Abstract
Daptomycin efficacy against clinical isolates of Enterococcus faecalis, Enterococcus faecium, and a lab-derived daptomycin-resistant isolate of E. faecalis was investigated in a mouse model of renal infection. The daptomycin MICs against these enterococci ranged from 0.5 to 50 μg/ml. The objective of this study was to determine the relationship between the MICs of drugs against E. faecalis and E. faecium and the level of daptomycin exposure needed to evaluate the drug's efficacy. Correlating the required therapeutic exposures of mice with the exposures achieved clinically allowed us to project enterococcal breakpoint values. Mice pretreated with carrageenan were infected intravenously with 3 × 108 to 4 × 108 CFU of E. faecalis or E. faecium. Daptomycin (5 to 50 mg of drug/kg of body weight) or saline control was administered 4 h postinfection and continued once daily for 2 days (three total doses). On day 4, infected kidneys were harvested, homogenized, and dilution plated. Efficacy was defined as a ≥2-log10 (99%) reduction in bacterial burden in infected kidneys. At clinically relevant dosages and exposures (area under the curve, 400 to 600 μg · hr/ml), daptomycin demonstrated similar and marked efficacy against all clinical enterococcal isolates tested. Daptomycin achieved efficacy with comparable doses against both vancomycin-sensitive (MIC, ≤4 μg/ml) and -resistant enterococcal strains tested. Efficacy was also established against the lab-derived daptomycin-resistant E. faecalis isolate. In this murine renal infection model, clinically relevant exposures of daptomycin were effective against E. faecalis and E. faecium strains for which MICs were ≤8 μg/ml. These murine efficacy data for daptomycin, along with surveillance data and human pharmacokinetic exposures achieved, suggest a breakpoint concentration value of ≤8 μg/ml (susceptible) and ≥16 μg/ml (resistant) for daptomycin against E. faecium and E. faecalis.
Enterococcus faecalis and Enterococcus faecium have emerged as important pathogens, with Enterococcus species accounting for approximately 12% of all nosocomial infections (10, 12). Furthermore, the percentage of enterococcal nosocomial organisms that are resistant to vancomycin is increasing. For example, from 1989 to 1998, the percentage of patients with nosocomial infections associated with vancomycin-resistant enterococci (VRE) increased from 0.4 to 22.6% and from 0.3 to 21.2% in hospital intensive-care and non-intensive-care settings, respectively (6). In particular, E. faecalis was observed in 85 to 89%, and E. faecium was observed in 10 to 15%, of identified enterococcal nosocomial isolates (10). The emerging threat of antibiotic resistance in enterococci and in other clinically relevant pathogens has resulted in renewed interest in the clinical development of novel, effective antibiotics.
Daptomycin is a new lipopeptide antibiotic with a unique mechanism of action and rapid in vitro bactericidal activity against most clinically relevant gram-positive pathogens, including drug-resistant isolates (1, 7, 15, 18; M. E. Jones, I. A. Critchley, D. C. Draghi, J. A. Karlowsky, C. Thornsberry, and D. F. Sahm, Abstr. 12th Eur. Congr. Clin. Microbiol. Infect. Dis., abstr. P812, 2002). Daptomycin is currently under clinical development for the treatment of serious gram-positive infections, including complicated skin and soft tissue infections, infectious endocarditis, and infections caused by VRE (18, 20). In vitro bactericidal activity has been demonstrated by daptomycin against both antibiotic-susceptible and -resistant organisms, including methicillin-resistant Staphylococcus aureus and VRE (1, 15). The mechanism of action of daptomycin involves depolarization of gram-positive bacterial membranes, and no cross-resistance has been demonstrated with any other drug class (20).
Daptomycin produces concentration-dependent bactericidal activity, which is predictive of the ratio of the maximum concentration of drug in serum (Cmax)/MIC or that of the area under the concentration-time curve (AUC)/MIC as key pharmacodynamic (PD) parameters (3). Increasing the ratio of the AUC/MIC for daptomycin optimizes the pharmacokinetic (PK) and PD strengths of the compound (9). In a neutropenic murine thigh model of S. aureus infection, investigators demonstrated that the AUC/MIC ratio for daptomycin was the PD parameter that best predicted outcome (11), whereas another study has indicated both the maximum concentration of the drug in serum and the AUC (20) ratios to be the best predictors.
The use of an animal model to investigate breakpoints incorporates host factors such as immune response in the evaluation of efficacy against enterococcal infection. The murine pyelonephritis model allows for an investigation of drug efficacy against enterococcal infection in a relevant target organ in nonimmunosuppressed animals. Enterococcal infections are often poorly virulent in the accepted mouse thigh model of infection. A previous PD study which tested daptomycin against E. faecalis thigh infection in mice indicated that a very low daptomycin exposure for efficacy was required, possibly due in part to the poor growth of the pathogen (N. Safdar, D. R. Andes, and W. A. Craig, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1769, 1999). This renal model allows for robust growth of the pathogen and a vigorous challenge to antibiotic efficacy.
Because daptomycin is excreted renally as intact drug, conclusions from this model of renal infection do not necessarily apply to other infection sites. Approximately 78% of the administered daptomycin is excreted in the urine as intact drug (22). For enterococci, urinary tract infections are salient; infections of other body sites, such as skin and skin structure, intra-abdominal region, bloodstream, and endocardium, are also important.
The goal of this study was to determine the efficacy of daptomycin against a number of E. faecalis and E. faecium clinical isolates that are representative of the MIC range found in surveillance studies. We determined the drug exposure levels that produce efficacy by using an animal model of renal infection. Correlation of the achieved clinical drug exposures with effective drug concentrations required in the animal model allowed for projections of breakpoints.
MATERIALS AND METHODS
Surveillance studies.
In 2000 through 2001, a total of 3,927 E. faecalis and 813 E. faecium isolates were collected from laboratories and hospital centers in the United States and Europe, and these isolates were centrally tested against daptomycin by the National Committee for Clinical Laboratory Standards broth microdilution method (6; I. A. Critchley, J. A. Karlowsky, R. S. Blosser, K. Murfitt, M. E. Jones, C. Thornsberry, and D. F. Sahm, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. 528, 2001).
Bacterial strains and antibiotics.
Clinical isolates of E. faecalis (n = 8) and E. faecium (n = 1) were used to induce infection (Table 1). Three of the E. faecalis strains and the E. faecium strain were vancomycin susceptible (MIC, <4 μg/ml), and five of the E. faecalis strains were vancomycin resistant (MIC, >100 μg/ml). The tenth strain tested was E. faecalis 312, a lab-derived isolate (MIC, 50 μg/ml) whose resistance to daptomycin was created by subjecting the strain to a 20-day serial passage in liquid culture in the presence of increasing concentrations of daptomycin (17). The E. faecium strain was obtained from the American Type Culture Collection. Strains were grown at 37°C for 24 h in Mueller-Hinton broth (Becton Dickinson, Cockeysville, Md.) supplemented with CaCl2 to provide physiologic levels (50 mg/liter) of free calcium. Daptomycin was manufactured by Cubist Pharmaceuticals, Inc. (Lexington, Mass.).
TABLE 1.
Bacterial counts (mean log10/mouse) of mouse kidney homogenates after 3 days of treatment
| Bacterial strain | MIC (μg/ml) | Log10 CFU/mouse at daptomycin dose (mg/kg/day)a
|
|||||
|---|---|---|---|---|---|---|---|
| 0 (control) | 5 | 10 | 25 | 50 | ED2logb | ||
| E. faecalis 588 | 0.5 | 6.4 | 2.7 | 1.9 | 1.7 | ND | 1.7 |
| E. faecalis 501 | 2 | 4.6 | <1.7 | <1.7 | <1.7 | ND | 3.6 |
| E. faecalis 201 | 2 | 6.0 | 4.9 | 4.2 | 1.9 | ND | 15.3 |
| E. faecium 14 | 2 | 4.0 | 3.5 | 1.9 | 1.2 | ND | 12.4 |
| E. faecium 80 | 4 | 5.5 | 4.5 | ND | 1.7 | ND | 13.7 |
| E. faecium 88 | 4 | 4.4 | 3.3 | 3.4 | 1.9 | ND | 22.4 |
| E. faecium 624 | 4 | 3.3 | 3.3 | ND | 1.1 | 1.3 | 23.9 |
| E. faecium 82 | 4 | 4.2 | 4.3 | ND | 2.2 | 1.5 | 29.2 |
| E. faecium 89 | 8 | 4.2 | 3.1 | ND | 2.6 | 1.6 | 32.4 |
| E. faecalis 312c | 50 | 5.9 | 5.8 | ND | 2.5 | 1.4 | 21.3 |
ND, not determined.
Values indicate the effective dose (mg/kg/day) required to produce a mean 2-log reduction in the log10 CFU count/mouse.
Laboratory-derived strain of Enterococcus.
Susceptibility studies.
MICs were determined by broth microdilution methodology as specified by the National Committee for Clinical Laboratory Standards (14). For MIC determinations, the final bacterial inoculum was 5 × 105 CFU/ml, and bacteria were incubated in Mueller-Hinton broth supplemented with CaCl2 as described above.
Murine renal infection model.
Female CD-1 mice (Charles River Laboratories, Wilmington, Mass.) 6 to 8 weeks of age and weighing 20 to 25 g were used in this study. Water and Agway (Syracuse, N.Y.) rodent chow was provided ad libitum throughout the study. On day −7, all mice were injected intravenously (i.v.) with 0.2 ml of 0.2% lambda carrageenan (Sigma Chemical Company, St. Louis, Mo.) to increase their susceptibility to bacterial renal infection.
Following i.v. injection, lambda carrageenan increases susceptibility to renal infection without the induction of nephrotoxicity. Injection of lambda carrageenan causes no increase in serum creatine or urea (19). Lambda carrageenan increases rodent susceptibility to renal infection following i.v. injection, possibly by forming a lattice structure in renal tissue and forming a support structure for bacterial attachment and growth (13). Bacterial strains that fail to cause renal infection in normal mice produce significant renal infections in lambda carrageenan-treated mice.
On day −1, bacterial strains were cultured in brain heart infusion broth (BBL Microbiology Systems, Hunt Valley, Md.) at 37°C for 18 h, and following dilution therein, the standard inoculum was adjusted to a concentration of 1 × 109 to 2 × 109 CFU/ml based on the optical density at 600 nm as described previously (13). Strains of enterococci were inoculated into the mice by a modification of the method used by Meulbroek et al. (13). On day 0, all mice were injected i.v. with 0.2 ml of the bacterial culture (3 × 108 to 4 × 108 CFU/mouse) through the tail vein. Four hours after the bacterial inoculation, mice were treated subcutaneously with 5, 10, 25, or 50 mg of daptomycin/kg of body weight or 0.2 ml of phosphate-buffered saline control. Daptomycin or saline was administered once daily for an additional 2 consecutive days for a total of three doses.
A total of 30 mice were infected with each enterococcal strain (n = 300). Ten mice from each group were subsequently treated with phosphate-buffered saline control, and five mice per group were administered 5, 10, 25, or 50 mg of daptomycin per kg.
Bacteriologic analysis.
All mice were sacrificed on day 3. Both kidneys from each mouse were removed aseptically and homogenized in 4 ml of distilled water. A 100-μl aliquot of the diluted homogenate was further diluted 100-fold and 10,000-fold with distilled water, and 100 μl of the three dilutions was plated separately on enterococcal agar (BBL Microbiology Systems) and incubated at 37°C for 48 h. Bacterial titers were calculated and subsequently averaged for each treatment group. In this study, bacterial counts of <20 CFU were below the detection limit. The daptomycin dose required to achieve a 2-log10 (99%) reduction in bacterial titer (ED2log) was calculated by linear-regression analysis. Correlations of log10 reduction in bacterial count versus PK exposures were also calculated by regression analysis and graphed as demonstrated previously by Louie et al. (11).
PKs.
Daptomycin (1, 5, 10, 25, 50, or 100 mg/kg) was administered as a single subcutaneous dose to groups of healthy female CD-1 mice. Terminal blood samples were collected from groups of three mice per time point at 5 min through 24 h after dosing. The blood was collected by cardiac puncture into heparinized tubes. Plasma was collected by centrifugation, and samples were stored at −20°C until analysis. A secondary analysis included daptomycin PK in carrageenan-pretreated mice compared with normal mice. This analysis was designed to determine whether the daptomycin PK analysis generated from normal mice also applied to animals with impaired renal function. On day −7, a subset of female CD-1 mice was injected with 0.2 ml of 0.2% lambda carrageenan IV to impair murine renal function. Daptomycin at a concentration of 25 mg/kg was administered as a single subcutaneous dose to groups of carrageenan-pretreated or healthy female CD-1 mice. Terminal blood samples were collected from both groups of mice and were processed as specified above.
Daptomycin analytical assay.
Daptomycin was detected by using an internal standard of ethylparaben and was isolated by protein precipitation with methanol followed by high-performance liquid chromatography. The mobile phase consisted of 90% mobile phase A (acetonitrile, 0.5% NH4H2PO4, 34:66 [vol/vol]) and 10% mobile phase B (acetonitrile, 0.5% NH4H2PO4, 20:80 [vol/vol]) at a flow rate of 1.5 ml/min. Concentrations of drug in serum were determined by reverse-phase high-performance liquid chromatography using a Metachem Hypersil C8 analytical column and a Waters Xterra RP18 guard column (ANSYS Technologies, Inc., Lake Forest, Calif.). At a flow rate of 1.5 ml/min, daptomycin shows a retention time of 14 to 16 min. The optical density of samples at 214 nm was analyzed. The detection of daptomycin concentrations in mouse plasma was linear across the range of 7.5 to 400 μg/ml. This method has been validated for daptomycin over the concentration range of 3 to 500 μg/ml, with a lower limit of quantitation equal to the lowest calibration level of 3 μg/ml.
RESULTS
Daptomycin in vitro activity.
Results from surveillance studies reported daptomycin MICs at which 90% of isolates are inhibited (MIC90s) of 2 μg/ml (range, 0.03 to 4 μg/ml) for vancomycin-sensitive E. faecalis and a MIC90 value of 2 μg/ml (range, ≤0.015 to 2 μg/ml) for vancomycin-resistant E. faecalis (20). The daptomycin MIC90s were 4 μg/ml (range, 0.06 to 8 μg/ml) for vancomycin-sensitive E. faecium and 4 μg/ml (range, 0.25 to 4 μg/ml) for vancomycin-resistant E. faecium. The daptomycin MICs for the clinical E. faecalis and E. faecium isolates used in this in vivo study ranged from 0.5 to 8 μg/ml (Table 1) and were representative of the values reported in the profiling study (Fig. 1A and B). The MIC for the lab-derived daptomycin-resistant E. faecalis 312 isolate was determined to be 50 μg/ml (Table 1).
FIG. 1.
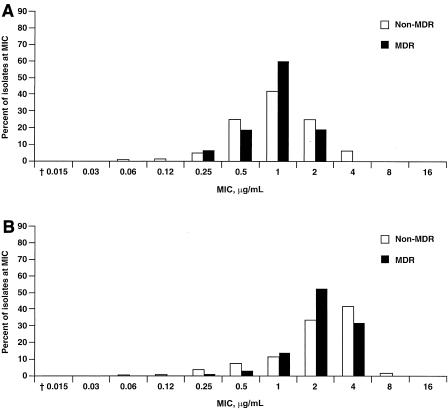
Distribution of MICs for daptomycin against vancomycin-susceptible (non-MDR, n = 2,049) and vancomycin-resistant (MDR, n = 40) E. faecalis (A) and vancomycin-susceptible (non-MDR, n = 147) and vancomycin-resistant (MDR, n = 219) E. faecium (B) in a multicenter U.S. study. MDR, multidrug resistant.
Daptomycin PKs.
The PK profile for daptomycin in mice was linear across the dose range of 1 to 100 mg/kg with respect to dose and AUC (Fig. 2). With increasing doses of daptomycin, there was a linear increase in the AUC. There was no difference between daptomycin PK profiles in normal mice and those of carrageenan-pretreated mice (Fig. 3).
FIG. 2.
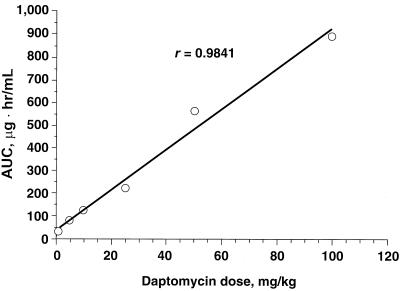
Correlation between daptomycin dose and AUC following subcutaneous administration in normal mice.
FIG. 3.
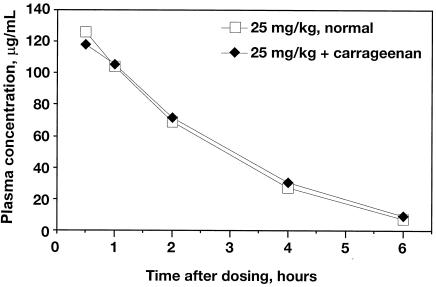
Correlation between daptomycin dose and exposure following subcutaneous administration to normal mice or mice that were pretreated with carrageenan.
Efficacy trials.
Daptomycin caused dose-dependent reductions in bacterial counts against all vancomycin-resistant and -sensitive strains of enterococci in the renal infection trials. The bacterial burden of the lab-derived E. faecalis 312 strain, against which the daptomycin MIC was 50 μg/ml, was also reduced by daptomycin treatment in a dose-dependent manner (Table 1). Mean reductions of ≥2 log10 (ED2log) in bacterial burden were obtained at calculated ED2log values of 1.7 to 32.4 mg/kg/day for the enterococcal isolates. There was a correlation between the mean effective dose (ED2log) and MIC for the isolates, although the daptomycin-resistant strain E. faecalis 312 (MIC, 50 μg/ml) had an ED2log dose lower than that predicted based on the MIC versus effective dose response curve (Fig. 4).
FIG. 4.
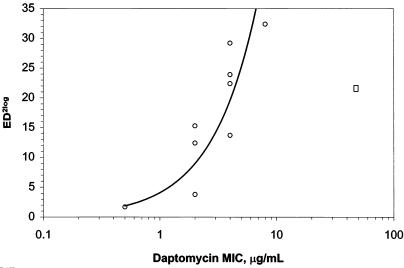
Correlation between daptomycin MIC and median effective dose (ED2log) in a model of murine renal infection that was induced by clinical isolates of enterococci (n = 9). ○, clinical isolates of E. faecalis or E. faecium; □, laboratory-derived daptomycin-resistant isolate of E. faecalis excluded from regression analysis.
A plot of the log change in bacterial titer for each mouse versus the corresponding AUC/MIC ratio showed a correlation with a reduction in bacterial burden against all clinical enterococcal isolates (Fig. 5). By extrapolating from the correlation curve, an ED2log (or −2-log change in bacterial titer) was achieved at a calculated AUC/MIC ratio of ≥42.7. This finding represents the daptomycin exposure required to obtain a 99% reduction in bacterial load in this enterococcal renal infection model.
FIG. 5.
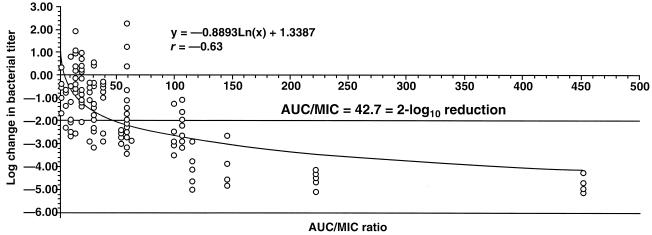
Correlation between AUC/MIC ratio and the log10 reduction in bacterial titer in murine renal tissue.
DISCUSSION
Daptomycin is a novel lipopeptide with rapidly bactericidal activity in vitro against clinically important gram-positive pathogens (1, 15, 18), including drug-resistant isolates. Previously published data have demonstrated that daptomycin is bactericidal in vitro against enterococci (15). Infections caused by E. faecalis and E. faecium strains, particularly VRE, have limited treatment options (8).
In this study, daptomycin produced significant efficacy against renal infection caused by both vancomycin-susceptible and -resistant E. faecalis and E. faecium clinical isolates. Efficacy was achieved at drug exposures that are routinely obtained clinically. The MICs of daptomycin against these nine clinical isolates ranged from 0.5 to 8 μg/ml, which correlates with larger surveillance studies of enterococci (1, 7, 20, 21; Jones et al., Abstr. 12th Eur. Congr. Clin. Microbiol. Infect. Dis.). This study demonstrated that daptomycin was effective against vancomycin-susceptible and -resistant enterococcal renal infection at pharmacologic drug exposures. Efficacy was achieved against all of the clinical isolates, with daptomycin MICs ranging from 0.5 to 8 μg/ml.
The laboratory-derived E. faecalis strain 312 was included in the study to determine the relationship between efficacy and high (50 μg/ml) MICs for daptomycin. Administration of daptomycin resulted in a dose-dependent reduction in enterococcus titers in infected kidneys. This reduction was observed for all E. faecalis and E. faecium strains tested, including the laboratory-derived daptomycin-resistant strain E. faecalis 312. Interestingly, E. faecalis 312 and the clinical isolates were effectively treated at similar daptomycin dose levels. This result may be related to the generally poor virulence of laboratory-derived daptomycin-resistant isolates (17). There was a correlation between the ED2log and the MIC for daptomycin against each of the nine clinical enterococcal strains (Fig. 4).
Lambda carrageenan pretreatment was used to increase mouse susceptibility to renal infection. Lambda carrageenan is not nephrotoxic but may instead function by forming a lattice support structure that allows for the attachment of bacteria (13, 22). The E. faecalis and E. faecium isolates used in this study would not induce renal infection in normal animals. The duration of the carrageenan effect is not known, but the infection remains stable for at least seven days.
PK analysis in this renal infection model indicated that effective daptomycin exposure encompassed the exposure (i.e., the AUC from 0 to 24 h) levels observed clinically in humans that were administered 4 mg/kg/day i.v. (494 μg · hr/ml) and 6 mg/kg/day i.v. (747 μg · hr/ml) (5). The exposures achieved in carrageenan-pretreated mice were equal to the exposures generated in normal mice (Fig. 3); therefore, the PK analysis generated in normal mice (Fig. 2) is valid. Analysis of the correlation between the log reduction in bactericidal titer and increasing AUC/MIC ratio indicated a 99% reduction in bacterial burden when the AUC/MIC ratio was ≥42.7. Applying the AUC/MIC ratio of 42.7 to treat E. faecalis or E. faecium strains, the MICs for which were 8 μg/ml, produces a required AUC value of 342 μg · hr/ml, which is achieved by the clinical dose of 4 mg/kg. In contrast, enterococci for which the MIC was 16 μg/ml would require an AUC of 683 μg · hr/ml, which is beyond the clinical exposures produced by the 4-mg/kg dose (15). Daptomycin was effective against all clinical enterococcal isolates tested at physiologic AUC values. The efficacy of daptomycin against these enterococci, at clinical exposures that represent a typical range of MICs, supports breakpoint values of 8 μg/ml (susceptible) and 16 μg/ml (resistant).
Because daptomycin is excreted renally, care must be taken when evaluating its efficacy in renal infections and in using its efficacy in the case of renal infection as a predictive model for efficacy at other body sites. However, the AUC/MIC ratio (42.7) calculated to produce efficacy in this renal infection study is markedly higher than the AUC/MIC ratio of 1.7 calculated to yield efficacy in an earlier study of daptomycin against E. faecalis thigh infection (Safdar et al., 39th ICAAC). Therefore, treatment of this murine enterococcal renal infection was a more challenging test, potentially due to the higher virulence of the enterococci in renal tissue.
The protein serum binding of daptomycin is approximately 90% across all species, including mice and humans (5). Wise et al. (21) have determined that human serum has little impact on the MIC of daptomycin in broth. In the presence of human serum, the MIC of daptomycin typically increased by only a single twofold dilution. Since the binding is relatively equal across species, the mouse model should provide a reasonable simulation of the dynamics of daptomycin response correlated to serum concentrations.
PDs determined in mice have been used to project human efficacy both for daptomycin (11; Safdar et al., 39th ICAAC) and for many other antibacterial drugs (3, 4, 16). The use of AUC is a proven parameter linked to clinical efficacy (3; Safdar et al., 39th ICAAC). Given that protein binding of daptomycin is consistent across species, PD projections made based on murine data should be similarly useful in predicting clinical efficacy.
The efficacy of daptomycin in the treatment of renal infection in a murine model is used to propose appropriate daptomycin susceptibility criteria for E. faecalis and E. faecium. Based on the distribution of the MICs against clinical isolates, the efficacy in the renal infection model, and the PK observed in humans, daptomycin breakpoint concentrations of 8 μg/ml for susceptible and ≥16 μg/ml for resistant E. faecium and E. faecalis are suggested (Table 2). The 8-μg/ml breakpoint value proposed here for E. faecium and E. faecalis strains is higher than previously proposed breakpoint concentrations that were based solely on the results of in vitro studies. In a study by Wise et al., application of the British Society of Antimicrobial Chemotherapy breakpoint formula suggested a breakpoint concentration range of 4 to 8 μg/ml, but the authors considered 2 μg/ml to be a reasonable value because few of the gram-positive isolates, including the E. faecalis and E. faecium strains, had a MIC that was >2 μg/ml (21). The present study included in vivo data against enterococci in the determination of potential breakpoint values. The breakpoint concentration (MIC, 8 μg/ml) recommended here for enterococci is within the 4- to 8-μg/ml range determined previously in vitro. Identical breakpoints against vancomycin-susceptible and -resistant enterococci are supported by surveillance data which show no cross-resistance to daptomycin.
TABLE 2.
Proposed daptomycin breakpoint concentrations
| Bacterial straina | MIC90 (μg/ml)b | Range (μg/ml) | Susceptible (μg/ml) | Resistant (μg/ml) |
|---|---|---|---|---|
| E. faecalis | 2 | 0.03-4 | 8 | 16 |
| E. faecium | 4 | 0.06-8 | 8 | 16 |
Indicated strains encompass both those that are vancomycin sensitive (MIC <4 μg/ml) and vancomycin resistant (MIC >100 μg/ml).
Data from Critchley et al., 41st ICAAC.
This study represents the first in vivo PD study of daptomycin against enterococcal renal infection designed to address the validity of potential breakpoint concentrations. This investigation concentrated on the AUC/MIC PD factor linked to efficacy determined previously (11). Efficacy was determined at a range of pharmacologic exposures against a clinically relevant array of enterococci, against which MICs ranged from 0.5 to 8 μg/ml. Previous studies have supported the rationale that PK and PD parameters required for efficacy are similar in animal models of infection and in clinical application (2, 3, 4; Safdar et al., 39th ICAAC). This present animal study suggests that daptomycin dosages of 4 or 6 mg/kg may be efficacious in the treatment of clinical enterococcal infections caused by vancomycin-susceptible and -resistant E. faecalis and E. faecium using a tentative breakpoint concentration of 8 μg/ml.
ADDENDUM IN PROOF
On 12 September 2003, daptomycin was approved for use in the United States for treatment of complicated skin and skin structure infections. The assigned breakpoints for E. faecalis (vancomycin susceptible only) are as follows: susceptible, ≤4 μg/ml; not susceptible, ≥8 μg/ml.
REFERENCES
- 1.Akins, R. L., and M. J. Rybak. 2001. Bactericidal activities of two daptomycin regimens against clinical strains of glycopeptide intermediate-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecium, and methicillin-resistant Staphylococcus aureus isolates in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 45:454-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alder, J. D. 1997. Determining the therapeutic potential of experimental antibacterial agents: the use of animal models. Curr. Pharm. Des. 3:143-158. [Google Scholar]
- 3.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-10; quiz 11-12. [DOI] [PubMed] [Google Scholar]
- 4.Drusano, G. L., S. L. Preston, C. Hardalo, R. Hare, C. Banfield, D. Andes, O. Vesga, and W. A. Craig. 2001. Use of preclinical data for selection of a phase II/III dose for evernimicin and identification of a preclinical MIC breakpoint. Antimicrob. Agents Chemother. 45:13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dvorchik, B. H., D. Brazier, M. F. DeBruin, and R. D. Arbeit. 2003. Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects. Antimicrob. Agents Chemother. 47:1318-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groseclose, S. L., C. M. Knowles, P. A. Hall, D. A. Adams, W. J. Anderson, K. Snavely, R. F. Fagan, G. F. Jones, C. A. Worsham, P. Gangarosa, M. K. Glynn, M.-H. Chang, T. Doyle, and R. A. Jajosky. 1999. Summary of notifiable diseases, United States, 1998. Morb. Mortal. Wkly. Rep. 47:1-93. [Google Scholar]
- 7.King, A., and I. Phillips. 2001. The in vitro activity of daptomycin against 514 gram-positive aerobic clinical isolates. J. Antimicrob. Chemother. 48:219-223. [DOI] [PubMed] [Google Scholar]
- 8.Levison, M. E., and S. Mallela. 2000. Increasing antimicrobial resistance: therapeutic implications for enterococcal infections. Curr. Infect. Dis. Rep. 2:417-423. [DOI] [PubMed] [Google Scholar]
- 9.Li, R. C., M. Zhu, and J. J. Schentag. 1999. Achieving an optimal outcome in the treatment of infections. The role of clinical pharmacokinetics and pharmacodynamics of antimicrobials. Clin. Pharmacokinet. 37:1-16. [DOI] [PubMed] [Google Scholar]
- 10.Linden, P. K., and C. B. Miller. 1999. Vancomycin-resistant enterococci: the clinical effect of a common nosocomial pathogen. Diagn. Microbiol. Infect. Dis. 33:113-120. [DOI] [PubMed] [Google Scholar]
- 11.Louie, A., P. Kaw, W. Liu, N. Jumbe, M. H. Miller, and G. L. Drusano. 2001. Pharmacodynamics of daptomycin in a murine thigh model of Staphylococcus aureus infection. Antimicrob. Agents Chemother. 45:845-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGeer, A. J., and D. E. Low. 2000. Vancomycin-resistant enterococci. Semin. Respir. Infect. 15:314-326. [DOI] [PubMed] [Google Scholar]
- 13.Meulbroek, J. A., A. Oleksijew, S. K. Tanaka, and J. D. Alder. 1996. Efficacy of ABT-719, a 2-pyridone antimicrobial, against enterococci, Escherichia coli, and Pseudomonas aeruginosa in experimental murine pyelonephritis. J. Antimicrob. Chemother. 38:641-653. [DOI] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. 2001. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, approved standard, 5th ed. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 15.Rybak, M. J., E. Hershberger, T. Moldovan, and R. G. Grucz. 2000. In vitro activities of daptomycin, vancomycin, linezolid, and quinupristin-dalfopristin against staphylococci and enterococci, including vancomycin-intermediate and -resistant strains. Antimicrob. Agents Chemother. 44:1062-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schentag, J. J. 1999. Antimicrobial action and pharmacokinetics/pharmacodynamics: the use of AUIC to improve efficacy and avoid resistance. J. Chemother. 11:426-439. [DOI] [PubMed] [Google Scholar]
- 17.Silverman, J. A., N. Oliver, T. Andrew, and T. Li. 2001. Resistance studies with daptomycin. Antimicrob. Agents Chemother. 45:1799-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tally, F. P., M. Zeckel, M. M. Wasilewski, C. Carini, C. L. Berman, G. L. Drusano, and F. B. Oleson, Jr. 1999. Daptomycin: a novel agent for gram-positive infections. Exp. Opin. Investig. Drugs 8:1223-1238. [DOI] [PubMed] [Google Scholar]
- 19.Thomson, A. W., and P. H. Whiting. 1981. A comparative study of renal and hepatic function in Sprague-Dawley rats following systemic injection of purified carrageenans (kappa, lambda and iota). Br. J. Exp. Pathol. 62:207-213. [PMC free article] [PubMed] [Google Scholar]
- 20.Thorne, G. M., and J. Alder. 2002. Daptomycin: a novel lipopeptide antibiotic. Clin. Microbiol. Newsl. 24:33-40. [Google Scholar]
- 21.Wise, R., J. M. Andrews, and J. P. Ashby. 2001. Activity of daptomycin against gram-positive pathogens: a comparison with other agents and the determination of a tentative breakpoint. J. Antimicrob. Chemother. 48:563-567. [DOI] [PubMed] [Google Scholar]
- 22.Woodworth, J. R., E. H. Nyhart, Jr., G. L. Brier, J. D. Wolny, and H. R. Black. 1992. Single-dose pharmacokinetics and antibacterial activity of daptomycin, a new lipopeptide antibiotic, in healthy volunteers. Antimicrob. Agents Chemother. 36:318-325. [DOI] [PMC free article] [PubMed] [Google Scholar]


