Abstract
Calreticulin (CRT) is a high-capacity, low-affinity Ca2+-binding protein located in the lumen of the endoplasmic reticulum (ER) of all eukaryotic cells investigated so far. Its high level of conservation among different species suggests that it serves functions fundamental to cell survival. The role originally proposed for CRT, i.e., the main Ca2+ buffer of the ER, has been obscured or even casted by its implication in processes as diverse as gene expression, protein folding, and cell adhesion. In this work we seek the role of CRT in Ca2+ storing and signaling by evaluating its effects on the kinetics and amplitude of the store-operated Ca2+ current (ICRAC). We show that, in the rat basophilic leukemia cell line RBL-1, overexpression of CRT, but not of its mutant lacking the high-capacity Ca2+-binding domain, markedly retards the ICRAC development, however, only when store depletion is slower than the rate of current activation. On the contrary, when store depletion is rapid and complete, overexpression of CRT has no effect. The present results are compatible with a major Ca2+-buffering role of CRT within the ER but exclude a direct, or indirect, role of this protein on the mechanism of ICRAC activation.
INTRODUCTION
The endoplasmic reticulum (ER) is the most relevant source of mobilizable Ca2+ in mammalian cells. The free intralumenal Ca2+ concentration has been estimated to range between 0.2 and 2 mM (Hofer et al., 1995; Montero et al., 1995, 1997; Hofer and Schulz 1996; Miyawaki et al., 1997). Among Ca2+-binding proteins that are known to be resident ER proteins, calreticulin (CRT) is among the most abundant (Lytton and Nigam, 1992) and may account for up to ∼1–2% of total ER proteins. CRT has been considered the best candidate to function as an ER Ca2+ buffer, because it mostly resembles calsequestrin, the well-known Ca2+-storing protein of the sarcoplasmic reticulum. The overall biochemical and functional properties of CRT have been thoroughly characterized. CRT is a soluble, small glycoprotein of 417 amino acids that contains three functional domains (Nash et al., 1994; Pozzan et al., 1994; Meldolesi et al., 1996; Krause and Michalak, 1997). In particular, the C-terminal domain binds Ca2+ with high capacity (25–50 mol/mol of protein) and low affinity (Kd ≅ 0.5–1 mM) through a negatively charged region (Ostwald and MacLennan, 1974; Macer and Koch, 1988; Damiani et al., 1989; Treves et al., 1990).
It has been shown previously that CRT overexpressed by transient transfection in HeLa cells is specifically targeted to the ER and selectively increases the Ca2+ content of thapsigargin-sensitive stores (Bastianutto et al., 1995). Along the same line, treatment of cells with antisense oligonucleotides reduces the peak level of [Ca2+]i increase caused by receptor stimulation (Liu et al., 1994). Recent findings, however, have challenged the role of CRT as a major Ca2+-storing protein (Meldolesi et al., 1996; Krause and Michalak, 1997). The effect on Ca2+ homeostasis has in fact been suggested to be independent of the C domain and instead to be attributable to an interaction with the inositol 1,4,5-trisphosphate (InsP3) receptor or the sarcoendoplasmic reticulum Ca2+ ATPases mediated by the high-affinity Ca2+-binding site and/or the lectin region of the central P domain (Camacho and Lechleiter, 1995). In addition, other unique functions have been attributed to this protein, including modulation of gene expression and cell adhesiveness (Dedhar, 1994; Opas et al., 1996; Coppolino et al., 1997) and involvement in protein folding as a selective chaperone of glycosylated proteins (Helenius et al., 1997).
A picture is emerging from all of these studies in which CRT is a multifunctional protein with a role in Ca2+ homeostasis, if any, that appears at the moment undetermined. Contradictions also exist concerning the effects of CRT on Ca2+ release and influx across the plasma membrane, particularly on the store-operated Ca2+ influx, which is the major pathway for Ca2+ entry in nonexcitable cells (Penner et al., 1993; Fasolato et al., 1994; Berridge, 1995). For instance, in CRT-overexpressing cells (Mery et al., 1996), as well as in CRT knockout embryonic stem cells (Coppolino et al., 1997), [Ca2+]i changes induced by sarcoendoplasmic reticulum Ca2+ ATPase inhibitors are similar to those measured in control cells. In CRT knockout cells the [Ca2+]i changes induced by agonists appear unchanged (Coppolino et al., 1997; but see Liu et al., 1994). Finally, Ca2+ influx induced by store depletion is unaffected in CRT knockout cells (Coppolino et al., 1997), whereas in CRT stably transfected cells, Mery et al. (1996) found that capacitative Mn2+ influx is drastically reduced even on full depletion of the stores.
In view of the importance of CRT in so many cellular activities, we decided to reinvestigate its role in cellular Ca2+ handling, in particular trying to definitively establish whether it serves as an important lumenal Ca2+ buffer within InsP3-sensitive Ca2+ stores. To this end, we transiently transfected cells with wild-type (wt) CRT or its C domain–deleted mutant and monitored the effect of the expressed proteins on the Ca2+ release-activated Ca2+ current (ICRAC) (Hoth and Penner, 1992, 1993; Zweifach and Lewis, 1993), a dynamic and highly sensitive reporter of free intralumenal [Ca2+] (Hofer et al., 1998). The data obtained support the notion that CRT can efficiently buffer Ca2+ within the stores, whereas its overexpression has no direct effect on the mechanism activating this current.
MATERIALS AND METHODS
Construction of CRT cDNAs (tCRT and tCRTΔ)
The cDNA coding for the cytosolic green fluorescent protein (cytGFP bright mutant S65T) in the expression vector VR1012 was kindly provided by Dr. T. MacDonald (Albert Einstein University, New York, NY). CRT cDNA including the HA1 tag at amino acid 225 of the wtCRT sequence (tCRT) (Bastianutto et al., 1995) was excised from the expression vector pcDNAI by EcoRI-XbaI digestion and cloned in pBluescript SK+ (pBSK+; Stratagene, La Jolla, CA). The whole coding sequence of tCRT was then cloned in the expression vector VR1012 using XbaI and EcoRV sites. The sequence encoding the tCRT deletion mutant without the C domain (tCRTΔ) was obtained by amplifying by PCR the vector tCRT/pBSK+ (see above) with the following primers: forward, 5′-GTAATACGACTCACTATAGGGC-3′; reverse, 5′-CTACAGCTCGTCCTTATAGGCATAGATACTGG-3′.
The forward primer is the T7 primer sequence present into the pBSK+ vector; the reverse primer corresponds, from 5′ to 3′, to the antisense orientation of nucleotides 971–988 and 1306–1320 of the wtCRT cDNA (McCauliffe et al., 1990). This primer hybridizes with the sequence coding for the amino acids (aa) SIYAY in position 304–308 and the KDEL sequence plus the stop codon, present in the end of the coding sequence of the wtCRT cDNA (aa 414–417), thus removing the C domain–coding region (aa 309–413) of CRT in the wt cDNA.
The PCR amplification was performed over 30 cycles (1 min at 95°C, 2 min at 55°C, and 1 min at 72°C), using 2 ng of template DNA. The PCR product, cloned in pCR2.1 (Invitrogen, San Diego, CA) and controlled by DNA sequencing, was excised via the SalI site present in the PCR product (between the CRT sequence and the T7 primer) and XbaI site located in the vector sequence downstream of the insert. This SalI-XbaI 1000 kb fragment was then cloned in the expression vector VR1012 and used together with the cytGFP bright/VR1012 for transfection experiments.
Cell Culture and Transfection
The rat basophilic leukemia cell line RBL-1 (from Dr. R. Penner, Max-Planck Institute, Göttingen, Germany) was cultured in DMEM supplemented with 10% fetal calf serum and penicillin/streptomycin. Transient transfection with recombinant cytGFP (bright mutant S65T) and CRT constructs (tCRT or tCRTΔ) was performed by electroporation (Kodak, Rochester, NY). Cells were harvested and resuspended in fresh medium in 4-mm cuvettes in the presence of 10 μg of cytGFP/VR1012 and tCRT/VR1012 (or tCRTΔ/VR1012) plasmids/2 × 106 cells. Cells were subjected to a single pulse characterized by an electric field of 300 V, 1500 microfarads. Cells were transferred to poly-l-lysine-coated glass coverslips (2.5 × 105 cells/coverslip, 13-mm diameter). After overnight incubation, the medium was changed, and after 24–48 h of incubation, the cells were used for immunofluorescence and patch-clamp experiments.
Current Measurements
Patch-clamp experiments were performed in the tight-seal whole-cell configuration in a standard external solution containing 145 mM NaCl, 10 mM CaCl2, 11 mM glucose, and 10 mM HEPES (pH 7.4 at 25°C); 5 mM CsCl was routinely added to block inwardly rectifying K+ channels (Hoth, 1995). Sylgard-coated patch pipettes had resistance between 2 and 4 MΩ after filling with the standard intracellular solution, which contained 145 mM Cs-glutamate, 8 mM NaCl, 1 mM MgCl2, 0.5 mM MgATP, 12 mM bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), and 10 mM HEPES (pH 7.2, 25°C). Drugs were added by local pressure from a wide-tipped micropipette (5–10 μm). Patch-clamp experiments were performed with an inverted microscope (Axiovert 100; Zeiss, Milan, Italy) equipped for epifluorescence and photometry (TILL Photonics, Planegg, Germany). The light source was a Xenon short-arc lamp (75X-O; Ushio Inc., Tokyo, Japan) and a diffraction grating mounted on a high-speed scanner, providing monochromatic light. For detection of the bright GFP fluorescence, the excitation light (470 nm) was directed through a quartz glass fiber to a gray filter (Oriel, Milan, Italy) before entering the microscope and then deflected by a 505DRLP (XF73; Omega Optical, Milan, Italy) dichroic mirror into the 40x oil immersion objective (Fluar, NA 1.3, Zeiss). The emitted light was directed through a 500- to 530-nm emission filter (Zeiss) to a photomultiplier tube (R928; Hamamatsu, Tokyo, Japan). To collect fluorescence from a single cell, a pinhole was placed in the image plane of the phototube. Standard transfection protocols usually resulted in GFP fluorescence intensity ranging from 2 to 20 times the background cell signal. Routinely, cells were selected with intensities between 5- and 10-fold of the background, to avoid either too low and too high CRT-overexpressing cells. High-resolution current recordings and GFP fluorescence were acquired by a computer-based patch-clamp amplifier system (EPC-9; HEKA, Lambrecht, Germany) controlled by Pulse software (HEKA). All voltages were corrected for a liquid junction potential of 8 mV between external and internal solutions. High-resolution currents were acquired at a sampling rate of 10 kHz, low-pass filtered at 2.3 kHz, and digitally filtered to 1 kHz for presentation. The holding current, the holding potential, the fluorescence, and other parameters were synchronously recorded, at low resolution (2 Hz), by X-Chart software (HEKA). Voltage ramps of 50 ms duration, from −100 to +100 mV, were delivered at 0.5 Hz. Capacitative currents were canceled before each voltage ramp using the automatic capacitance compensation of the EPC-9. Uncompensated series resistance was in the range of 5–12 MΩ.
Immunolocalization and Fluorescence Detection
RBL-1 cells were fixed and immunostained as described previously (Brini et al., 1995). The antibodies (Abs) used were mouse monoclonal anti-HA1 Ab (Boehringer Mannheim, Milan, Italy) and rabbit polyclonal anti-CRT Ab (a kind gift from, Dr. E. Clementi, University of Catanzaro, Catanzaro, Italy), both used at 1:100 dilution. Ab binding was revealed with Texas Red-labeled anti-mouse and anti-rabbit IgG Ab, respectively. Fluorescence was analyzed with the Nikon RCM 8000 confocal microscope using a krypton ion laser at the 488-nm excitation band for GFP fluorescence (emission, 520 nm) and the 532-nm excitation band for Texas Red-conjugated secondary Abs (emission, 590 nm).
Materials
Culture media and sera were from Technogenetics (Milan, Italy); InsP3 was from Calbiochem (San Diego, CA); other chemicals were from Sigma (St. Louis, Missouri, USA).
Statistical Analysis
Analysis has been performed by Pulse (HEKA) and Igor-Pro3 (Wavemetrics, Lake Oswego, OR) software. The reported traces and data are the average ± SE (mean ± SE) of five to eight experiments for each condition.
RESULTS
Although the Ca2+-binding properties of CRT have been well characterized in vitro, its role in vivo as an intralumenal Ca2+ buffer is still highly debated. To solve this important issue we decided to investigate the effect of overexpressing CRT on the kinetics and amplitude of ICRAC, the selective Ca2+ current that is activated by depletion of intracellular Ca2+ stores (for reviews, see Penner et al., 1993; Fasolato et al., 1994; Berridge, 1995). The exquisite sensitivity of ICRAC to the Ca2+ content of the intracellular stores makes it ideally suited to monitor the role of alterations in Ca2+ handling within this compartment (Hofer et al., 1998).
RBL-1 cells, which are known to express a robust ICRAC (Fasolato et al., 1993; Hoth, 1995; Innocenti et al., 1996; Parekh and Penner, 1995; Parekh et al., 1997), were thus cotransfected by electroporation with CRT-cDNA and, as a marker of transfection, with the bright mutant S65T-cDNA of cytGFP, as described in MATERIALS and METHODS. Twenty-four to 48 h after transfection, cells positive for the GFP signal were identified and selected for patch-clamp experiments as described below.
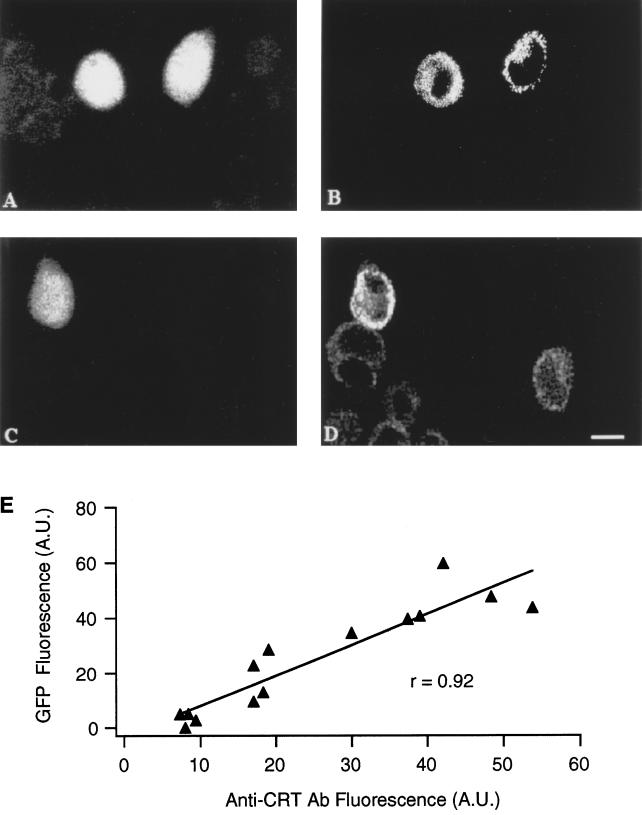
Figure 1 shows an example of RBL-1 cells positive for the bright mutant of cytGFP (Figure 1A) and decorated with the Ab specific for the HA1-tag inserted in the sequence of wtCRT (tCRT) (Figure 1B). Although the overall efficiency of transfection was rather low (8–10%), practically all cells positive for GFP were also positive for the tCRT. The same batch of cotransfected cells were decorated with anti-CRT Ab to track the subcellular distribution of the endogenous and transfected proteins. The cell positive for GFP (Figure 1C) had a more intense staining with the anti-CRT Ab (Figure 1D). As shown in Figure 1E, there is a good correlation in signal intensities when comparing GFP fluorescence and CRT overexpression. It is worth noting that both the Ab signals indicate a clear nuclear exclusion and a distinct reticular pattern distributed to a large part of the cytoplasm (cf. Figure 1, B and D). A rough estimation of the increase in CRT expression can be obtained by comparing the staining with the anti-CRT Ab in both GFP-positive and -negative cells. On average, CRT-overexpressing cells show an increase in fluorescence of 5.4 ± 1.4-fold over the basal signal because of the endogenous CRT (n = 14; cf. Figure 1, D and E).
Figure 1.
Immunofluorescence of cotransfected RBL-1 cells. CytGFP- and tCRT-cotransfected cells were analyzed for GFP (A and C), anti-tag (B), and anti-CRT (D) fluorescence signals by confocal microscope as described in MATERIALS AND METHODS. Note that the GFP-positive cell (C) shows a CRT signal clearly higher compared with control cells, which exhibit a moderate reticular signal attributable to the endogenous CRT (D; bar, 15 μm). E shows the correlation between fluorescence of the cytGFP and the antiCRT Ab in cotransfected cells. Each point represents the fluorescence intensity of a cell, obtained by averaging the signal of a standard digital box positioned on different parts of the cell. The fluorescence of GFP was subtracted from the signal intensity of a parallel batch of untransfected cells. The average increase in CRT expression was 5.4 ± 1.4-fold over the basal signal (n = 14 cells). A.U., arbitrary units of fluorescence.
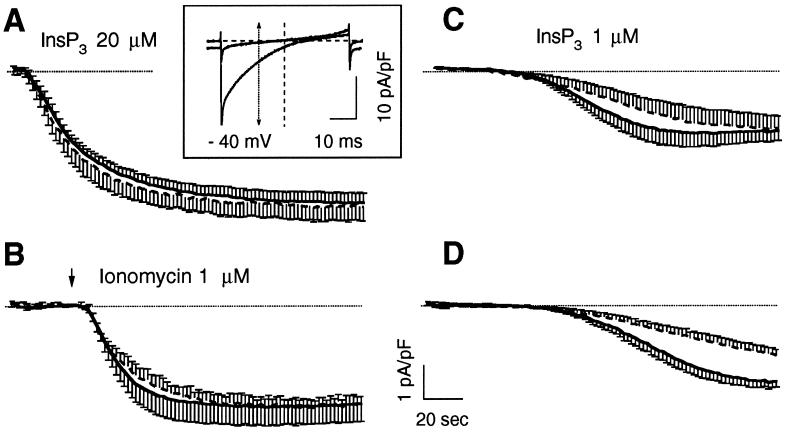
Cells with comparable GFP signal (normalized to the cell size) were selected for patch-clamp experiments. Two main protocols were initially used to compare ICRAC activation in controls (expressing only cytGFP) and tCRT-cotransfected cells. RBL-1 cells were voltage clamped at 0-mV holding potential in the whole-cell configuration of the patch-clamp technique with an internal solution containing a high concentration of the Ca2+ chelator BAPTA (12 mM; [Ca2+]i ≤ 10−9 nM) to prevent current inactivation (see MATERIALS AND METHODS). A rapid depletion of intracellular stores was achieved either with a maximal concentration of InsP3 (20 μM) added to the intracellular solution or with a maximal dose of the Ca2+ ionophore ionomycin (1 μM) applied from a puff pipette close to the cell surface. As shown in Figure 2, A and B, the average time course of ICRAC activation, under these conditions, was similar in kinetics and amplitudes in cells transfected with cytGFP alone or together with tCRT. In particular, when ICRAC was activated by InsP3 (Figure 2A), the current density (measured at −40 mV from fast voltage ramps acquired every 2 sec; see Figure 2A, inset, and MATERIALS AND METHODS) was −4.19 ± 0.32 and −4.37 ± 0.45 pA/pF in controls and CRT-overexpressing cells, respectively. Similarly, when ionomycin was applied within 40–50 sec from the establishment of the whole-cell configuration (Figure 2B), the current was maximally activated and reached −3.15 ± 0.31 and −3.2 ± 0.51 pA/pF in controls and CRT-transfected cells, respectively. (Note also that the current density obtained with optimal doses of ionomycin is smaller than that obtained with maximal doses of InsP3 [−3.2 and −4.4 pA/pF, respectively]. This reduction is likely attributable to the washout caused by the whole-cell dialysis [also see DISCUSSION].) Noteworthy, expression of cytGFP alone did not modify the kinetics and the overall properties of ICRAC if compared with untransfected cells (Fasolato et al., 1993; Innocenti et al., 1996). A protocol such as that presented in Figure 2, A and B, however, causes such a rapid and complete discharge of the Ca2+ pools that the Ca2+-buffering effect of CRT might be obscured (see DISCUSSION).
Figure 2.
Activation ICRAC by different procedures in control and CRT-overexpressing cells. RBL-1 cells transfected with the cDNA for cytGFP alone (control) or together with the cDNA for human tCRT (tCRT), as described in MATERIALS AND METHODS, were selected for GFP fluorescence and voltage clamped at 0 mV holding potential with an internal solution based on Cs-glutamate, containing 12 mM BAPTA, as described in MATERIALS AND METHODS. In A and C, InsP3 was also included at the specified concentrations. In B, current activation was boosted by application of ionomycin (1 μM) from a wide-tipped pipette positioned close to the cell. In D, ICRAC activation was induced simply by the presence of the Ca2+ chelator BAPTA. Traces represent the time courses of ICRAC activation measured at −40 mV from the instantaneous voltage ramps, used to monitor ICRAC development every 2 s, as shown in the inset. Currents from single cells were normalized to the cell capacitance, as indicator of the cell size, and plotted as average ± SE (from five to eight cells averaged for each condition); continuous trace, control; dashed trace, tCRT-transfected cells. The dotted line shows the zero current level. Inset, normalized current-voltage relationships, obtained by voltage ramps from −100 to +100 mV, lasting 50 ms and delivered at 0.5 Hz. The traces show the normalized current recorded before and after maximal activation in a typical experiment. The arrow shows the normalized current values measured at −40 mV and used to plot the time course of ICRAC activation.
To address the problem of whether CRT can significantly buffer the lumenal Ca2+, we designed a protocol in which the release of stored Ca2+ is much slower. Figure 2, C and D, shows that, when current activation was obtained by including in the patch pipette a submaximal dose of InsP3 (1 μM; Figure 2C), or when store depletion was allowed to occur spontaneously, as an effect of the prolonged intracellular perfusion with high BAPTA concentrations (Figure 2D), CRT overexpression grossly modified the kinetics of ICRAC activation. In particular, Figure 2D shows that, when depletion was caused by BAPTA alone, the time required to approximate the maximal current amplitude was doubled, from ∼1 to 2 min in CRT overexpressers versus control cells. Given that the difference between controls and tCRT-transfected cells was best appreciated using the passive depletion protocol, the latter was routinely used in the experiments described below, although qualitatively similar data were obtained with low InsP3 concentrations in the pipette (our unpublished data).
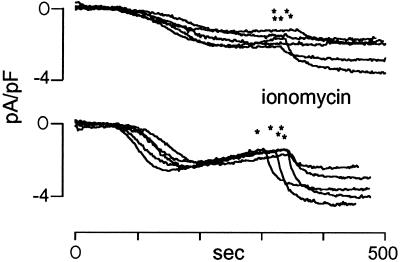
In the experiment presented in Figure 2D, it is clear that CRT overexpression modified not only the kinetics of ICRAC but also the extent of the current. Figure 3 shows a few examples of the time course of ICRAC activation in single cells during a typical experiment. It can be noted that in CRT-overexpressing cells the steady-state current, obtained by passive store depletion, did not reach the same level of control cells (−2.21 ± 0.10 and −1.75 ± 0.19 pA/pF in control and CRT overexpressers, respectively). Moreover, subsequent addition of a maximal dose of ionomycin (1 μM) did not result in recovery of the maximal current (−3.44 ± 0.41 and −2.37 ± 0.38 pA/pF in control and CRT overexpressers, respectively).
Figure 3.
Time course of ICRAC activation in single cells induced by passive store depletion. RBL-1 cells were transfected as described in Figure 1 and MATERIALS AND METHODS and voltage-clamped at 0 mV holding potential. ICRAC was slowly activated by BAPTA diffusing inside the cell as shown in Figure 2D. At the times indicate by the asterisks, ionomycin (1 μM) was applied from the external solution to induce maximal current activation. The traces show the normalized current measured as described in Figure 2.
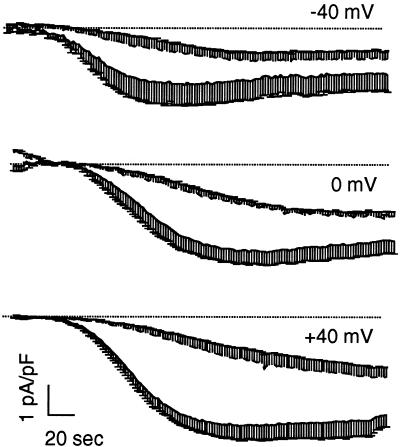
The effect induced by CRT overexpression was also tested in cells voltage clamped at either negative (−40 mV) or positive (+40 mV) holding potentials. Figure 4 shows the average time course of current activation at the three different holding potentials. Note that, as in the previous figures, the traces show the average current measured at −40 mV from the fast voltage ramps. It appears that the slowdown induced by CRT overexpression can be appreciated at each holding potential.
Figure 4.
Effect of the holding potential on ICRAC kinetics in CRT-overexpressing cells. RBL-1 cells were transfected and voltage clamped at different potentials. ICRAC was activated as described in Figure 2D. Traces are the average ± SE (n = 5 for each condition) of the normalized current, measured at −40 mV from the instantaneous voltage ramps delivered at 0.5 Hz. Continuous traces, control; dashed traces, tCRT-transfected cells; the dotted line shows the zero current level.
The reduction of ICRAC amplitude by CRT overexpression (Figure 3) is not easily explained by a simple buffering role of CRT. To determine whether CRT affects ICRAC by mechanisms other than, or in addition to, its capacity of buffering ER Ca2+, experiments were performed as presented in Figure 5.
Figure 5.
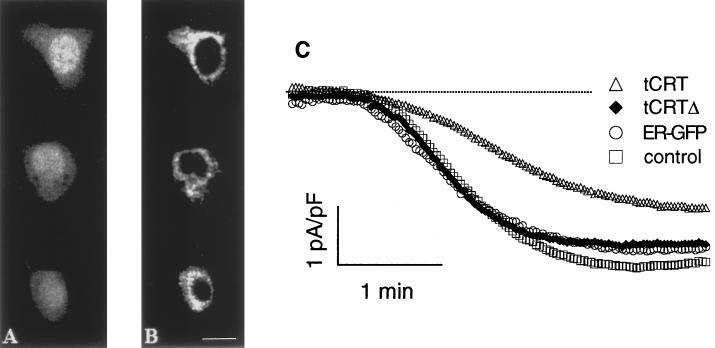
Immunolocalization of tCRTΔ cotransfected with cytGFP and its effect on ICRAC. Confocal images of RBL-1 cells cotransfected with cytGFP (A) and tCRTΔ cDNAs (B, anti-tag Ab) show that the tCRT deletion mutant was correctly expressed in the ER. Bar, 15 μm. C, ICRAC induced by passive store depletion, as described in Figure 2D, was monitored in cells transfected with cytGFP alone (control) or together with, respectively, tCRT, tCRTΔ, and ER-targeted GFP (ER-GFP) as described in MATERIALS AND METHODS. The traces show the normalized current, plotted as the pool average from three transfections; the dotted line shows the zero current level. For legibility SE is not shown (see RESULTS).
We generated a tCRT deletion mutant (tCRTΔ) from which the C domain with its low-affinity and high-capacity Ca2+-binding region had been removed. The mutant was further engineered to maintain, at the C terminus, the KDEL ER retention motif to ensure proper localization (see MATERIALS AND METHODS). As shown in Figure 5, cotransfected cells positive for cytGFP (Figure 5A) were also decorated with the anti-tag Ab against the mutant tCRTΔ (Figure 5B). A clear nuclear exclusion and a distinct reticular pattern confirm that the mutant was correctly targeted to the ER. Induction of ICRAC by passive store depletion was then followed with the protocol described in Figure 2D. Figure 5C shows the average time course of ICRAC activation in cells cotransfected with tCRTΔ (or tCRT) and cytGFP. The development of ICRAC was clearly delayed only by tCRT overexpression but not by transfection of its mutant, tCRTΔ. In cells transfected with tCRTΔ there was a marginal, statistically not significant, reduction in the extent of ICRAC (−3.06 ± 0.24, −2.67 ± 0.22, and −2.04 ± 0.21 pA/pF in control, tCRTΔ-, and tCRT-transfected cells, respectively). To test whether this small effect was indeed attributable to tCRTΔ or simply to the overexpression of a protein within the ER, cells were transfected with a recombinant protein targeted to the ER but with no Ca2+-binding capacity, that is, a GFP specific for the ER (ER-GFP; De Giorgi et al., 1998). The amplitude (as well as the kinetics) of ICRAC in ER-GFP–expressing cells was indistinguishable from that of tCRTΔ (−2.77 ± 0.38 pA/pF).
DISCUSSION
In the last years the role of CRT has been thoroughly investigated, and new functions for this protein have been implicated in a variety of diverse biological processes (Meldolesi et al., 1996; Helenius et al., 1997; Krause and Michalak, 1997).
In this study we show that ICRAC activation, induced by high doses of InsP3 or ionomycin, reaches maximal amplitudes at almost identical rates in control and CRT-overexpressing cells. Thus, a drastic increase in ER CRT does not interfere per se with the signaling pathway that activates the CRAC channels. On the contrary, procedures that slowly deplete the stores, such as simply buffering the cytosolic Ca2+ at nanomolar levels with or without submaximal doses of InsP3, result in a marked delay and slowdown of ICRAC activation that is significantly higher in CRT-overexpressing cells. The slowdown of ICRAC, induced by CRT overexpression, was consistently observed when cells were clamped at different holding potentials, confirming that the phenomenon is not attributable to current modulation but is causally linked to the depletion process.
The question thus arises of whether these observations are compatible with a simple Ca2+-buffering role of CRT, or whether CRT affects ICRAC by other means. To answer this question, let us first consider the basic features of ER Ca2+ handling.
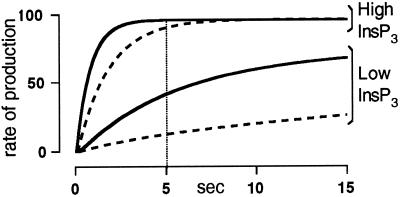
To a first approximation, the steady-state [Ca2+] of the ER is the result of a pump and leak equilibrium, whereby an increase in Ca2+ buffering, by CRT or any other means, results in an increase in the total Ca2+ content with no effect on the steady-state free lumenal Ca2+ concentration. Given that the rate and extent of ICRAC activation depends on the free Ca2+ concentration in the ER (Hofer et al., 1998), one would have expected that the kinetics of current activation should be slower in CRT-overexpressing cells, independently of the rate at which ER depletion occurs. To account for the experimental observations, we propose a simple model that takes into account a few established facts but makes no assumptions about the molecular mechanism of ICRAC activation. In this model we assume that the kinetics of ICRAC development follow, to a first approximation, the cellular concentration of the ICRAC-activating factor. If the production of this factor is directly correlated with the [Ca2+] of the ER, the maximal rate of its production will be reached faster at high InsP3 concentrations and will be lowered by overexpression of CRT, as depicted in Figure 6. The cellular level of ICRAC-activating factor will also increase with time in a similar manner, reaching a steady state when the rate of production equals that of degradation. Using these two simple assumptions and taking into account that, even at maximal rates of store depletion, there is an intrinsic lag time of 5 sec between the drop in the ER [Ca2+] and the development of ICRAC (Hofer et al., 1998), we can easily explain the experimental results. In fact, if store depletion occurs within the intrinsic lag time required for ICRAC activation, the rate of production of the factor will approach its maximal value before current development. Accordingly, at high InsP3 concentrations maximal rate of its production will be achieved also in tCRT-overexpressing cells. The kinetics of current activation will be indistinguishable from control cells despite an initial reduced rate (Figure 6). On the other hand, procedures that induce a slow depletion, such as cell perfusion with low InsP3 concentrations or BAPTA alone, will take a much longer time to approach the maximal rate of production of the ICRAC-activating factor, allowing the difference in lumenal Ca2+ buffering between control and CRT-overexpressing cells to become obvious. This is indeed what we observed experimentally.
Figure 6.
Model for the production of the ICRAC-activating factor. Hypothetical rate of production of the ICRAC-activating factor, at low and high concentrations of InsP3, in control (continuous line) and tCRT-overexpressing cells (dashed line). The vertical line represents the lag time for ICRAC activation for fast depletion protocols (Hofer et al., 1998).
The conclusion that CRT affects ICRAC because of its Ca2+-buffering role and not because of other functions of this protein is further strengthened by the demonstration that tCRTΔ, which lacks the C-terminal Ca2+-binding domain, does not mimic the slowdown of the current observed with intact tCRT.
The model presented in Figure 6 also predicts that eventually the extent of ICRAC should be identical, independent of the rate at which store depletion occurred. The finding that a slow activation of the current results in a reduction of current amplitude is in large part attributable to the fact that ICRAC is subjected to a washout effect; i.e., procedures that activate ICRAC at longer times after the establishment of the whole-cell configuration are known to produce currents of progressively reduced size (Fasolato et al., 1993). Also in this work, the current density in control cells is smaller if activation is triggered by passive store depletion compared with activation by maximal doses of InsP3. Passive store depletion in CRT-overexpressing cells is even more affected, because channel recruitment occurs at a much slower rate.
The washout phenomenon, however, does not account entirely for the difference in ICRAC amplitude between control and CRT-overexpressing cells when, after partial emptying of the stores by passive depletion, ionomycin is added to both cells (Figure 3). In this case, a simple washout effect would predict that the current density should be identical in the two conditions. We have no simple explanation for this finding. A plausible hypothesis is that the rate of channel recruitment dictates the extent of the current that can be eventually activated. This phenomenon cannot, however, be attributed to an effect of CRT overexpression per se, given that 1) it is not observed with tCRTΔ; and 2) it can be induced by other depletion protocols that imply a delay in current activation (Fasolato and Innocenti, unpublished observations).
How can one reconcile the present data (and the previous data along the same line) with those 1) negating a Ca2+-buffering role of CRT and 2) sustaining a direct effect of CRT on the mechanism of ICRAC activation? The simplest explanation is that in this study (and that of Bastianutto et al., 1995) control cells are compared with cells acutely transfected with CRT (or with cells in which CRT had been acutely down-regulated, as in the study by Liu et al., 1994). On the contrary, Coppolino et al. (1997) and Mery et al. (1996) used knockout or stably transfected cells, thus allowing for intrinsic homeostatic mechanisms to develop and for differences between clones to appear. In those studies, therefore, the lack of effects of CRT down-regulation, or the apparent inhibition of ICRAC by CRT overexpression, most likely reflects adaptive mechanisms to CRT expression levels rather than being indicative of CRT functions.
Admittedly, our data provide evidence only in favor of a Ca2+-buffering role of overexpressed CRT and do not address the question of whether endogenous CRT plays an important role as an intralumenal Ca2+ buffer. In fact, van de Put and Elliott (1997) have recently questioned this role in pancreatic acinar cells. A few considerations, however, suggest that in RBL-1 cells, as well as in other cells, endogenous CRT is a significant component of the ER Ca2+-buffering system. In particular: 1) CRT is ∼1–2% of total ER proteins (Krause and Michalak, 1997), and thus its lumenal concentration approaches 50–100 μM; 2) given that CRT can account for up to 50 Ca2+-binding sites per mole of protein, its total Ca2+-binding capacity could be as high as 2.5–5 mM in ER water; and 3) Considering that the free Ca2+ concentration in the ER lumen is ∼0.3–0.6 mM, and the total mobilizable Ca2+ is 3–6 mM in ER (Pizzo et al., 1997), it derives that endogenous CRT has the potential to buffer a substantial amount of it.
A final question concerns whether CRT overexpression is only an experimental tool to investigate the function of this protein or whether it can offer some paradigms to understand more physiological or pathological conditions. In this respect, it is worth mentioning that CRT is up-regulated during proliferation, and high levels of CRT expression were detected in B16 melanoma cells (Gersten et al., 1990). Noteworthy, the promoter region of CRT contains a retinoblastoma control element (Krause and Michalak, 1997). In addition, up to a fourfold increase in the level of CRT can be induced by prolonged exposure to Ca2+-depleting agents and other stress treatments (Conway et al., 1995; Nguyen et al., 1996; Llewellyn et al., 1996; Tsutsui et al., 1997; Waser et al., 1997). Thus, overexpression of CRT might be a widespread phenomenon with important consequences on Ca2+ homeostatic mechanisms.
ACKNOWLEDGMENTS
We are grateful to G. Ronconi and M. Santato for skillful assistance and Drs. R. Rizzuto and M. Murgia for kindly providing recombinant ER-GFP. We thank Dr. T. MacDonald for generously supplying the expression vector VR1012/cytGFP and Dr. C. Bastianutto for the tag-CRT sequence. This work was supported by Telethon grant 845 and grants from the European Union Human Capital and Mobility and Copernicus, the Human Frontier Science Program and the Armenise Foundation (Harvard University) to T.P. C.F. and P.P. contributed equally to this work.
REFERENCES
- Bastianutto C, Clementi E, Codazzi F, Podini P, De Giorgi F, Rizzuto R, Meldolesi J, Pozzan T. Overexpression of calreticulin increases the Ca2+ capacity of rapidly exchanging Ca2+ stores and reveals aspects of their lumenal microenvironment and function. J Cell Biol. 1995;130:847–855. doi: 10.1083/jcb.130.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. Capacitative calcium entry. Biochem J. 1995;312:1–11. doi: 10.1042/bj3120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brini M, Marsault R, Bastianutto C, Alvarez J, Pozzan T, Rizzuto R. Transfected aequorin in the measurement of cytosolic Ca2+ concentration ([Ca2+]c). A critical evaluation. J Biol Chem. 1995;270:9896–9903. doi: 10.1074/jbc.270.17.9896. [DOI] [PubMed] [Google Scholar]
- Camacho P, Lechleiter JD. Calreticulin inhibits repetitive intracellular Ca2+ waves. Cell. 1995;82:765–771. doi: 10.1016/0092-8674(95)90473-5. [DOI] [PubMed] [Google Scholar]
- Conway EM, Liu L, Nowakowski B, Steiner-Mosonyi M, Ribeiro SP, Michalak M. Heat shock-sensitive expression of calreticulin. In vitro and in vivo up-regulation. J Biol Chem. 1995;270:17011–17016. doi: 10.1074/jbc.270.28.17011. [DOI] [PubMed] [Google Scholar]
- Coppolino MG, Woodside MJ, Demaurex N, Grinstein S, St-Arnaud R, Dedhar S. Calreticulin is essential for integrin-mediated calcium signalling and cell adhesion. Nature. 1997;386:843–847. doi: 10.1038/386843a0. [DOI] [PubMed] [Google Scholar]
- Damiani E, Heilmann C, Salvatori S, Margreth A. Characterization of high-capacity low-affinity calcium binding protein of liver endoplasmic reticulum: calsequestrin-like and divergent properties. Biochem Biophys Res Commun. 1989;165:973–980. doi: 10.1016/0006-291x(89)92698-3. [DOI] [PubMed] [Google Scholar]
- Dedhar S. Novel functions for calreticulin: interaction with integrins and modulation of gene expression? Trends Biochem Sci. 1994;19:269–271. doi: 10.1016/0968-0004(94)90001-9. [DOI] [PubMed] [Google Scholar]
- De Giorgi, F., Ahmed, Z., Bastianutto, C., Brini, M., Jouaville, L.S., Marsault, R., Murgia, M., Pinton, P., Pozzan, T., and Rizzuto, R. (1998). Targeting GFP to organelles. Methods Cell Biol. (in press). [DOI] [PubMed]
- Fasolato C, Hoth M, Penner R. A GTP-dependent step in the activation mechanism of capacitative calcium influx. J Biol Chem. 1993;268:20737–20740. [PubMed] [Google Scholar]
- Fasolato C, Innocenti B, Pozzan T. Receptor-activated Ca2+ influx: how many mechanisms for how many channels? Trends Pharmacol Sci. 1994;15:77–83. doi: 10.1016/0165-6147(94)90282-8. [DOI] [PubMed] [Google Scholar]
- Gersten DM, Bijwaard KE, Law LW, Hearing VJ. Homology of the B50 murine melanoma antigen to the Ro/SS-A antigen of human systemic lupus erythematosus and to calcium-binding proteins. Biochim Biophys Acta. 1990;1096:20–25. doi: 10.1016/0925-4439(90)90007-c. [DOI] [PubMed] [Google Scholar]
- Helenius A, Trombetta SE, Hebert DN, Simons JF. Calnexin, calreticulin and the folding of glycoproteins. Trends Cell Biol. 1997;7:193–200. doi: 10.1016/S0962-8924(97)01032-5. [DOI] [PubMed] [Google Scholar]
- Hofer AM, Fasolato C, Pozzan T. Capacitative Ca2+ entry is closely linked to the filling state of internal Ca2+ stores: a study using simultaneous measurements of ICRAC and intraluminal [Ca2+] J Cell Biol. 1998;140:325–334. doi: 10.1083/jcb.140.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer AM, Schlue WR, Curci S, Machen TE. Spatial distribution and quantitation of free luminal [Ca2+] within the InsP3-sensitive internal store of individual BHK-21 cells. Ion dependence of InsP3-induced Ca2+ release and reloading. FASEB J. 1995;9:788–798. doi: 10.1096/fasebj.9.9.7601343. [DOI] [PubMed] [Google Scholar]
- Hofer AM, Schulz I. Quantification of intraluminal free [Ca2+] in the agonist-sensitive internal calcium store using compartmentalized fluorescent indicators: some considerations. Cell Calcium. 1996;20:235–242. doi: 10.1016/s0143-4160(96)90029-9. [DOI] [PubMed] [Google Scholar]
- Hoth M. Calcium and barium permeation through calcium release-activated calcium (crac) channels. Pfluegers Archiv Eur J Physiol. 1995;430:315–322. doi: 10.1007/BF00373905. [DOI] [PubMed] [Google Scholar]
- Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol. 1993;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti B, Pozzan T, Fasolato C. Intracellular ADP modulates the Ca2+ release-activated calcium current in a temperature and Ca2+ dependent way. J Biol Chem. 1996;271:8582–8587. doi: 10.1074/jbc.271.15.8582. [DOI] [PubMed] [Google Scholar]
- Krause KH, Michalak M. Calreticulin. Cell. 1997;88:439–443. doi: 10.1016/s0092-8674(00)81884-x. [DOI] [PubMed] [Google Scholar]
- Liu N, Fine RE, Simons E, Johnson RJ. Decreasing calreticulin expression lowers the Ca2+ response to bradykinin and increases sensitivity to ionomycin in NG-108–15 cells. J Biol Chem. 1994;269:28635–28639. [PubMed] [Google Scholar]
- Llewellyn DH, Kendall JM, Sheikh FN, Campbell AK. Induction of calreticulin expression in HeLa cells by depletion of the endoplasmic reticulum Ca2+ store and inhibition of N-linked glycosylation. Biochem J. 1996;318:555–560. doi: 10.1042/bj3180555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytton J, Nigam SK. Intracellular calcium: molecules and pools. Curr Opin Cell Biol. 1992;4:220–226. doi: 10.1016/0955-0674(92)90036-c. [DOI] [PubMed] [Google Scholar]
- Macer DR, Koch GL. Identification of a set of calcium-binding proteins in reticuloplasm, the luminal content of the endoplasmic reticulum. J Cell Sci. 1988;91:61–70. doi: 10.1242/jcs.91.1.61. [DOI] [PubMed] [Google Scholar]
- McCauliffe DP, et al. Molecular cloning, expression, and chromosome 19 localization of a human Ro/SS-A autoantigen. J Clin Invest. 1990;85:1379–1391. doi: 10.1172/JCI114582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldolesi J, Krause KH, Michalak M. Calreticulin: how many functions in how many cellular compartments? Cell Calcium. 1996;20:83–86. doi: 10.1016/s0143-4160(96)90053-6. [DOI] [PubMed] [Google Scholar]
- Mery L, Mesaeli N, Michalak M, Opas M, Lew DL, Krause KH. Overexpression of calreticulin increases intracellular Ca2+-storage and decreases store-operated Ca2+ influx. J Biol Chem. 1996;271:9332–9339. doi: 10.1074/jbc.271.16.9332. [DOI] [PubMed] [Google Scholar]
- Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- Montero M, Barrero MJ, Alvarez J. [Ca2+]i microdomains control agonist-induced Ca2+ release in intact HeLa cells. FASEB J. 1997;11:881–885. doi: 10.1096/fasebj.11.11.9285486. [DOI] [PubMed] [Google Scholar]
- Montero M, Brini M, Marsault R, Alvarez J, Sitia R, Pozzan T, Rizzuto R. Monitoring dynamic changes in free Ca2+ concentration in the endoplasmic reticulum of intact cells. EMBO J. 1995;14:5467–5475. doi: 10.1002/j.1460-2075.1995.tb00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash PD, Opas M, Michalak M. Calreticulin: not just another calcium-binding protein. Mol Cell Biochem. 1994;135:71–78. doi: 10.1007/BF00925962. [DOI] [PubMed] [Google Scholar]
- Nguyen TO, Capra JD, Sontheimer RD. Calreticulin is transcriptionally upregulated by heat shock, calcium and heavy metals. Mol Immunol. 1996;33:379–386. doi: 10.1016/0161-5890(95)00149-2. [DOI] [PubMed] [Google Scholar]
- Opas M, Szewczenko-Pawlikowski M, Jass GK, Mesaeli N, Michalak M. Calreticulin modulates cell adhesiveness via regulation of vinculin expression. J Cell Biol. 1996;135:1913–1923. doi: 10.1083/jcb.135.6.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostwald TJ, MacLennan DH. Isolation of a high affinity calcium-binding protein from sarcoplasmic reticulum. J Biol Chem. 1974;249:974–976. [PubMed] [Google Scholar]
- Parekh AB, Fleig A, Penner R. The store-operated calcium current Icrac: Nonlinear activation by InsP3 and dissociation from calcium release. Cell. 1997;89:973–980. doi: 10.1016/s0092-8674(00)80282-2. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Penner R. Depletion-activated calcium current is inhibited by protein kinase in RBL-2H3 cells. Proc Natl Acad Sci USA. 1995;92:7907–7911. doi: 10.1073/pnas.92.17.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner R, Fasolato C, Hoth M. Calcium influx and its control by calcium release. Curr Opin Neurobiol. 1993;3:368–374. doi: 10.1016/0959-4388(93)90130-q. [DOI] [PubMed] [Google Scholar]
- Pizzo P, Fasolato C, Pozzan T. Dynamic properties of an inositol 1,4,5-trisphosphate-and thapsigargin-insensitive calcium pool in mammalian cell lines. J Cell Biol. 1997;136:355–366. doi: 10.1083/jcb.136.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzan T, Rizzuto R, Volpe P, Meldolesi J. Molecular and cellular physiology of Ca2+ stores. Physiol Rev. 1994;74:595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- Treves S, De Mattei M, Landfredi M, Villa A, Green NM, MacLennan DH, Meldolesi J, Pozzan T. Calreticulin is a candidate for a calsequestrin-like function in Ca2+-storage compartments (calciosomes) of liver and brain. Biochem J. 1990;271:473–480. doi: 10.1042/bj2710473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui H, Ishibashi Y, Imanakayoshida K, Yamamoto S, Yoshida T, Sugimachi M, Urabe Y, Takeshita A. Alterations in sarcoplasmic reticulum calcium storing protein in pressure overload cardiac hypertrophy. Am J Physiol. 1997;41:H168–H175. doi: 10.1152/ajpheart.1997.272.1.H168. [DOI] [PubMed] [Google Scholar]
- van de Put FHMM, Elliott AC. The endoplasmic reticulum can act as a functional Ca2+ store in all subcellular regions of the pancreatic acinar cell. J Biol Chem. 1997;272:27764–27770. doi: 10.1074/jbc.272.44.27764. [DOI] [PubMed] [Google Scholar]
- Waser M, Mesaeli N, Spencer C, Michalak M. Regulation of calreticulin gene expression by calcium. J Cell Biol. 1997;138:547–557. doi: 10.1083/jcb.138.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifach A, Lewis RS. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci USA. 1993;90:6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]