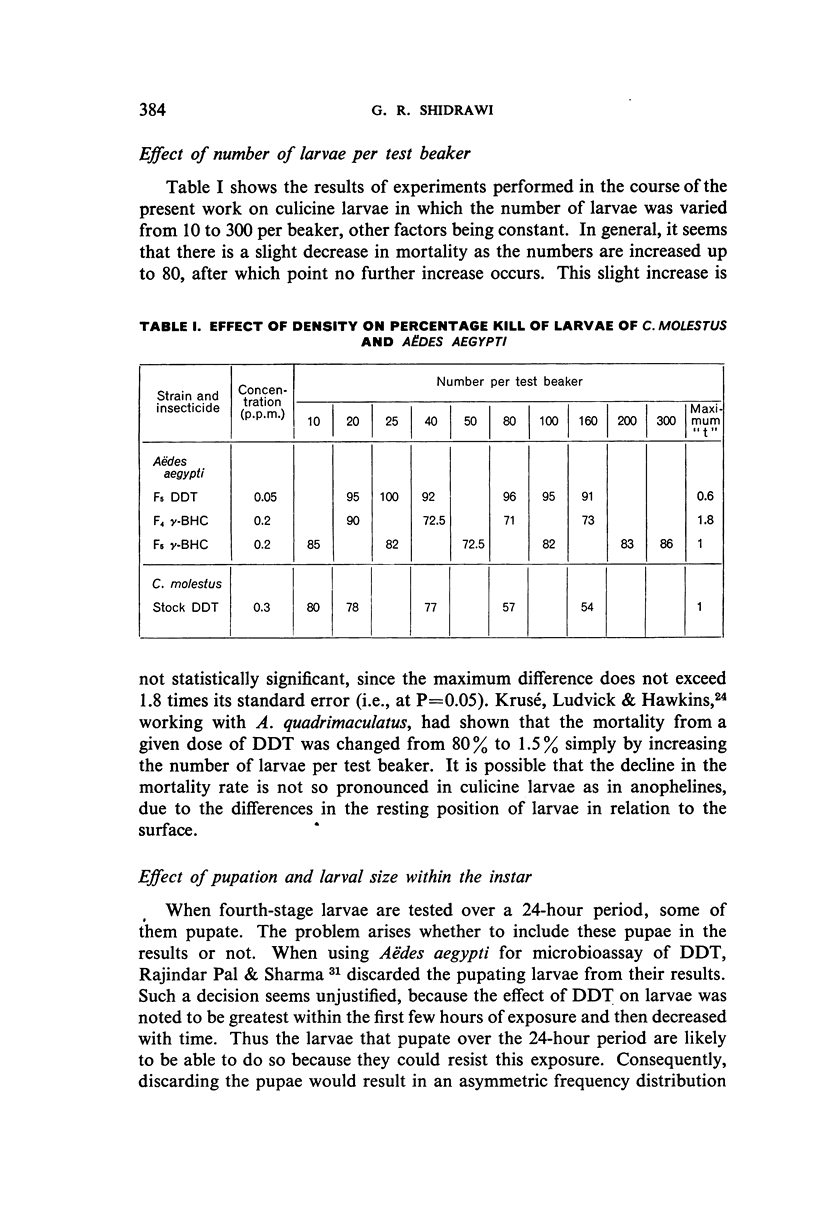
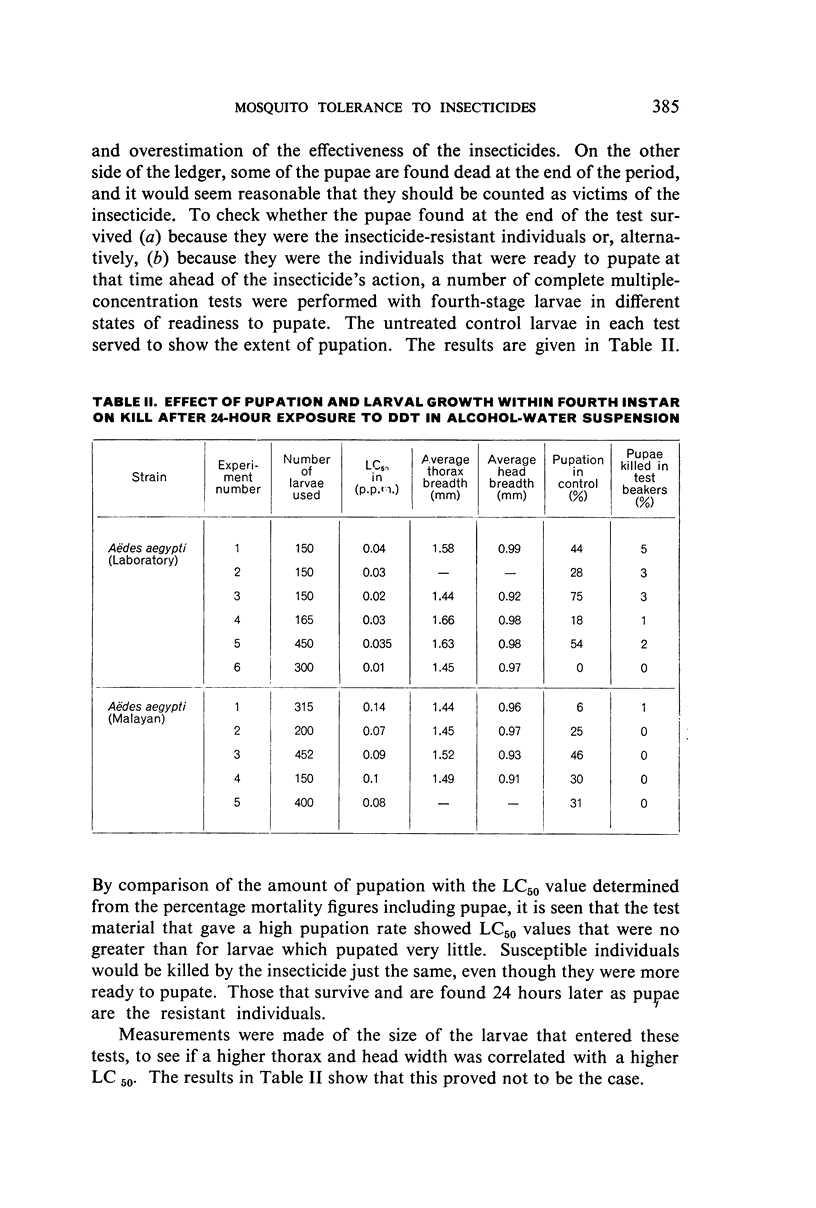
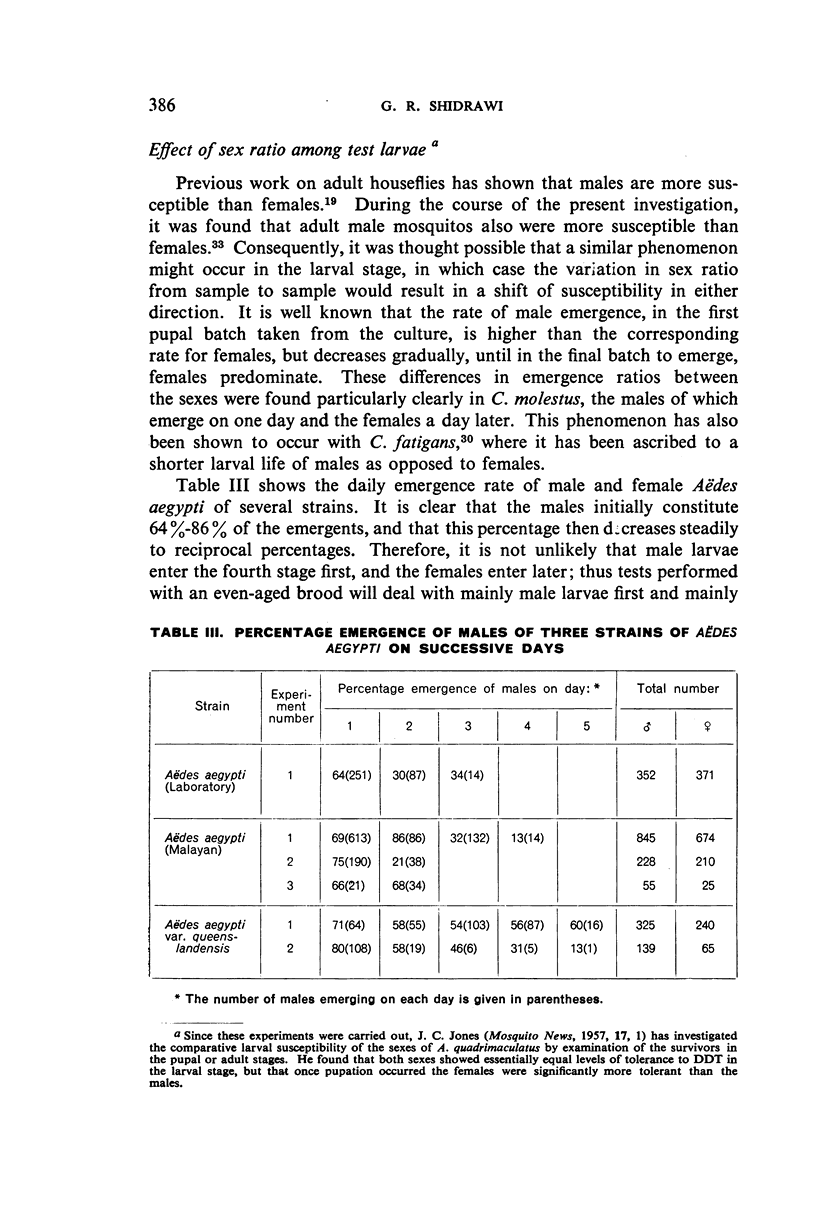
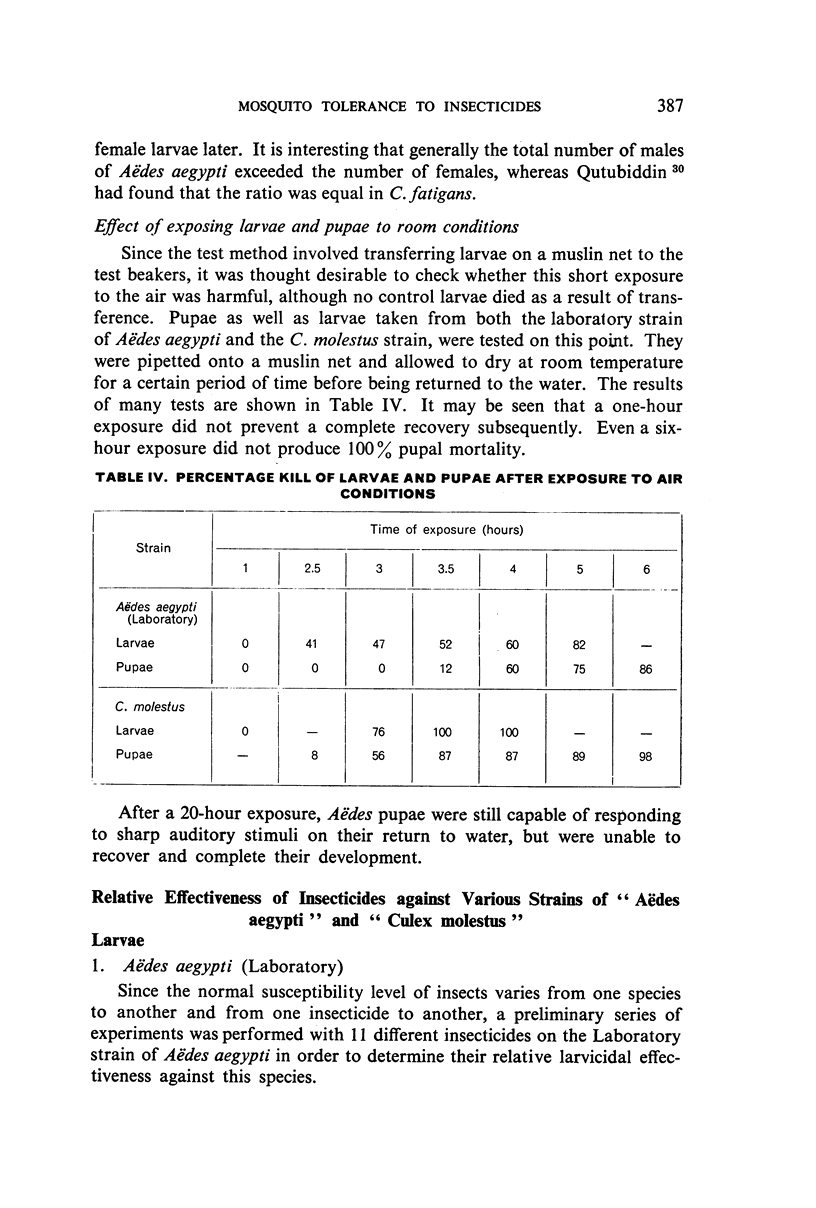
Abstract
Three strains of Aëdes aegypti and a strain of Culex molestus were used in tests to determine the relative susceptibility levels of the larvae to various insecticides and to investigate the development of resistance in the laboratory through selection by larval treatment only. The procedures and test methods used are described and the results discussed and set out in tabular and graphic form.
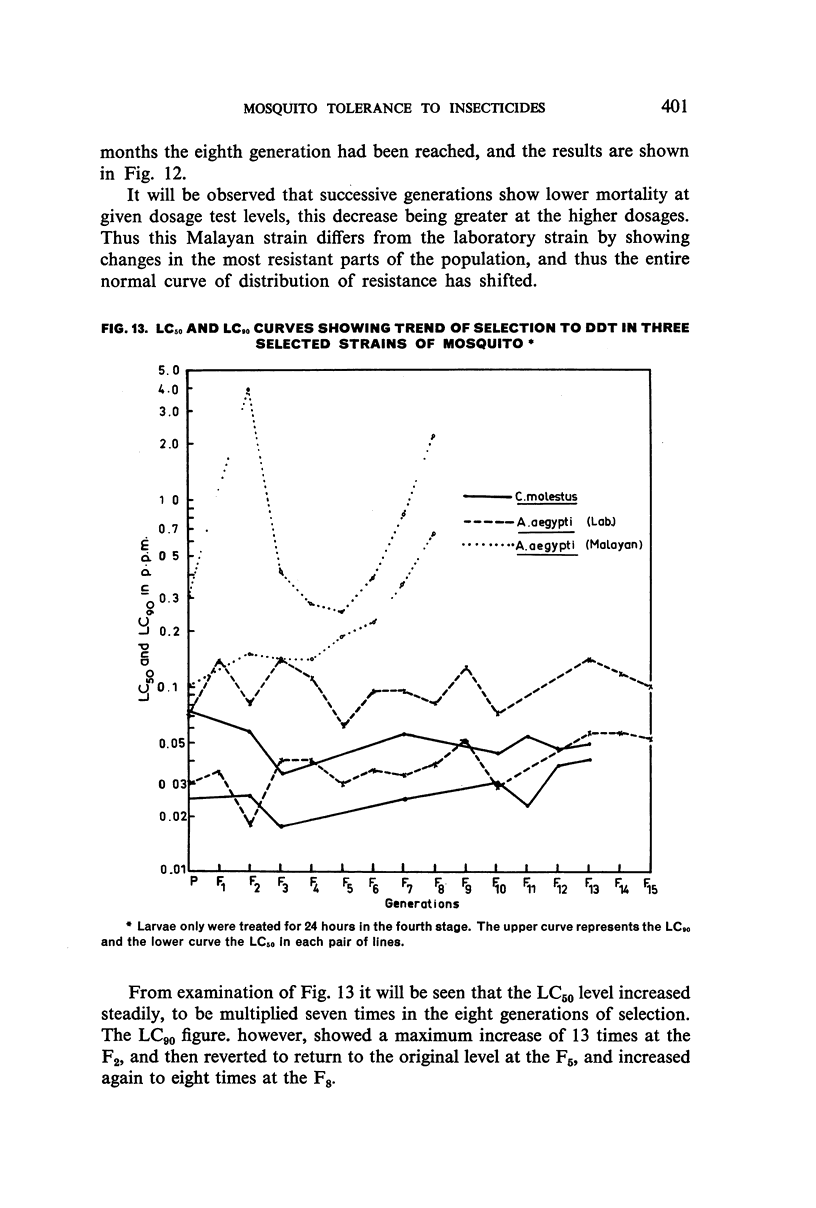
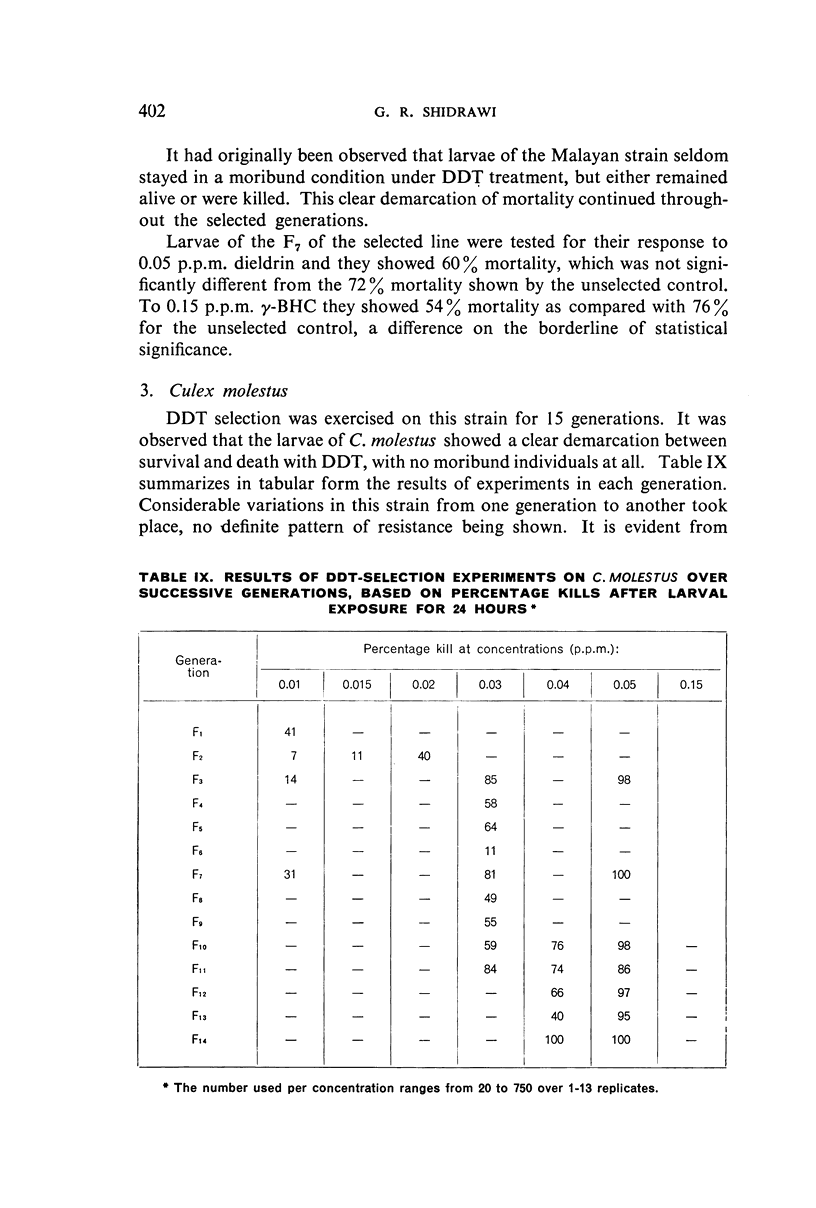
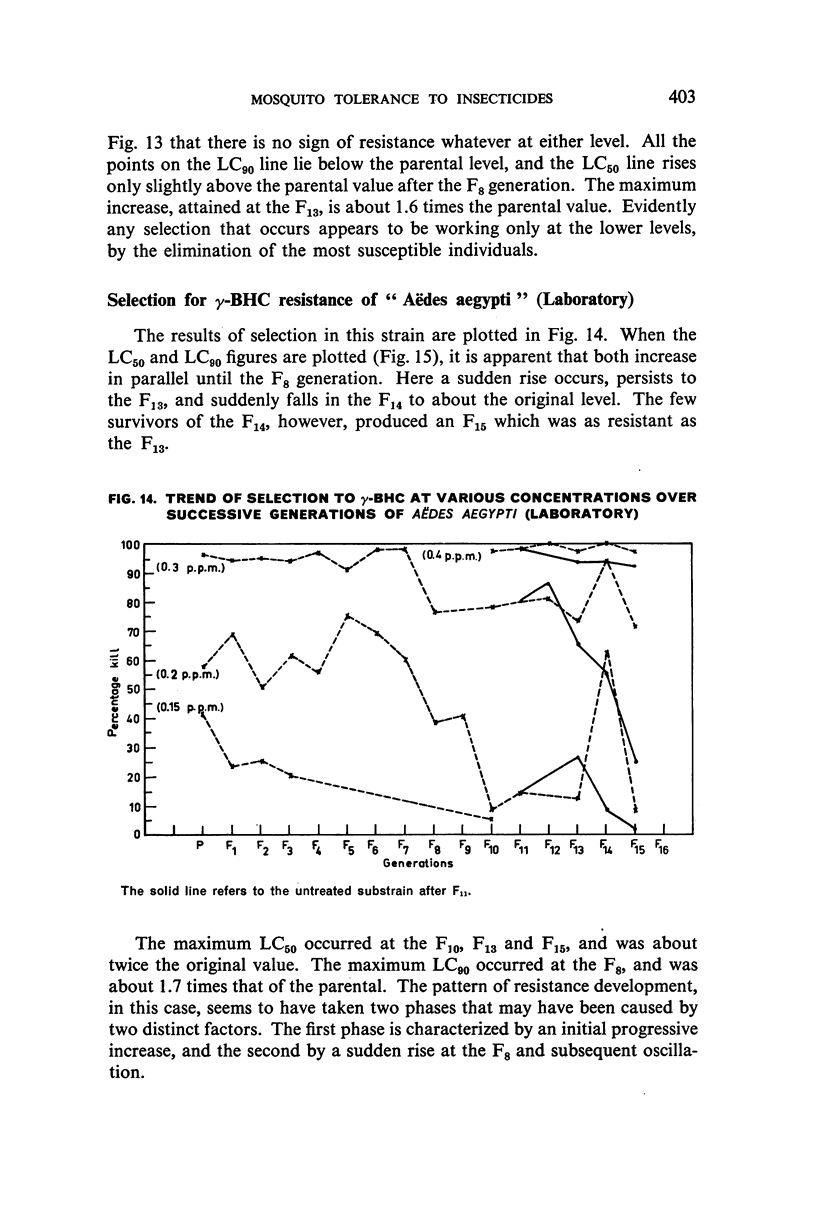
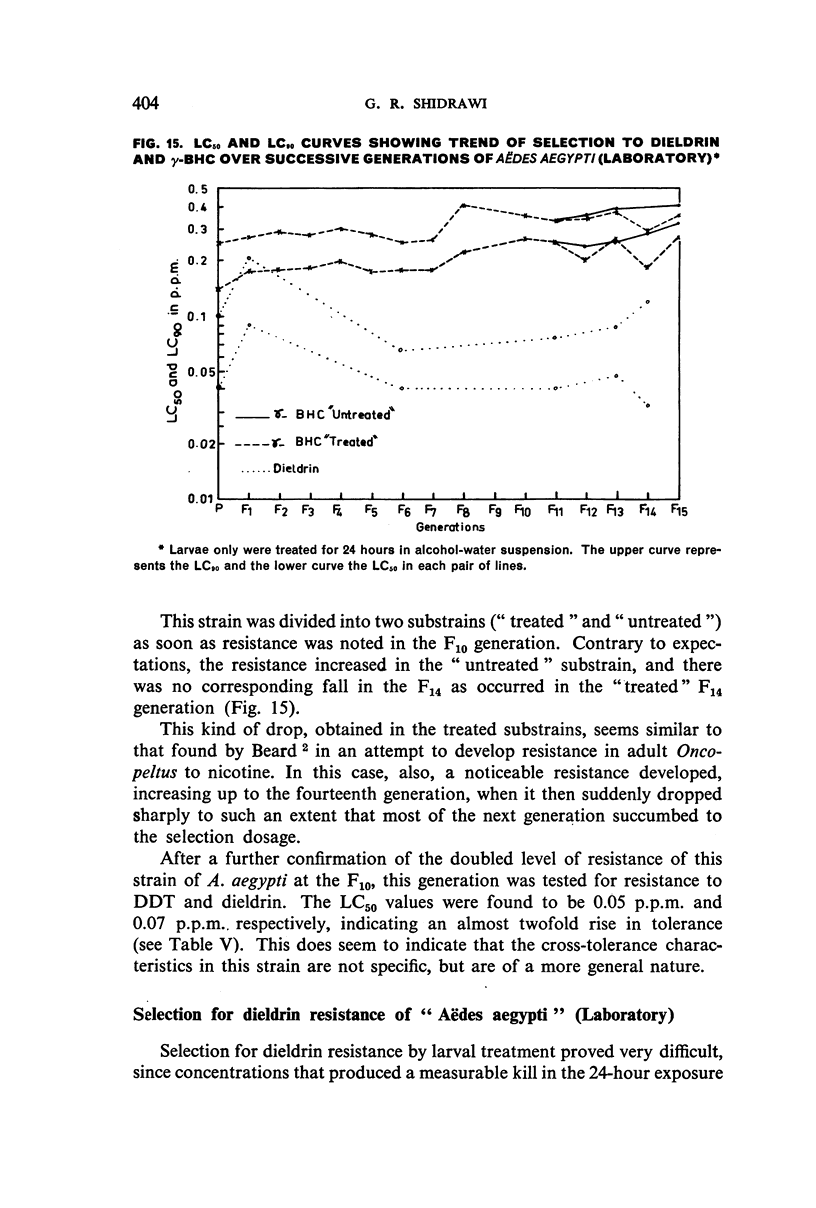
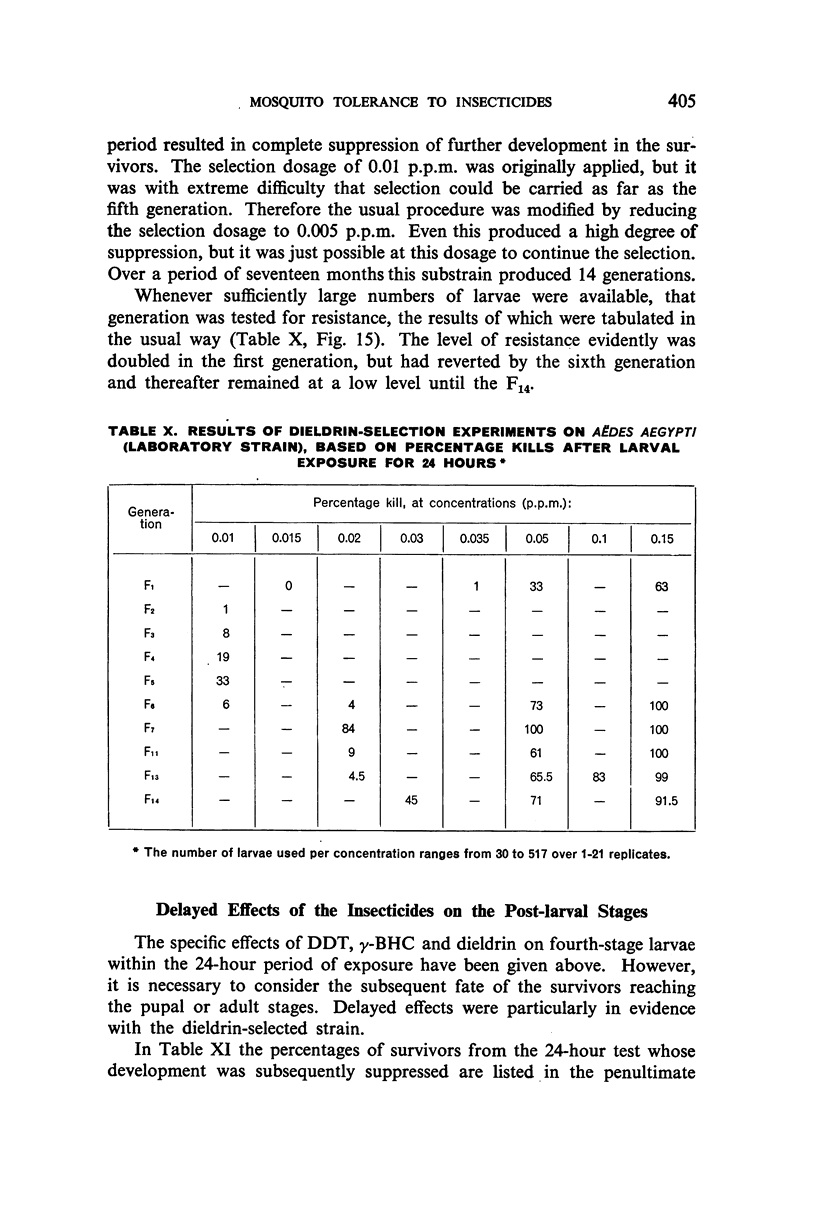
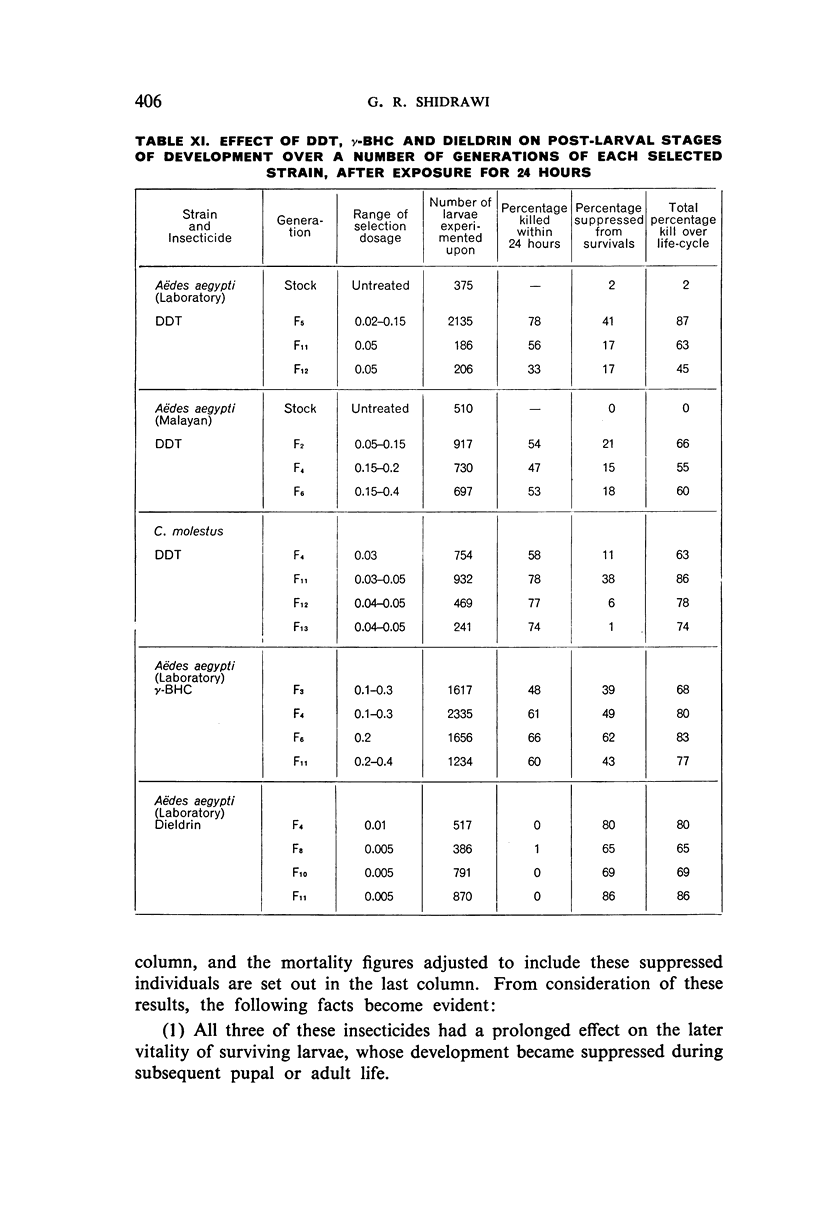
It was found, inter alia, that selection of fourth-stage larvae of a laboratory strain of Aëdes and Culex for 15 generations with DDT at the LC50 level did not induce any appreciable decrease in susceptibility; that with another Aëdes strain, naturally three times as resistant to DDT as the laboratory strain, selection for eight generations further increased this resistance to seven times its natural level; that selection of the laboratory strain of Aëdes with γ-BHC for 15 generations induced a twofold increase in resistance to this compound, which also extended to DDT and dieldrin; and that selection of the same strain with dieldrin, over 14 generations at a low mortality level to avoid after-effects of this insecticide, did not induce any appreciable decrease in susceptibility.
Full text
PDF






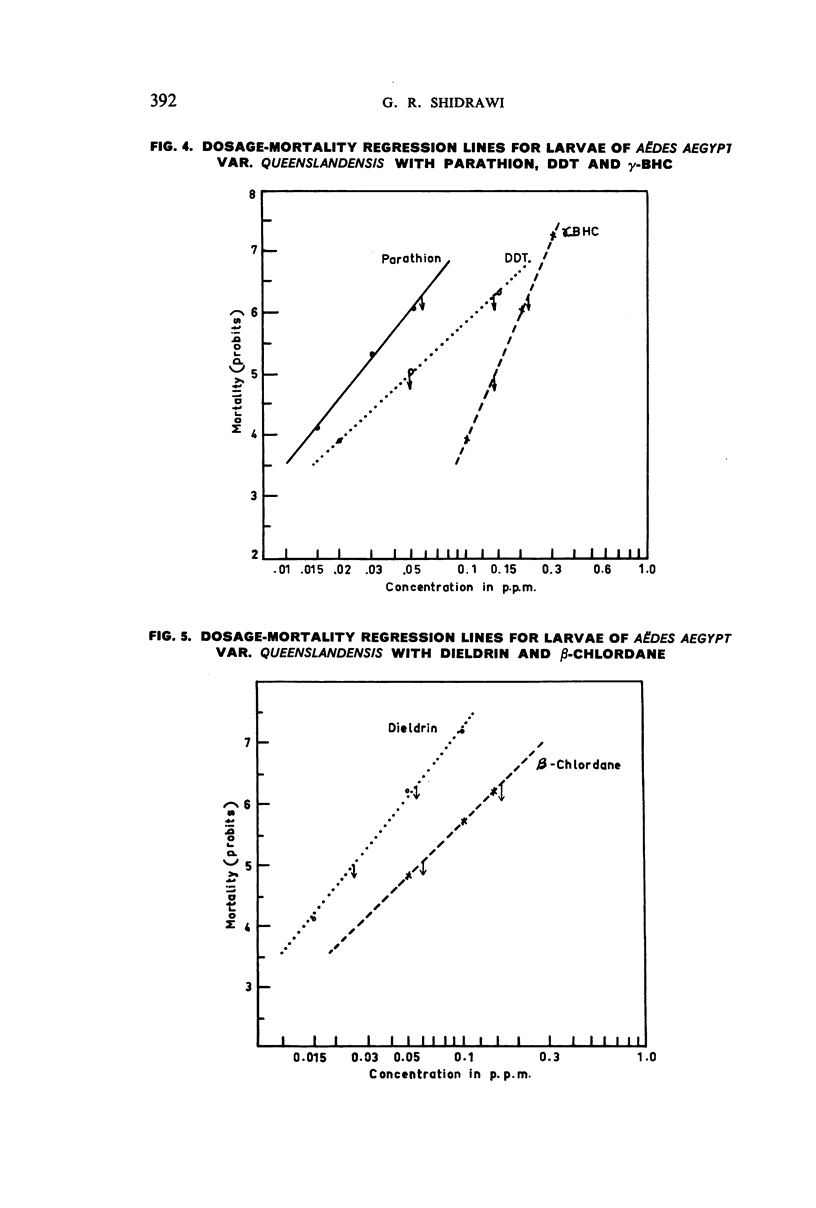
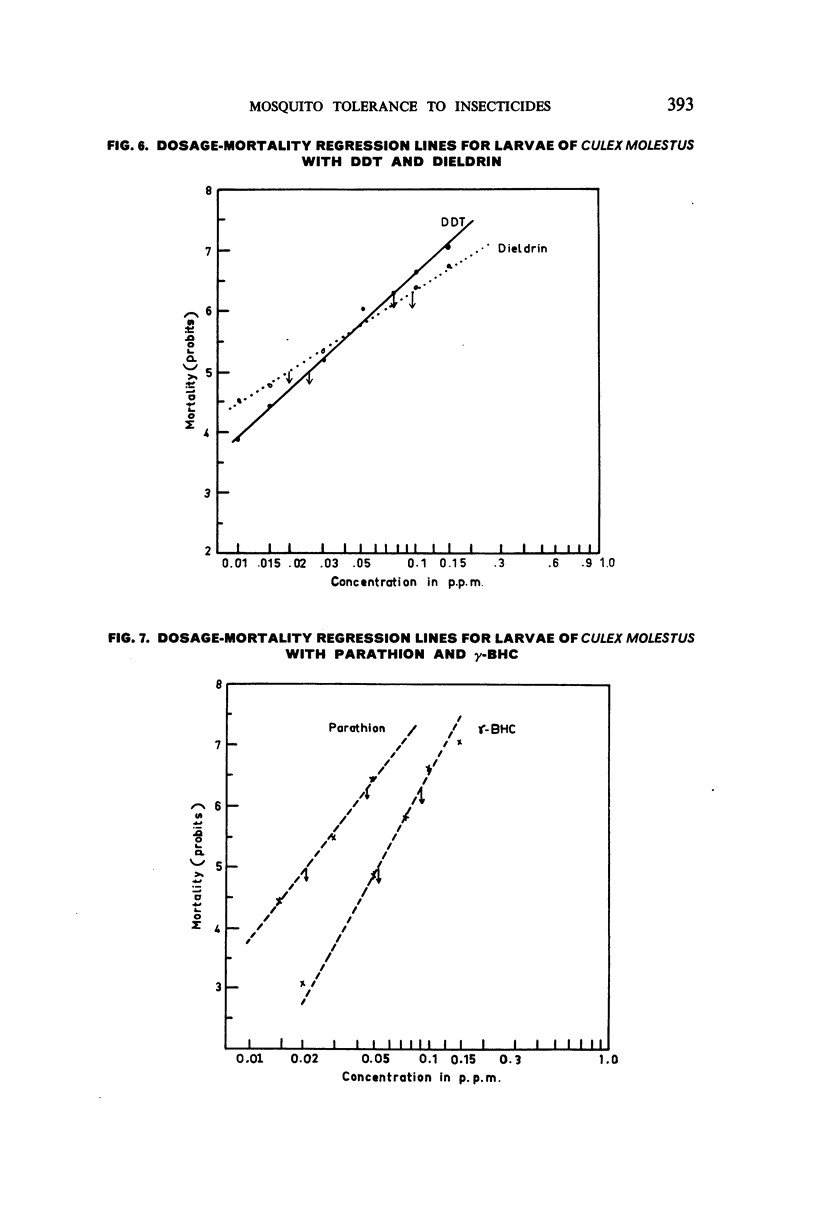
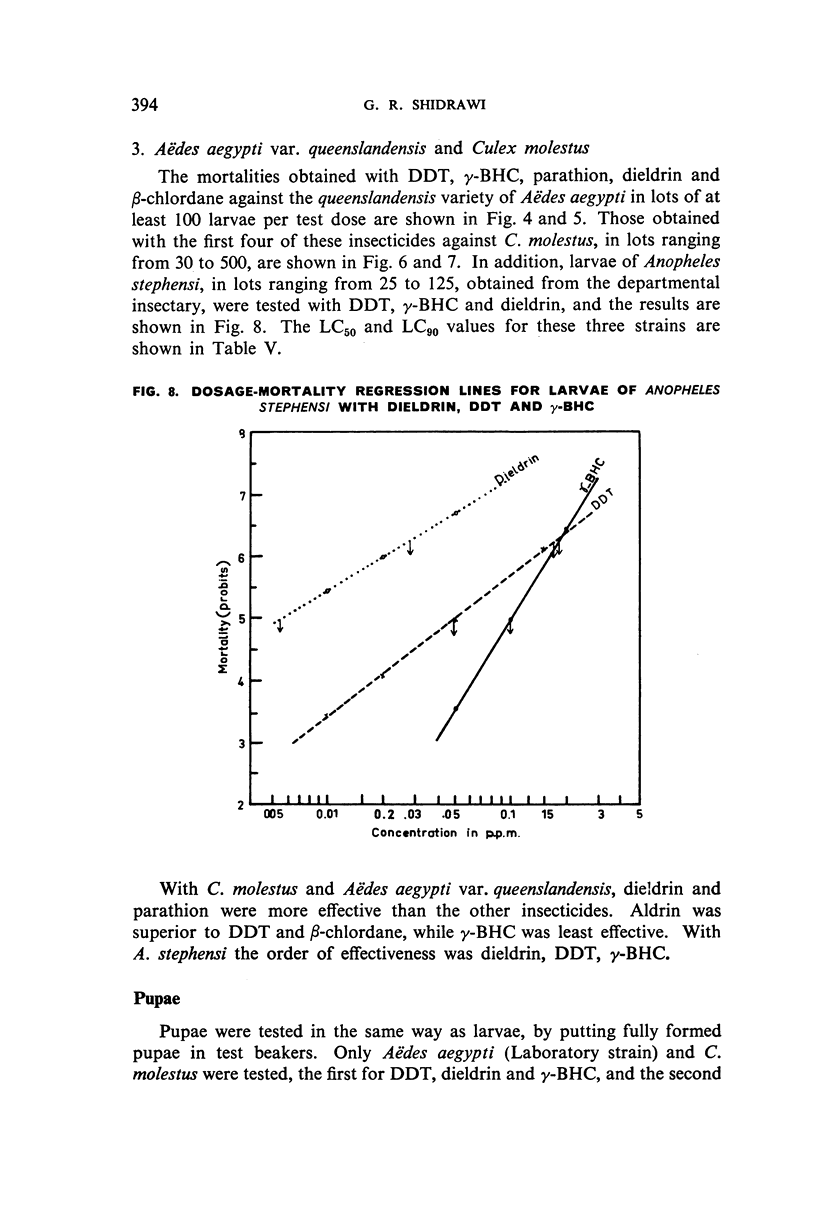
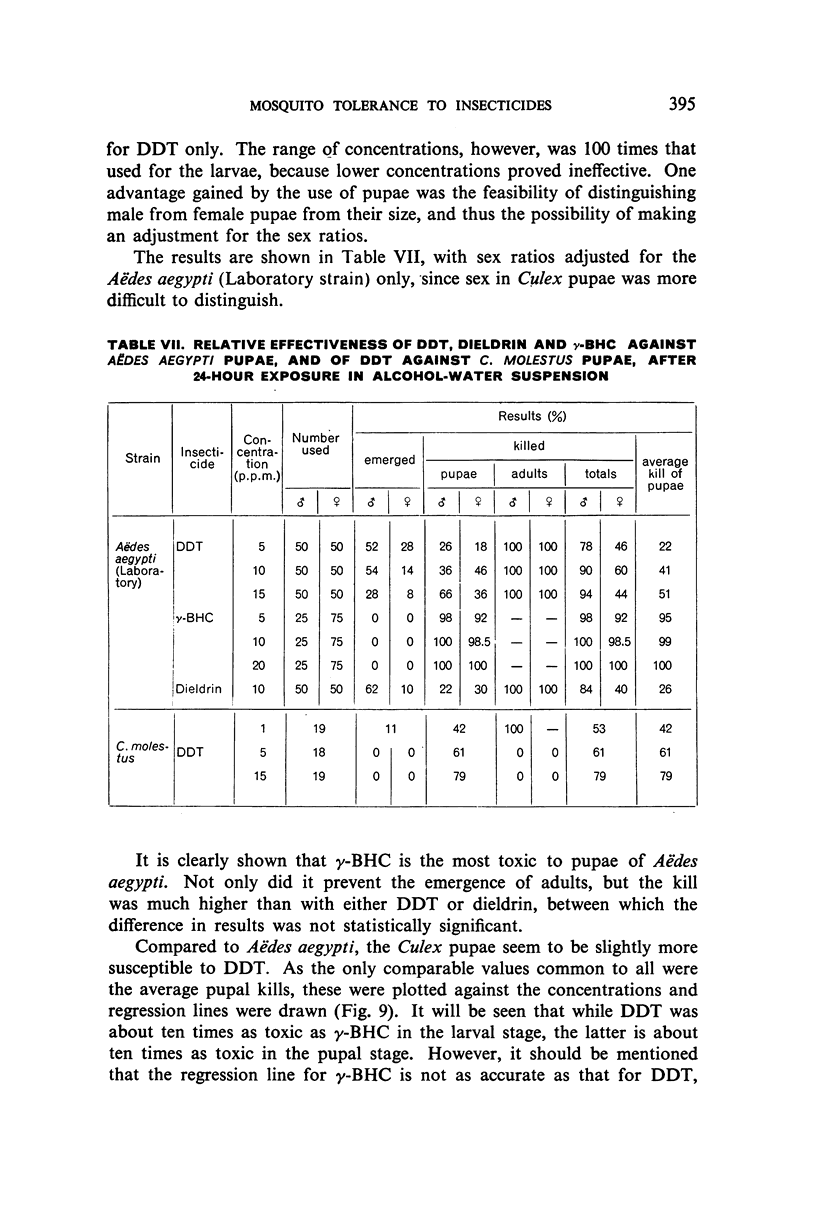






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Deonier C. C., Jones H. A. TDE, 1,1-Dichloro-2,2-bis(p-chlorophenyl) ethane, as an Anopheline Larvicide. Science. 1946 Jan 4;103(2662):13–14. doi: 10.1126/science.103.2662.13. [DOI] [PubMed] [Google Scholar]
- FAY R. W., BAKER W. C., GRAINGER M. M. Laboratory studies on the resistance of Anopheles quadrimaculatus to DDT and other insecticides. J Natl Malar Soc. 1949 Jun;8(2):137–146. [PubMed] [Google Scholar]
- GILKES C. D., GILLETTE H. P., KELLETT F. R. Yellow fever in Trinidad and the development of resistance in Aedes aegypti Linn, to D.D.T. formulations. West Indian Med J. 1956 Jun;5(2):73–89. [PubMed] [Google Scholar]
- HESS A. C. The significance of insecticide resistance in vector control programs. Am J Trop Med Hyg. 1952 May;1(3):371–388. doi: 10.4269/ajtmh.1952.1.371. [DOI] [PubMed] [Google Scholar]
- HESS A. D. Current status of insecticide resistance in insects of public health importance. Am J Trop Med Hyg. 1953 Mar;2(2):311–318. doi: 10.4269/ajtmh.1953.2.311. [DOI] [PubMed] [Google Scholar]
- METCALF R. L. Physiological basis for insect resistance to insecticides. Physiol Rev. 1955 Jan;35(1):197–232. doi: 10.1152/physrev.1955.35.1.197. [DOI] [PubMed] [Google Scholar]
- MUIRHEAD-THOMSON R. C., GORDON R. M., DAVEY T. H. A plea for the standardization of experimental work on residual insecticides. Trans R Soc Trop Med Hyg. 1952 May;46(3):271–274. doi: 10.1016/0035-9203(52)90075-8. [DOI] [PubMed] [Google Scholar]
- TRAPIDO H. Recent experiments on possible resistance to DDT by Anopheles albimanus in Panama. Bull World Health Organ. 1954;11(4-5):885–889. [PMC free article] [PubMed] [Google Scholar]


