which revealed that lysostaphin successfully disrupted the biofilms of both MRSA strains. Upon microscopic observation of chamber slides of similar experiments, the residual staining in the lysostaphin-treated wells was determined to be debris and contained no intact staphylococci (data not shown).
When biofilms were treated with 25 μg of lysostaphin/ml, the biofilms of lysostaphin-sensitive S. aureus strains MBT 5040 and SA113 were disrupted; however, when biofilms of in vitro-isolated, lysostaphin-resistant variants of two S. aureus strains, MBT 5040 LysoR and SA5 LysoR, were treated with the same concentration of lysostaphin, the biofilms were not affected by the lysostaphin treatment (Fig. 2). Microscopic examination of S. aureus biofilms on chamber slides treated with 25 μg of lysostaphin/ml revealed that there were no remaining intact bacteria associated with the slide, and in most cases, the biofilm glycocalyx was not evident on the treated slides (data not shown).
The capacity of lysostaphin to disrupt the biofilms of another prolific biofilm producer, P. aeruginosa, was also evaluated. P. aeruginosa biofilms grown in plastic chamber slides were treated with lysostaphin at concentrations as high as 200 μg/ml for 5 h. This treatment had no visible effect on the biofilms; i.e., the staining intensity was the same with or without lysostaphin treatment, and microscopic examination of the P. aeruginosa biofilm revealed no change in the biofilm following lysostaphin treatment (data not shown).
SEM reveals that lysostaphin eradicates S. aureus biofilms.
To further assess the extent of lysostaphin's effects on the S. aureus biofilms, biofilm assays were conducted in polycarbonate transwells to allow SEM observation. Transwells were found to cultivate confluent biofilms and were the best substrate on which to observe these biofilms by SEM, as they were small and flat enough to view under an SEM without damaging the biofilm. SEM observation revealed that S. aureus biofilms grew as prolifically on a polycarbonate surface as on a polystyrene surface (Fig. 3A and B) and that 100 μg of lysostaphin/ml eradicated the S. aureus biofilm (Fig. 3C and D), releasing both sessile cells and the extracellular matrix from the polycarbonate transwells. Similar observations were made for six separate wells of each sample in two separate experiments. In similar polycarbonate transwell experiments observed by SEM, the biofilms produced by S. aureus strains MBT 5040, Col, and ATCC 35556 were also found to be eradicated; i.e., no sessile cells or glycocalyx remained after treatment with 100 μg of lysostaphin/ml in all wells examined (data not shown).
FIG. 3.
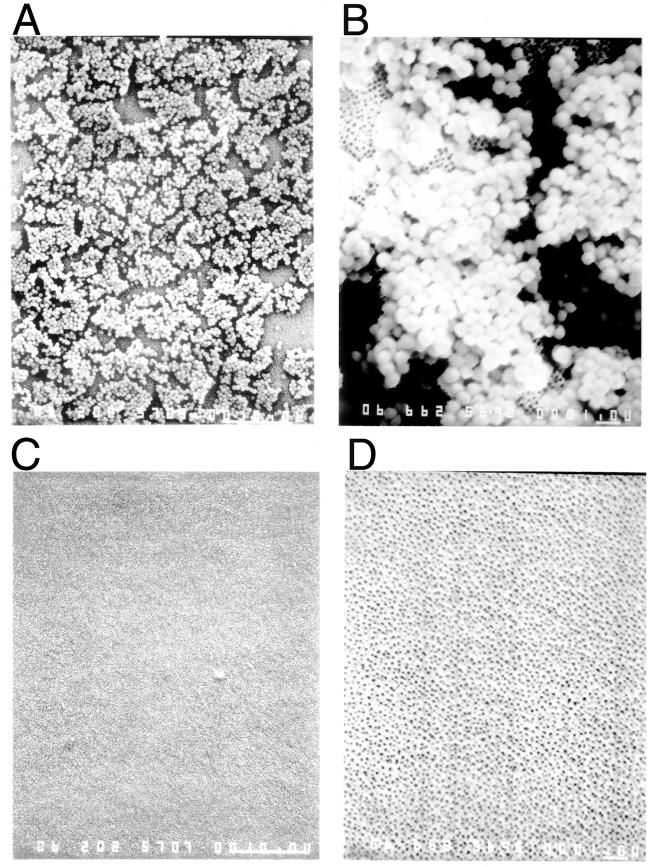
SEM revealed that lysostaphin eradicates S. aureus biofilms, removing both the sessile cells and the extracellular matrix. Polycarbonate transwells were inoculated with 5 × 108 CFU of S. aureus ATCC 49521 per ml. Following 48 h of biofilm formation, transwells were washed and then treated with either PBS (A and B) or 100 μg of lysostaphin/ml in PBS (C and D) for 3 h. The treated wells were washed again and then fixed with gluteraldehyde prior to SEM. The results shown are representative of the entire well. Magnifications, ×1,800 (A and C) and ×5,940 (B and D).
Lysostaphin begins to disrupt S. aureus biofilms immediately and does so more effectively than other antibiotics.
Oxacillin and vancomycin have often been used in antibiotic susceptibility studies of S. aureus biofilms (1, 18, 20, 31, 41). These antibiotics were compared to lysostaphin to determine whether lysostaphin was more effective in disrupting S. aureus strain ATCC 35556 biofilms than the conventional antibiotics. Twenty-four-hour biofilms in polystyrene 96-well tissue culture plates were treated with serial dilutions of lysostaphin, oxacillin, and vancomycin (Fig. 4 and 5).
FIG. 4.
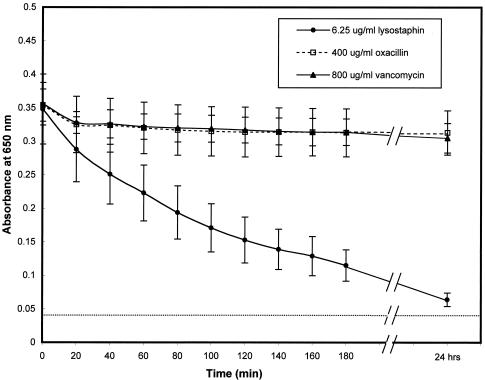
Lysostaphin caused an immediate and continuous drop in the absorbance of S. aureus biofilms, which continued over time. Polystyrene 96-well tissue culture wells were inoculated, per ml, with ∼108 CFU of S. aureus ATCC 35556 (MIC of lysostaphin, 0.004 μg/ml; MIC of oxacillin, 0.125 μg/ml; and MIC of vancomycin, 1 μg/ml). Following 24 h of biofilm formation, wells were washed twice with PBS and then treated with either lysostaphin (6.25 μg/ml), oxacillin (400 μg/ml), or vancomycin (800 μg/ml) in PBS. The absorbance at 650 nm following treatment was monitored by a microtiter plate reader every 20 min for 3 h and then again at 24 h. The data are the means of results for six samples from three separate experiments ± the standard deviations.
In order to examine the kinetic effects of lysostaphin, oxacillin, and vancomycin on biofilms, the absorbances at 650 nm of established biofilms in a 96-well tissue culture plate were measured over time (0 to 3 h and 24 h). Tissue culture wells containing biofilms of S. aureus SA113 were incubated with serial dilutions of lysostaphin (0.8 to 200 μg/ml), oxacillin (1.6 to 400 μg/ml), or vancomycin (3.2 to 800 μg/ml) for 24 h. The absorbance of the lysostaphin-treated biofilms dropped from approximately 0.35 at time zero to 0.125 after 3 h of treatment and dropped to near baseline (0.04) by 24 h when the biofilms were treated with a dose of lysostaphin of 6.25 μg/ml in PBS (Fig. 4). The absorbances of the biofilms treated with oxacillin or vancomycin for 24 h showed minimal change, remaining around 0.325, despite the fact that the biofilms were treated with as much as 400 μg of oxacillin/ml or 800 μg of vancomycin/ml in PBS (Fig. 4). Since antimicrobials like oxacillin and vancomycin are known to be more effective against actively metabolizing bacteria, a similar experiment was conducted but with incubation of the biofilms with the three antimicrobials in bacterial medium (TSB). Very similar results were found when the assay was conducted with TSB rather than PBS. Lysostaphin reduced the absorbance of biofilms to near background by 24 h, while oxacillin and vancomycin had little or no effect even after 24 h of incubation (data not shown).
The capacity of the three agents to disrupt S. aureus biofilms in polystyrene wells could be visualized by comparing the staining intensities of treated wells with those of control (buffer-treated) wells. Biofilms from the above-described kinetics experiment that were treated for 24 h stained darkly on the bottom of the wells (Fig. 5), while wells cleared of biofilms did not stain with safranin. Lysostaphin concentrations as low as 0.8 μg/ml in PBS (Fig. 5A) and 12.5 μg/ml in TSB plus 0.25% glucose (Fig. 5B) appeared to clear biofilms from the transwells, while 400 μg of oxacillin/ml or 800 μg of vancomycin/ml in PBS or TSB had no obvious effect on established biofilms even after 24 h of treatment (Fig. 5). The antibiotic clindamycin has also been shown to be somewhat effective for the disruption of some biofilms (27, 35). When this antibiotic was tested in a similar 96-well plate assay, concentrations of clindamycin as high as 800 μg/ml had little or no effect on the S. aureus biofilms even after 24 h of treatment (data not shown).
Lysostaphin disrupts S. epidermidis biofilms.
While lysostaphin demonstrated activity against S. aureus biofilms, it was of interest to explore whether biofilms of S. epidermidis, known to be less sensitive to lysostaphin (25, 42), were also sensitive to the biofilm-disrupting effect of lysostaphin. Three S. epidermidis strains with various capacities for glycocalyx (slime) production were examined, including S. epidermidis strain Hay (a low slime producer), S. epidermidis strain SE1175 (a moderate slime producer), and S. epidermidis ATCC 35984 (34) (a high slime producer). All three of these S. epidermidis strains produced biofilms on a glass chamber slide (Fig. 6), with ATCC 35984 producing the thickest and most darkly staining biofilm, as expected. Incubation of these S. epidermidis biofilms with 200 μg of lysostaphin/ml for 3 h disrupted the biofilms of all three strains of S. epidermidis (Fig. 6). S. aureus strain SA113 was included in this experiment as a control. Microscopic examination of the disrupted biofilms revealed that there were no intact bacteria left on the artificial surface (Fig. 6, inset). The stained material visible in lysostaphin-treated wells was extracellular glycocalyx, which stained pink by safranin and contained no intact gram-positive S. epidermidis cells, only cellular debris.
DISCUSSION
Lysostaphin's activity against planktonic staphylococci has been well documented (3, 7, 8, 25, 26, 42; J. F. Kokai-Kun, T. Chanturiya, and J. J. Mond, Abstr. 102nd Gen. Meet. Am. Soc. Microbiol. abstr. A-62, p. 12, 2002); it was not known, however, whether lysostaphin would be any more effective in disrupting staphylococcal biofilms than conventional antibiotics. We have now demonstrated that, in vitro, lysostaphin disrupted S. aureus biofilms on polystyrene, polycarbonate, and glass surfaces and also disrupted S. epidermidis biofilms on glass. When S. aureus biofilms were visualized by SEM, lysostaphin eradicated both the sessile S. aureus cells of the biofilm and the extracellular matrices (Fig. 3).
The exact mechanism of lysostaphin's action against S. aureus biofilms remains unclear. It has been shown that, even if an antimicrobial agent can kill some of the bacteria embedded in the biofilm, the biofilm is not disrupted unless the extracellular matrix is also destroyed (1, 18, 20, 36, 41). Lysostaphin may, therefore, be able to target and disrupt both the individual sessile cells of the S. aureus biofilms and the extracellular matrix. While there is no evidence that lysostaphin acts directly on the extracellular matrices of staphylococcal biofilms, it is possible that lysostaphin may target some components of these matrices. Since lysostaphin's primary enzymatic substrate is the pentaglycine cross bridges of the peptidoglycan of staphylococci (42), the extracellular staphylococcal biofilm matrix may contain components of cell walls, including polyglycines, that might be affected by lysostaphin, thus disrupting the extracellular biofilm matrix. Indeed, other macromolecules have been found in the extracellular matrix of biofilms; extracellular DNA is required for P. aeruginosa biofilm formation (40). A more likely explanation, however, is that the disruption of staphylococcal biofilms by lysostaphin occurs through the rapid lysis of the sessile staphylococci, which may be sufficient to destabilize the entire biofilm matrix in such a manner as to allow detachment from artificial surfaces. This explanation was supported by the finding that lysostaphin's capacity to disrupt S. aureus biofilms appeared to be specific for lysostaphin-sensitive S. aureus (Fig. 2), since biofilms formed by lysostaphin-resistant S. aureus were not disrupted by lysostaphin. The finding that biofilms from three strains of S. epidermidis, each with a different capacity to form slime, could also be disrupted by lysostaphin further supported the theory that lysostaphin attacks the actual cells of the biofilm, since the glycocalyxes of biofilms of various S. epidermidis strains are thought to have different chemical compositions (17).
The resistance to antibiotics of bacteria growing in biofilms remains an incompletely understood process and is an area of active research. The antibiotic resistance of bacteria in established biofilms may be due to a number of factors, including the multilayer structure of biofilms (33) and/or the unique genetic characteristics of bacteria in biofilms compared to those of planktonic cells (9). Antibiotics used to treat biofilm infections in hospitals also have to overcome the potential emergence of antibiotic resistance as well as the increased risk to the patient of developing allergies to the antibiotic therapies or other problems associated with antibiotics, such as diarrhea, as higher doses and longer courses of antibiotic treatments are used to try to eradicate biofilms (37). Antibiotic efficacy also decreases as treatment time increases (1). Thus, the recommended course of action for treatment of staphylococcal biofilm infections is to remove the infected device, treat the patient with rigorous antibiotic therapy, and reinsert a new device (2, 5). Despite this treatment regimen, the recurrence of infection is high (23, 41). Biofilm bacteria can usually survive antibiotics at concentrations 1,000 to 1,500 times higher than antibiotic concentrations used to treat bactericidal planktonic bacteria (9).
Using lysostaphin to treat staphylococcal-biofilm-associated infections may prove to be preferable to using antibiotics such as oxacillin, vancomycin, and clindamycin to treat these infections. It may be possible to administer lysostaphin at relatively low doses and disrupt a staphylococcal biofilm (Fig. 5), obviating the need for surgical removal of the infected device. Lysostaphin also has the advantage of a relatively short response time in terms of disrupting the biofilm; within 20 min of application there is measurable disruption of the biofilm (Fig. 4), and depending on the concentration of lysostaphin used, this disruption may be completed within hours. This time to biofilm disruption is relatively short compared to those with oxacillin and vancomycin, both of which have been reported to require approximately 24 h to disrupt biofilms (1, 28), an exposure time which had very little effect on S. aureus biofilms in this study (Fig. 4 and 5). The specific activity of lysostaphin for staphylococci has the added benefit of not disrupting normal flora, thus minimizing the occurrence of antibiotic-associated diarrheas or selection of antibiotic resistance in other opportunistic pathogens. Whether lysostaphin proves to be an effective therapy for staphylococcal-biofilm infections, however, will depend on results of preclinical testing that is under way (J. F. Kokai-Kun, T. Chanturiya, A. Shah, S. M. Walsh, and J. J. Mond, Abstr. 103rd Gen. Meet. Am. Soc. Microbiol., abstr. A-026, p. 6, 2003) and clinical testing which is planned for the future.
Lysostaphin is not the only enzyme to have been examined for its capacity to disrupt biofilms. Various combinations of enzymes have been studied for the removal and disinfection of bacterial biofilms in various systems (22). This process, however, requires a minimum of two enzymes, one enzyme for removal of the adherent bacteria of the biofilms and a second enzyme with bactericidal activity. Lysostaphin bridges this two-step process by both disrupting the S. aureus and S. epidermidis biofilms and killing the released bacteria.
It has been shown that coating medical implants with antimicrobials may effectively prevent the initial adherence of staphylococcal biofilms to the implants (29, 32, 37). Coating biomedical materials with lysostaphin may also prove successful in preventing early adherence of staphylococci to the implants, thus averting biofilm formation. Work is under way to determine whether lysostaphin-coated medical implants, such as catheters, will prevent staphylococcal adherence and the formation of biofilms (A. Shah and S. M. Walsh, unpublished data).
Bacterial biofilms in medical systems are thought to be virtually identical to biofilms in any other aquatic system (9). Our data demonstrate that lysostaphin disrupted both S. aureus and S. epidermidis biofilms in vitro and suggest that lysostaphin may be effective against staphylococcal-biofilm infections in vivo. We are currently examining the effectiveness of lysostaphin for the in vivo clearance of staphylococcal-biofilm-associated catheter infections by using a catheterized mouse model (Kokai-Kun et al., Abstr. 103rd Gen. Meet. Am. Soc. Microbiol.).
Acknowledgments
We thank Mike Sepanski for conducting SEM and Andreas Peschel for providing various strains of S. aureus.
J. A. Wu and C. Kusuma contributed equally to this work.
All of the authors of the manuscript are, or formerly were, employees of Biosynexus Incorporated. Biosynexus Incorporated is currently developing commercial applications of lysostaphin.
REFERENCES
- 1.Amorena, B., E. Gracia, M. Monzón, J. Leiva, C. Oteiza, M. Pérez, J. Alabart, and J. Hernández-Yago. 1999. Antibiotic susceptibility assay for Staphylococcus aureus in biofilms developed in vitro. J. Antimicrob. Chemother. 44:43-55. [DOI] [PubMed] [Google Scholar]
- 2.Bellón, J. M., N. G-Honduvilla, F. Jurado, A. G-Carranza, and J. Buján. 2001. In vitro interaction of bacteria with polypropylene/ePTFE prostheses. Biomaterials 22:2021-2024. [DOI] [PubMed] [Google Scholar]
- 3.Browder, H., W. Zygmunt, J. Young, and P. Travormina. 1965. Lysostaphin: enzymatic mode of action. Biochem. Biophys. Res. Commun. 19:383-389. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1998. Preventing emerging infectious diseases: a strategy for the 21st century. Centers for Disease Control and Prevention, Atlanta, Ga.
- 5.Chamis, A., G. Peterson, C. Cabell, G. Corey, G. Sorrentino, R. Greenfield, R. Thomas, L. Reller, and V. Fowler. 2001. Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter-defibrillators. Circulation 104:1029-1033. [DOI] [PubMed] [Google Scholar]
- 6.Christensen, G. D, W. A. Simpson, J. J. Younger, L. M. Baddour, F. F. Barrett, D. M. Melton, and E. H. Beachey. 1985. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 22:996-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Climo, M. W., K. Ehlert, and G. L. Archer. 2001. Mechanism and suppression of lysostaphin resistance in oxacillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:1431-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Climo, M. W., R. L. Patron, B. P. Goldstein, and G. L. Archer. 1998. Lysostaphin treatment of experimental methicillin-resistant Staphylococcus aureus aortic valve endocarditis. Antimicrob. Agents Chemother. 42:1355-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costerton, J. 1999. Introduction to biofilm. Int. J. Antimicrob. Agents 11:217-221. [DOI] [PubMed] [Google Scholar]
- 10.Costerton, J., P. Stewart, and E. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 11.Cucarella, C., C. Solano, J. Valle, B. Amorena, Í. Lasa, and J. R. Penadés. 2001. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J. Bacteriol. 183:2888-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dajcs, J., E. Hume, J. Moreau, A. Caballero, B. Cannon, and R. O'Callaghan. 2000. Lysostaphin treatment of methicillin-resistant Staphylococcus aureus keratitis in rabbits. Investig. Ophthalmol. Vis. Sci. 41:1432-1437. [PubMed] [Google Scholar]
- 13.de Silva, G. D. I., M. Kantzanou, A. Justice, R. C. Massey, A. R. Wilkinson, N. P. J. Day, and S. J. Peacock. 2002. The ica operon and biofilm production in coagulase-negative staphylococci associated with carriage and disease in a neonatal intensive care unit. J. Clin. Microbiol. 40:382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiTizio, V., G. Feurguson, M. Mittleman, A. Khoury, A. Bruce, and F. DiCosmo. 1998. A liposomal hydrogel for the prevention of bacterial adhesion to catheters. Biomaterials 19:1877-1884. [DOI] [PubMed] [Google Scholar]
- 15.Donlan, R. M., and J. W. Costerton. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donlan, R. M., R. Murga, M. Bell, C. M. Toscano, J. H. Carr, T. J. Novicki, C. Zuckerman, L. C. Corey, and J. M. Miller. 2001. Protocol for detection of biofilms on needleless connectors attached to central venous catheters. J. Clin. Microbiol. 39:750-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunne, W. M., Jr. 2002. Bacterial adhesion: seen any good biofilms lately? Clin. Microbiol. Rev. 15:155-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gander, S., and R. Finch. 2000. The effects of exposure at constant (1 h) or exponentially decreasing concentrations of quinupristin/dalfopristin on biofilms of gram-positive bacteria. J. Antimicrob. Chemother. 46:61-67. [DOI] [PubMed] [Google Scholar]
- 19.Gross, M., S. E. Cramton, F. Götz, and A. Peschel. 2001. Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. Infect. Immun. 69:3423-3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton-Miller, J., and S. Shah. 1997. Activity of quinupristin/dalfopristin against Staphylococcus epidermidis in biofilms: a comparison with ciprofloxacin. J. Antimicrob. Chemother. 39:103-108. [DOI] [PubMed] [Google Scholar]
- 21.Huber, M. M., and T. W. Huber. 1989. Susceptibility of methicillin-resistant Staphylococcus aureus to lysostaphin. J. Clin. Microbiol. 27:1122-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansen, C., P. Falholt, and L. Gram. 1997. Enzymatic removal and disinfection of bacterial biofilms. Appl. Environ. Microbiol. 63:3724-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones, S., M. Morgan, T. Humphrey, and H. Lappin-Scott. 2001. Effect of vancomycin and rifampicin on methicillin-resistant Staphylococcus aureus biofilms. Lancet 357:40-41. [DOI] [PubMed] [Google Scholar]
- 24.Kerr, D., K. Plaut, A. Bramley, C. Williamson, A. Lax, K. Moore, K. Wells, and R. Wall. 2001. Lysostaphin expression in mammary glands confers protection against staphylococcal infection in transgenic mice. Nat. Biotechnol. 19:66-70. [DOI] [PubMed] [Google Scholar]
- 25.Kiri, N., G. Archer, and M. W. Climo. 2002. Combinations of lysostaphin with β-lactams are synergistic against oxacillin-resistant Staphylococcus epidermidis. Antimicrob. Agents Chemother. 46:2017-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kokai-Kun, J. F., S. M. Walsh, T. Chanturiya, and J. J. Mond. 2003. Lysostaphin cream eradicates Staphylococcus aureus nasal colonization in a cotton rat model. Antimicrob. Agents Chemother. 47:1589-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lima, K., L. Fava, and J. Siqueira. 2001. Susceptibilities of Enterococcus faecalis biofilms to some antimicrobial medications. J. Endod. 27:616-619. [DOI] [PubMed] [Google Scholar]
- 28.Mack, D., H. Rohde, S. Dobinsky, J. Riedewald, M. Nedelmann, J. K.-M. Knobloch, H.-A. Elsner, and H. H. Feucht. 2000. Identification of three essential regulatory gene loci governing expression of Staphylococcus epidermidis polysaccharide intercellular adhesion and biofilm formation. Infect. Immun. 68:3799-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Grady, N., M. Alexander, E. Dellinger, J. Gerberding, S. Heard, D. Maki, H. Masur, R. McCormick, L. Mermel, M. Pearson, I. Raad, A. Randolph, and R. Weinstein. 2002. Guidelines for the prevention of intravascular catheter related infections. Pediatrics 110:e51. [DOI] [PubMed] [Google Scholar]
- 30.Patron, R. L., M. W. Climo, B. P. Goldstein, and G. L. Archer. 1999. Lysostaphin treatment of experimental aortic valve endocarditis caused by a Staphylococcus aureus isolate with reduced susceptibility to vancomycin. Antimicrob. Agents Chemother. 43:1754-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polonio, R. E., L. A. Mermel, G. E. Paquette, and J. F. Sperry. 2001. Eradication of biofilm-forming Staphylococcus epidermidis (RP62A) by a combination of sodium salicylate and vancomycin. Antimicrob. Agents Chemother. 45:3262-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raad, I., R. Darouiche, R. Hachem, D. Abi-Said, H. Safar, T. Darnule, M. Mansouri, and D. Morck. 1998. Antimicrobial durability and rare ultrastructural colonization of indwelling central catheters coated with minocycline and rifampin. Crit. Care Med. 26:219-224. [DOI] [PubMed] [Google Scholar]
- 33.Rachid, S., K. Ohlsen, U. Wallner, J. Hacker, M. Hecker, and W. Ziebuhr. 2000. Alternative transcription factor σB is involved in regulation of biofilm expression in a Staphylococcus aureus mucosal isolate. J. Bacteriol. 182:6824-6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rachid, S., K. Ohlsen, W. Witte, J. Hacker, and W. Ziebuhr. 2000. Effect of subinhibitory antibiotic concentrations on polysaccharide intercellular adhesion expression in biofilm-forming Staphylococcus epidermidis. Antimicrob. Agents Chemother. 44:3357-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramerez de Arellano, E., A. Pascual, L. Martinez-Martinez, and E. Perea. 1994. Activity of eight antibacterial agents on Staphylococcus epidermidis attached to Teflon catheters. J. Med. Microbiol. 10:43-47. [DOI] [PubMed] [Google Scholar]
- 36.Rupp, M., and K. Hamer. 1998. Effect of subinhibitory concentrations of vancomycin, cefazolin, ofloxacin, l-ofloxacin and d-ofloxacin on adherence to intravascular catheters and biofilm formation by Staphylococcus epidermidis. J. Antimicrob. Chemother. 41:155-161. [DOI] [PubMed] [Google Scholar]
- 37.Schierholz, J., H. Steinhauser, A. Rump, R. Berkels, and G. Pulverer. 1997. Controlled release of antibiotics from biomedical polyurethanes: morphological and structural features. Biomaterials 18:839-844. [DOI] [PubMed] [Google Scholar]
- 38.Schindler, C., and V. Schuhardt. 1964. Lysostaphin: a new bacteriolytic agent for the Staphylococcus. Proc. Natl. Acad. Sci. USA 51:414-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sugita, J., N. Yokoi, N. Fullwood, A. Quantock, Y. Takada, Y. Nakamura, and S. Kinoshita. 2001. The detection of bacteria and bacterial biofilms in punctal plug holes. Cornea 20:362-365. [DOI] [PubMed] [Google Scholar]
- 40.Whitchurch, C., T. Tolker-Nielsen, P. Ragas, and J. Mattick. 2002. Extracellular DNA required for bacterial biofilm formation. Science 295:1487. [DOI] [PubMed] [Google Scholar]
- 41.Zekeriya, I., R. Darouiche, G. Landon, and T. Beck. 1996. Efficacy of antibiotics alone for orthopedic device related infections. Clin. Orthop. Relat. Res. 332:184-189. [DOI] [PubMed] [Google Scholar]
- 42.Zygmunt, W., and P. Tavormina. 1972. Lysostaphin: model for specific enzymatic approach to infectious disease. Prog. Drug Res. 16:309-333. [DOI] [PubMed] [Google Scholar]


