Abstract
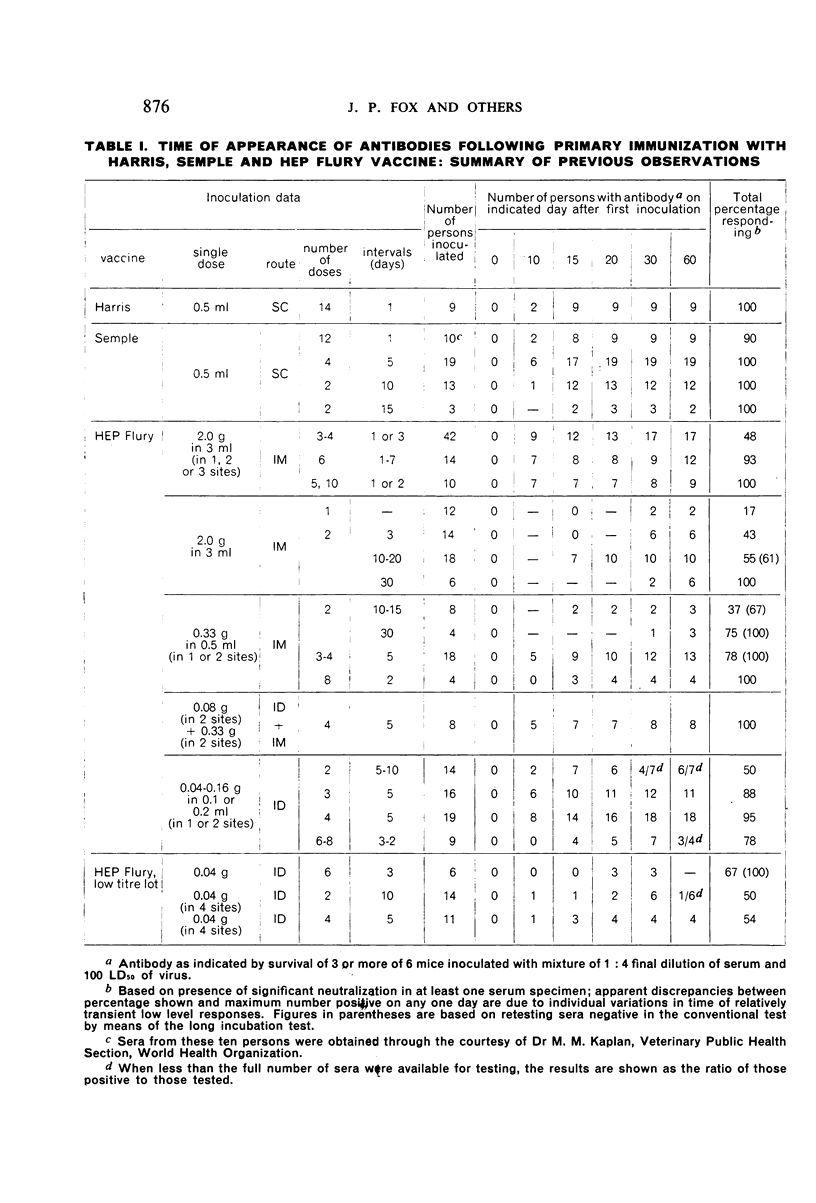
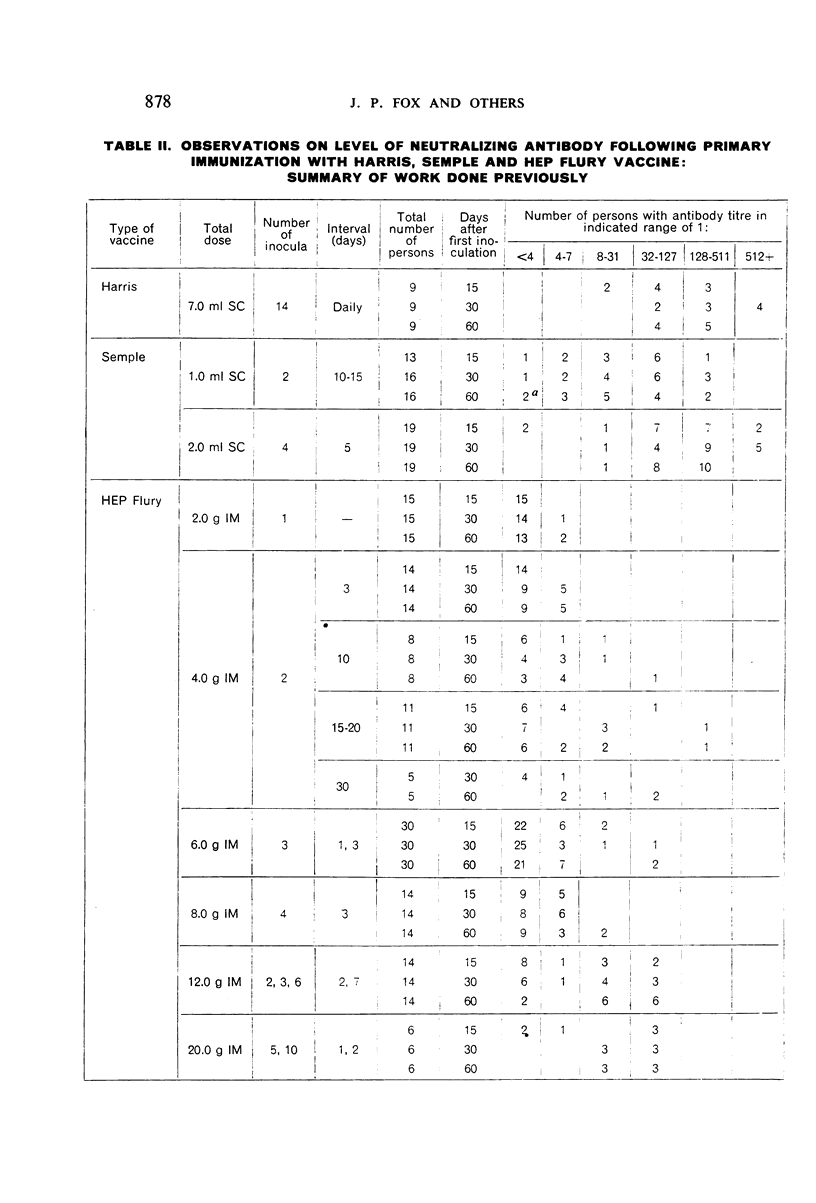
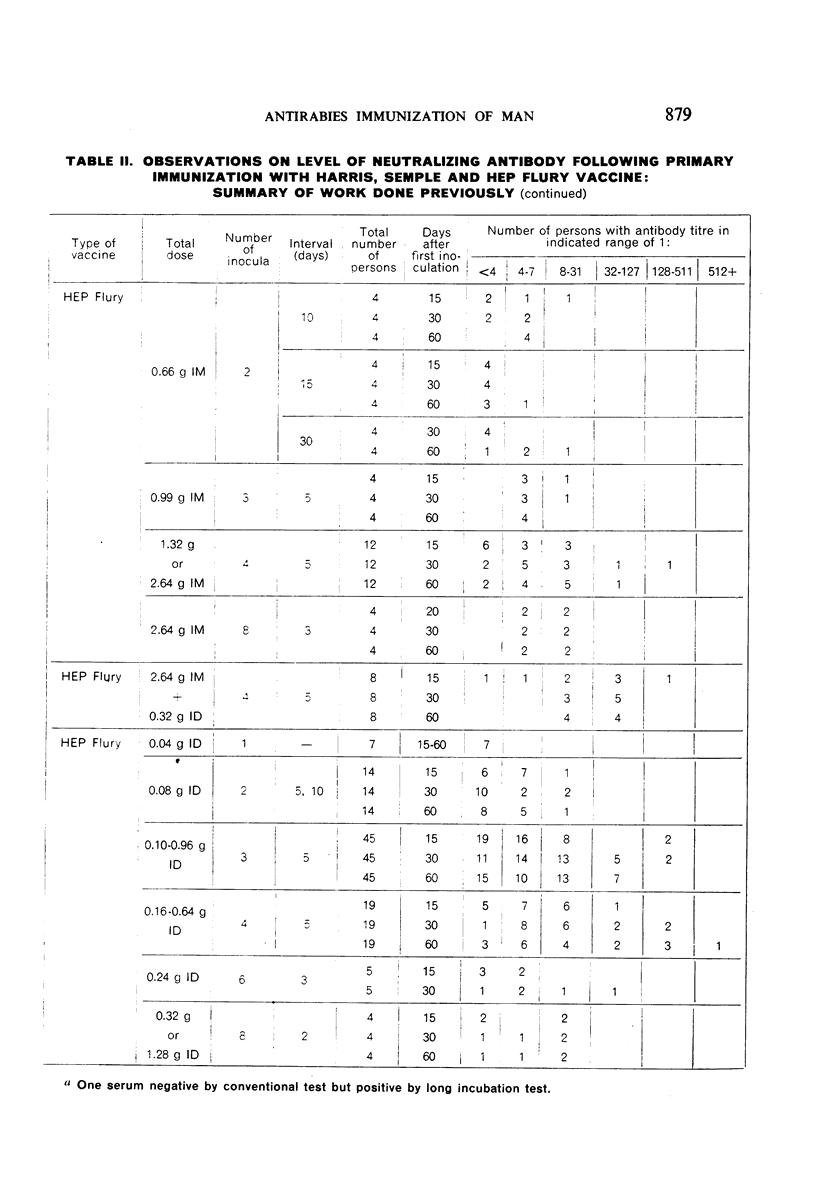
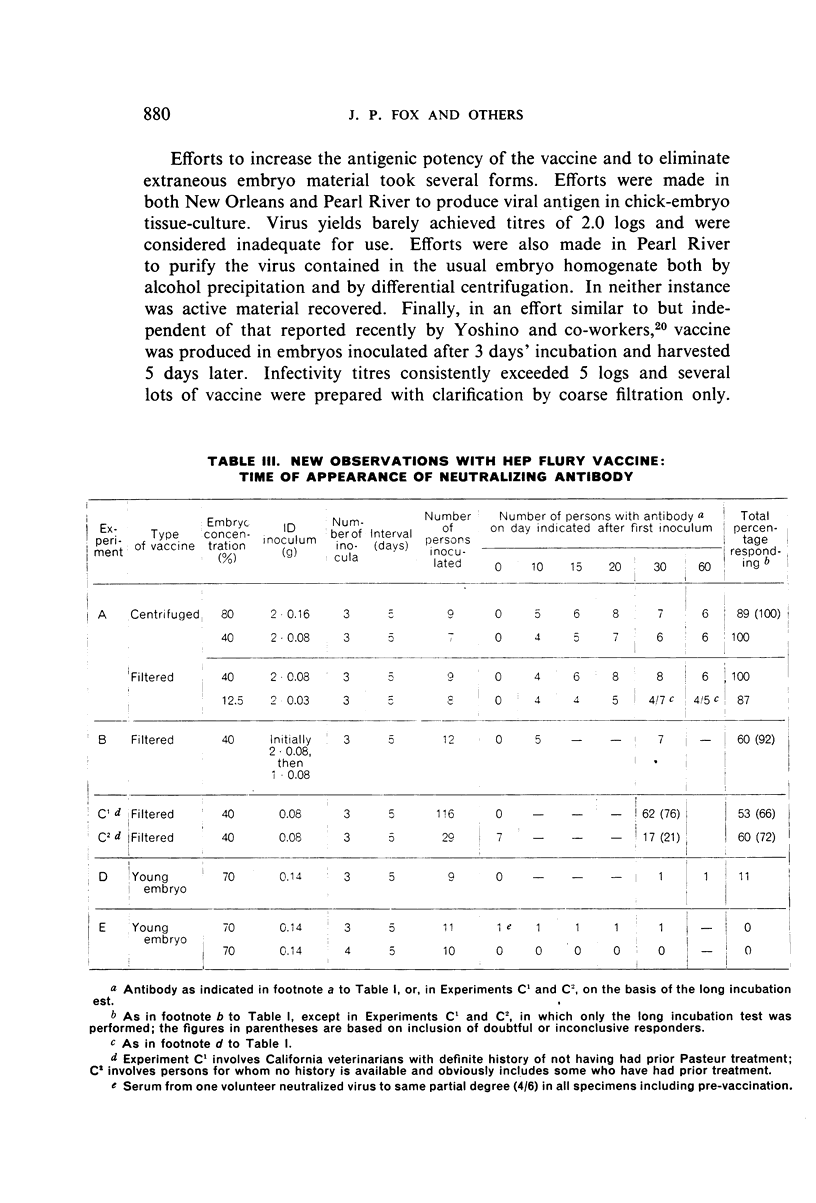
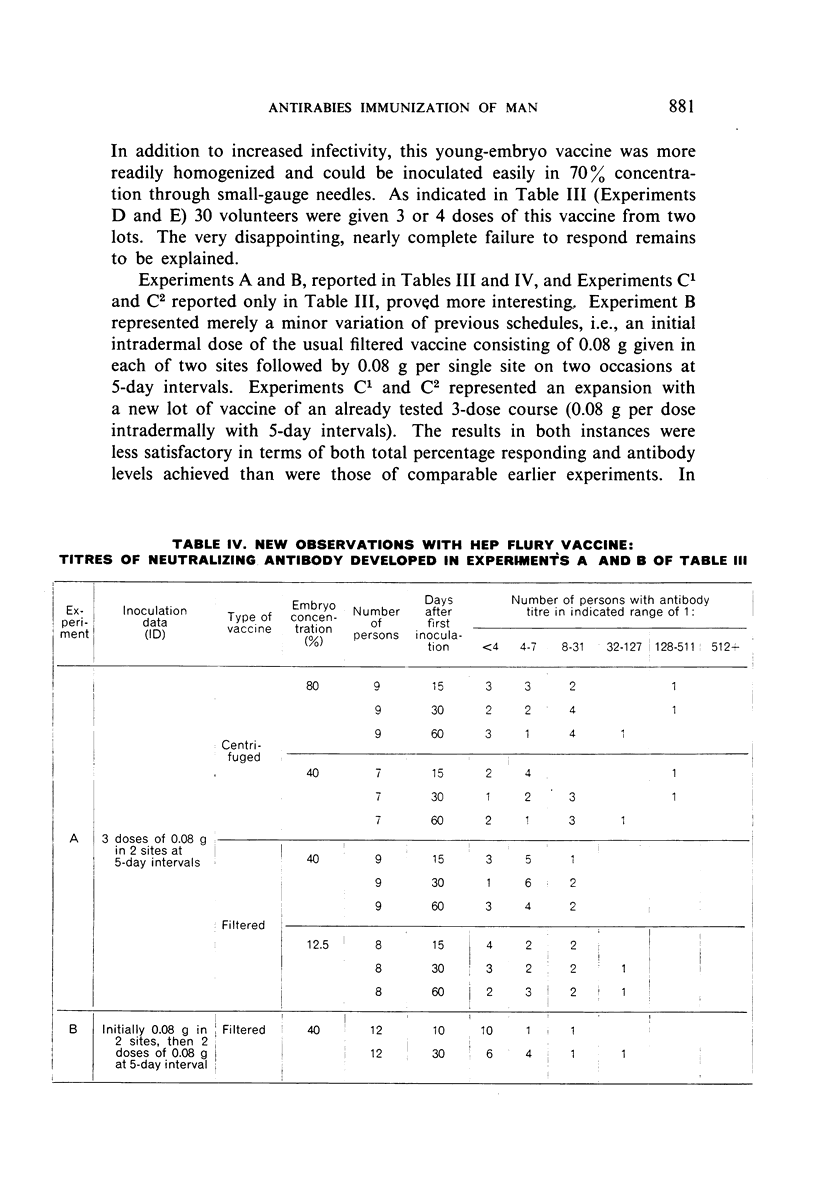
Detailed results are presented of primary immunizations of 387 persons with various courses of HEP Flury vaccine and of 54 persons with Harris- or Semple-type vaccines. Antibody response to HEP Flury vaccine was at least as rapid as that to the conventional type, but fell short in uniformity and level of response. The most promising course involved a 4-dose schedule, intradermal alone or combined with intramuscular, at 5-day intervals. A similar subcutaneous course of Semple vaccine yielded results completely equivalent to those of a 14-dose course of Harris vaccine. It is concluded that, although living, the HEP Flury virus does not multiply in man and that its lesser antigenic potency, as compared with Semple or Harris vaccines, is due to its relatively small content of viral antigen.
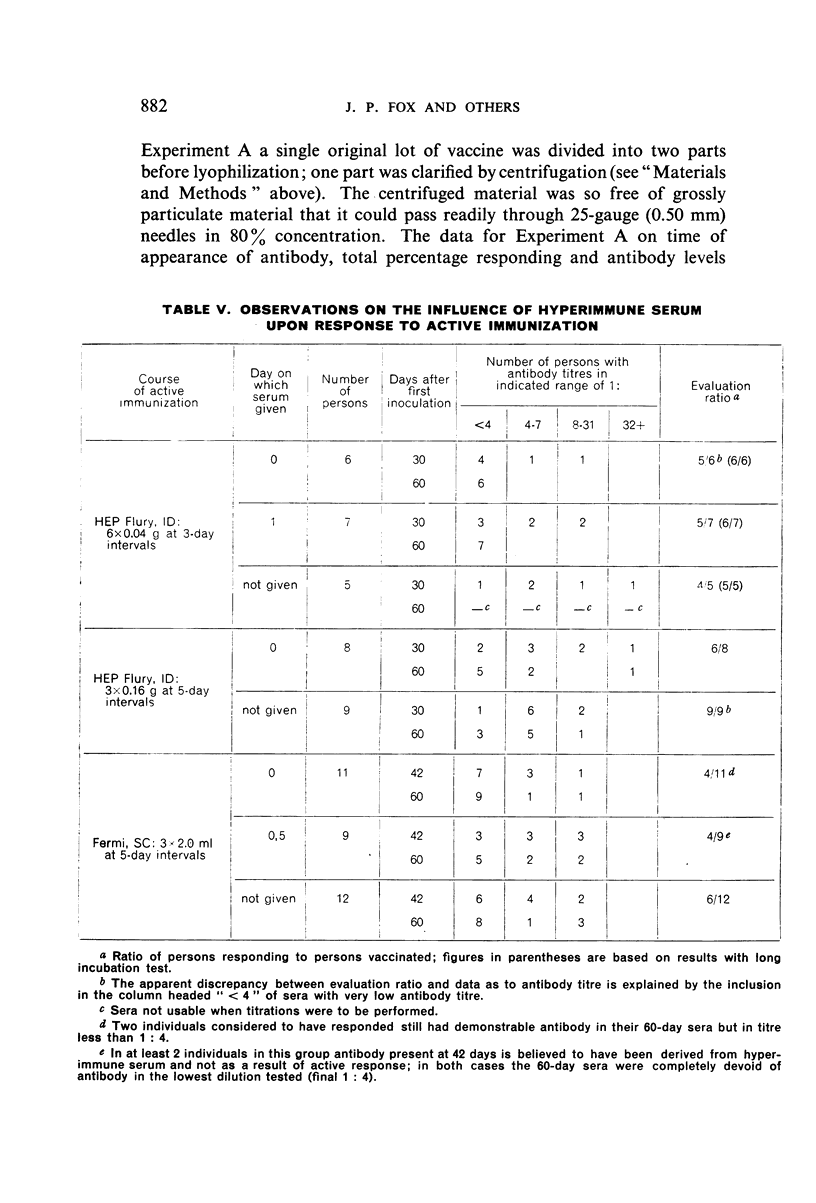
Further evidence has been obtained that hyperimmune serum may exert a slight suppressive effect on active response, but the opinion is expressed that, with vaccines of full potency, this will not be of practical significance.
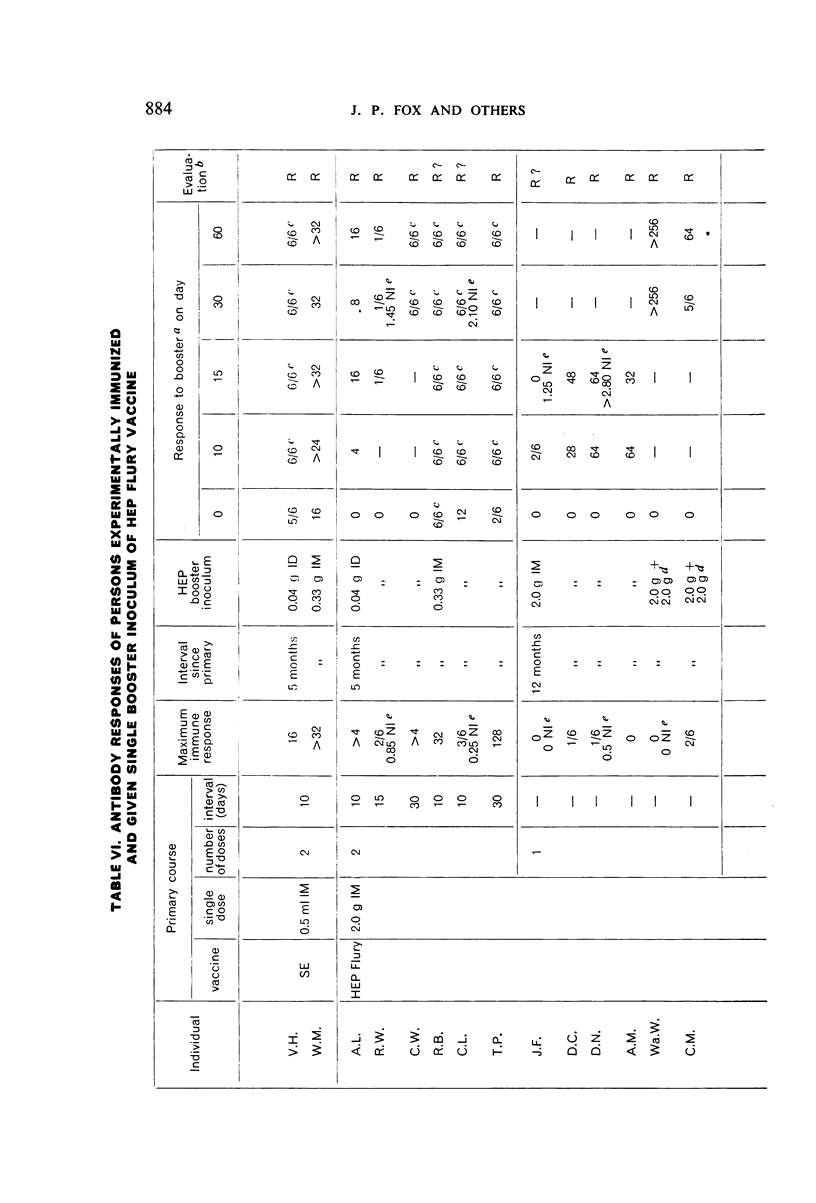
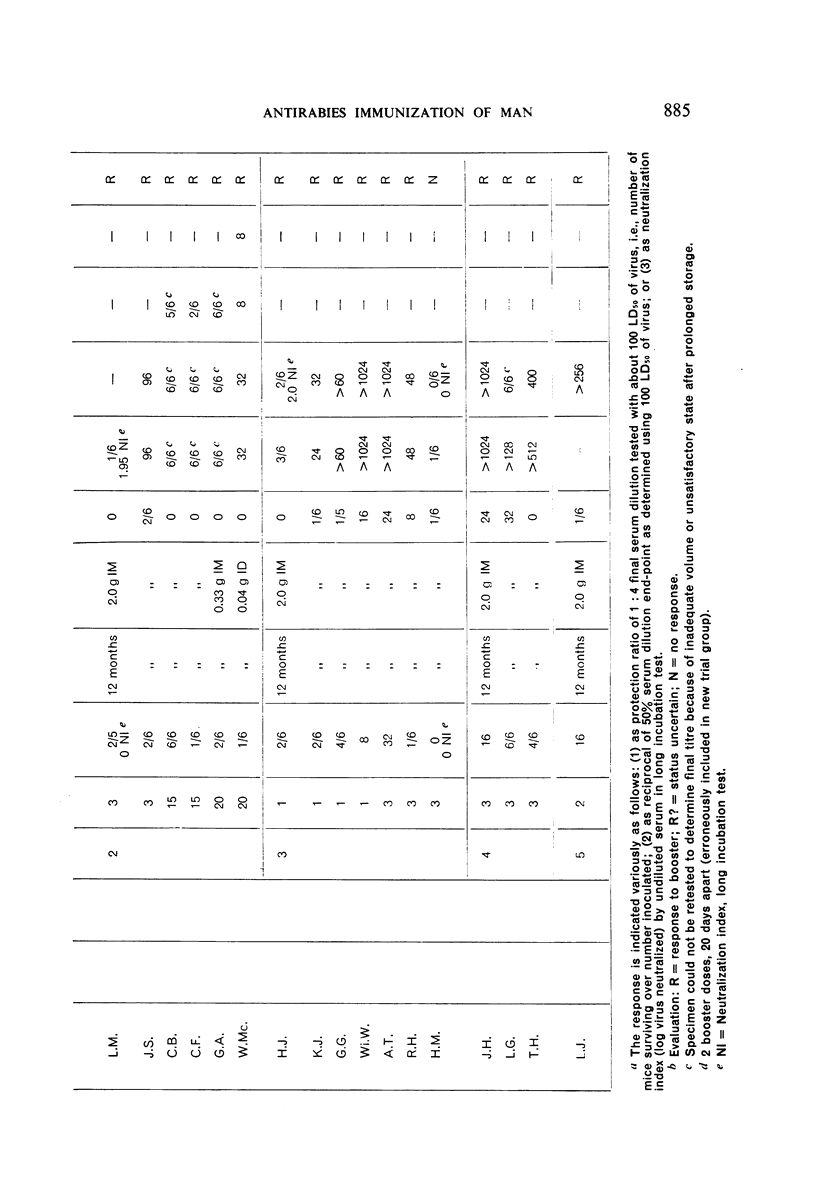
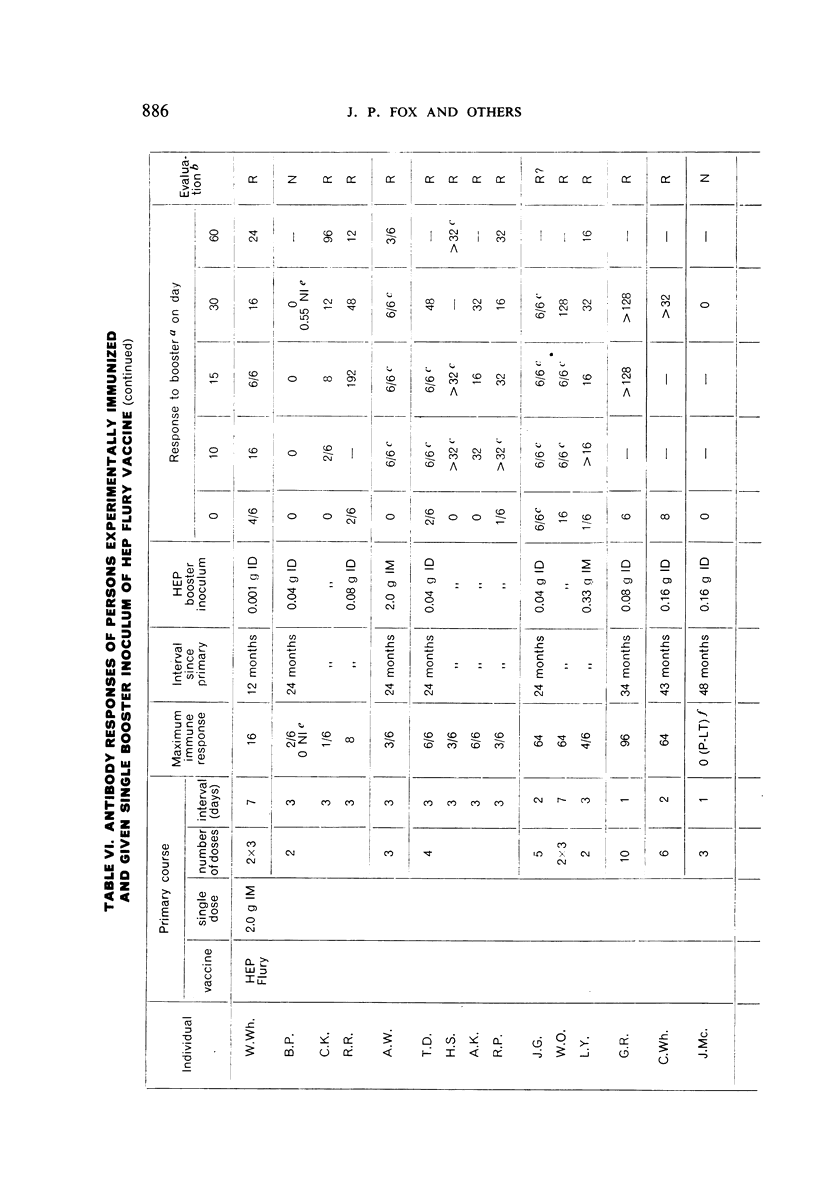
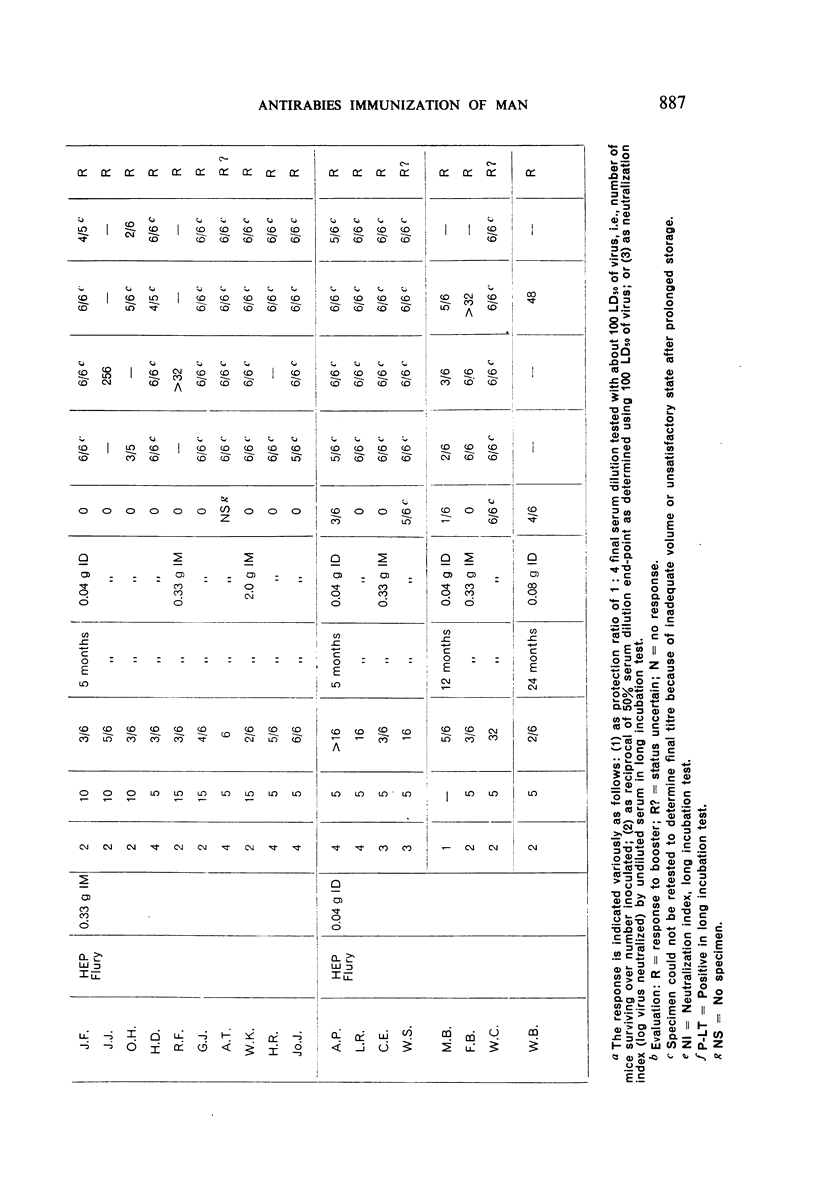
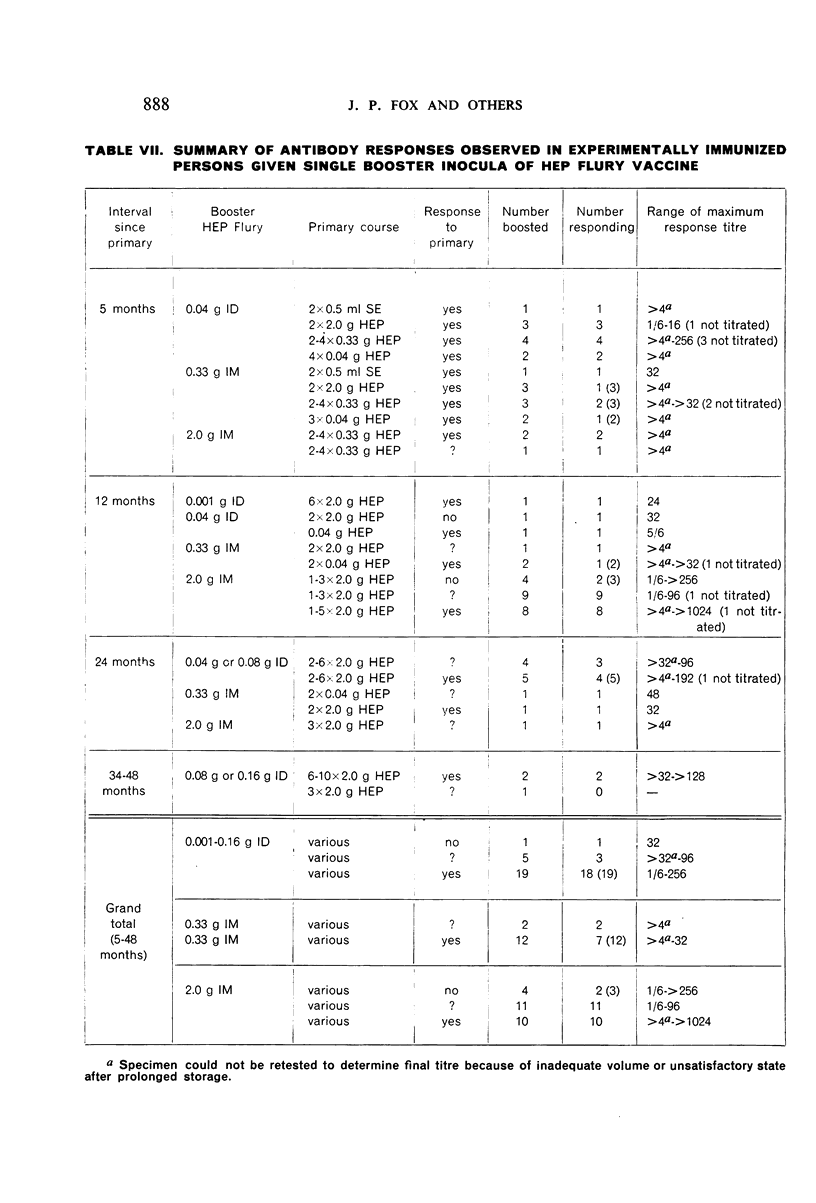
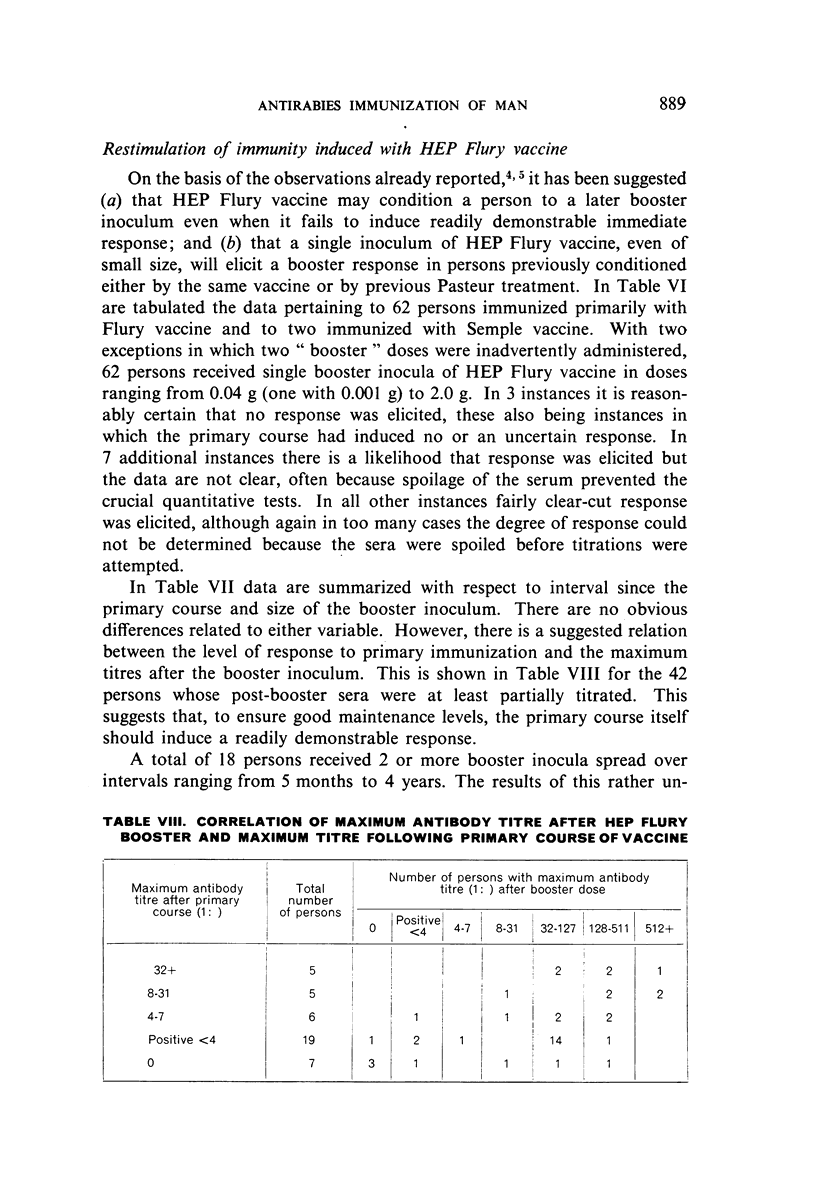
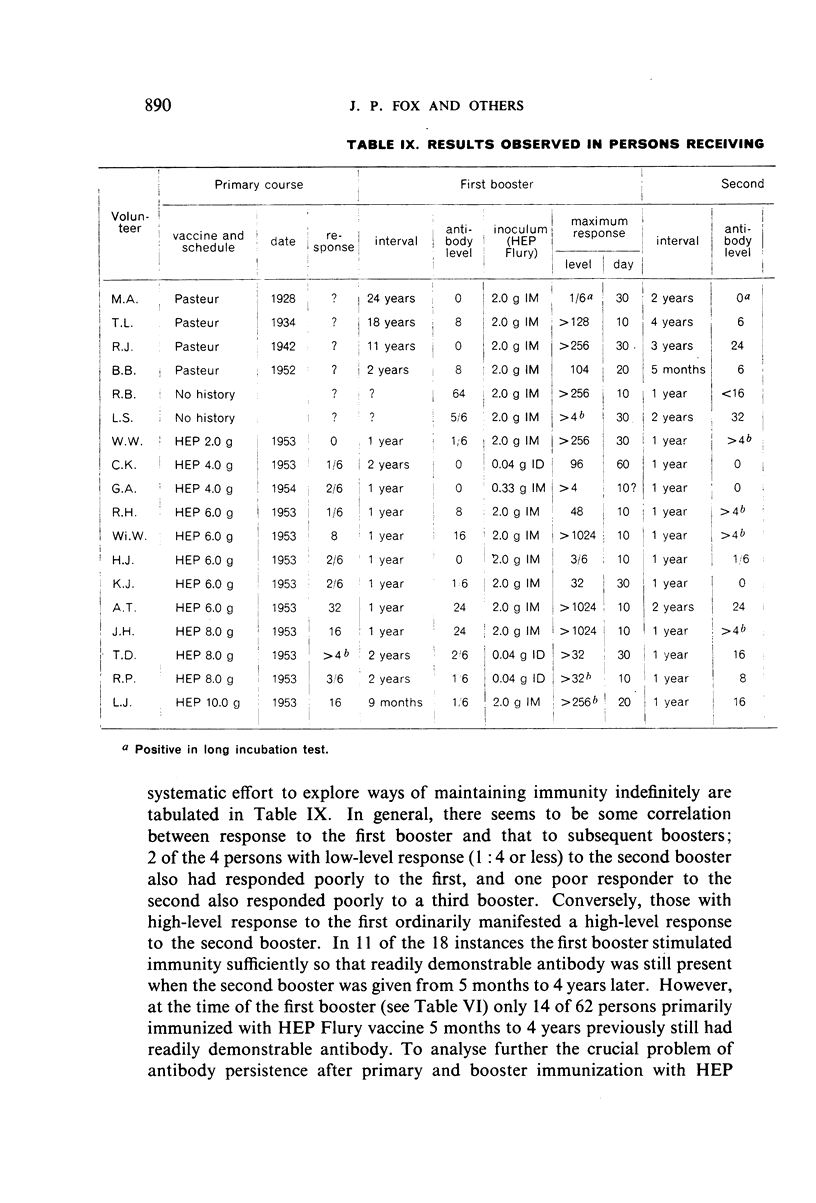
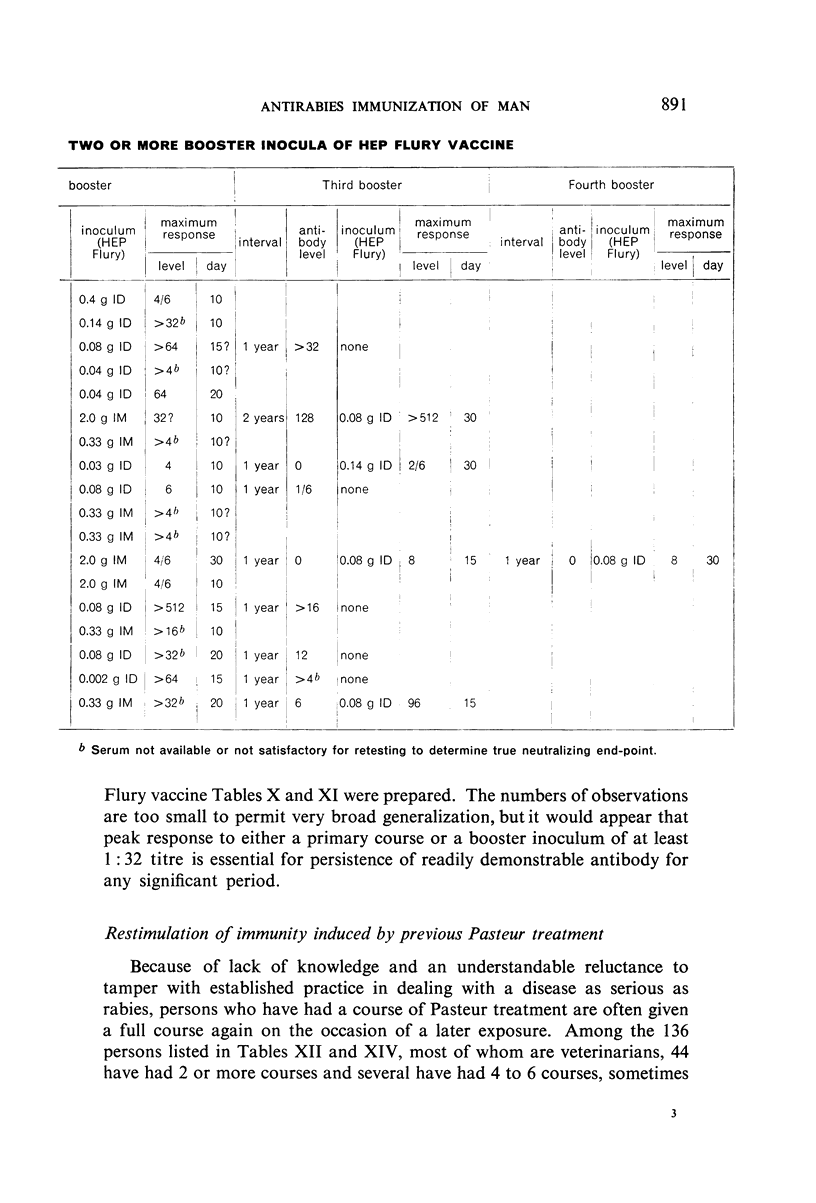
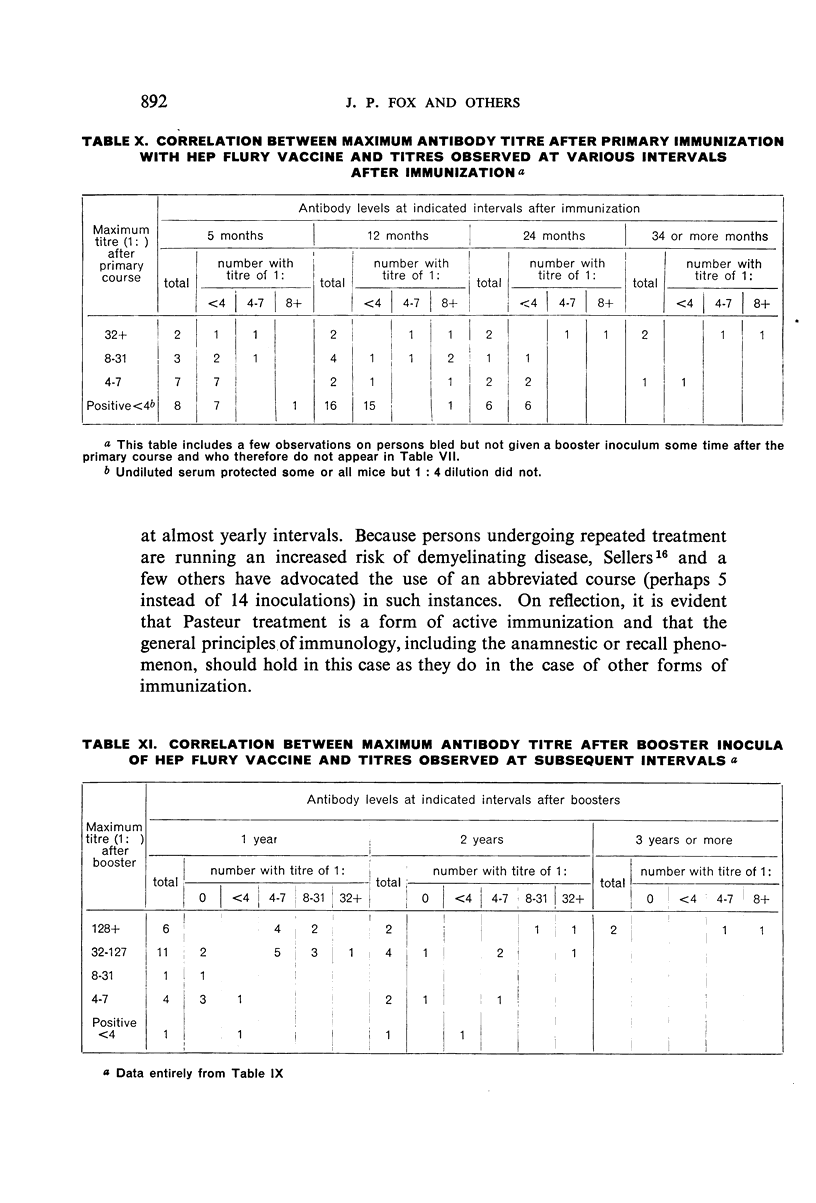
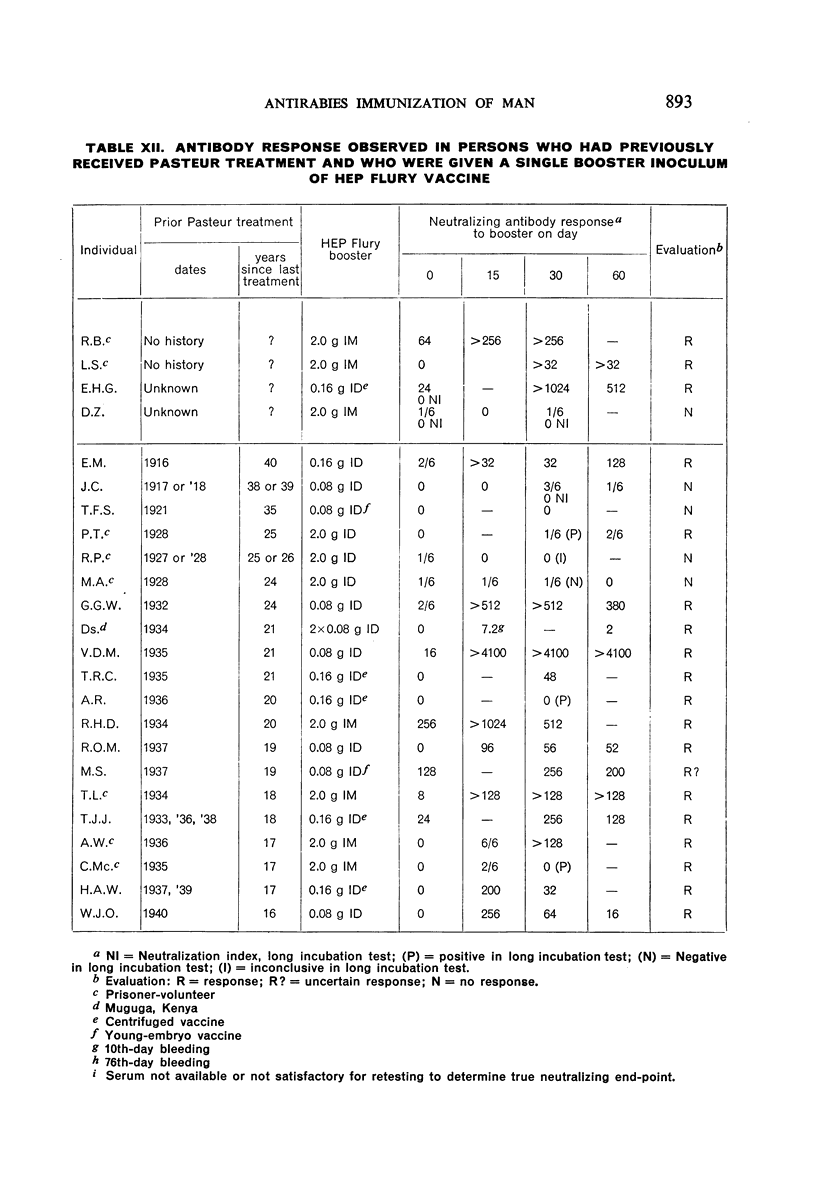
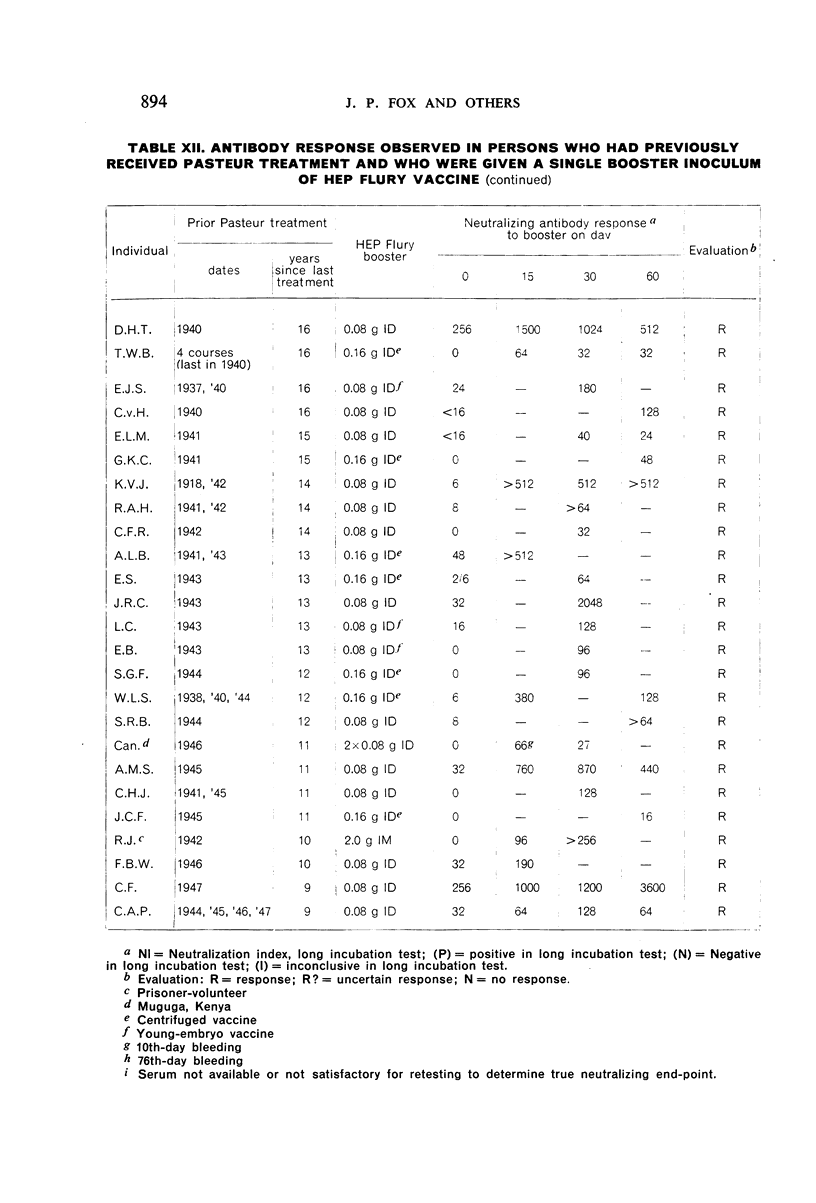
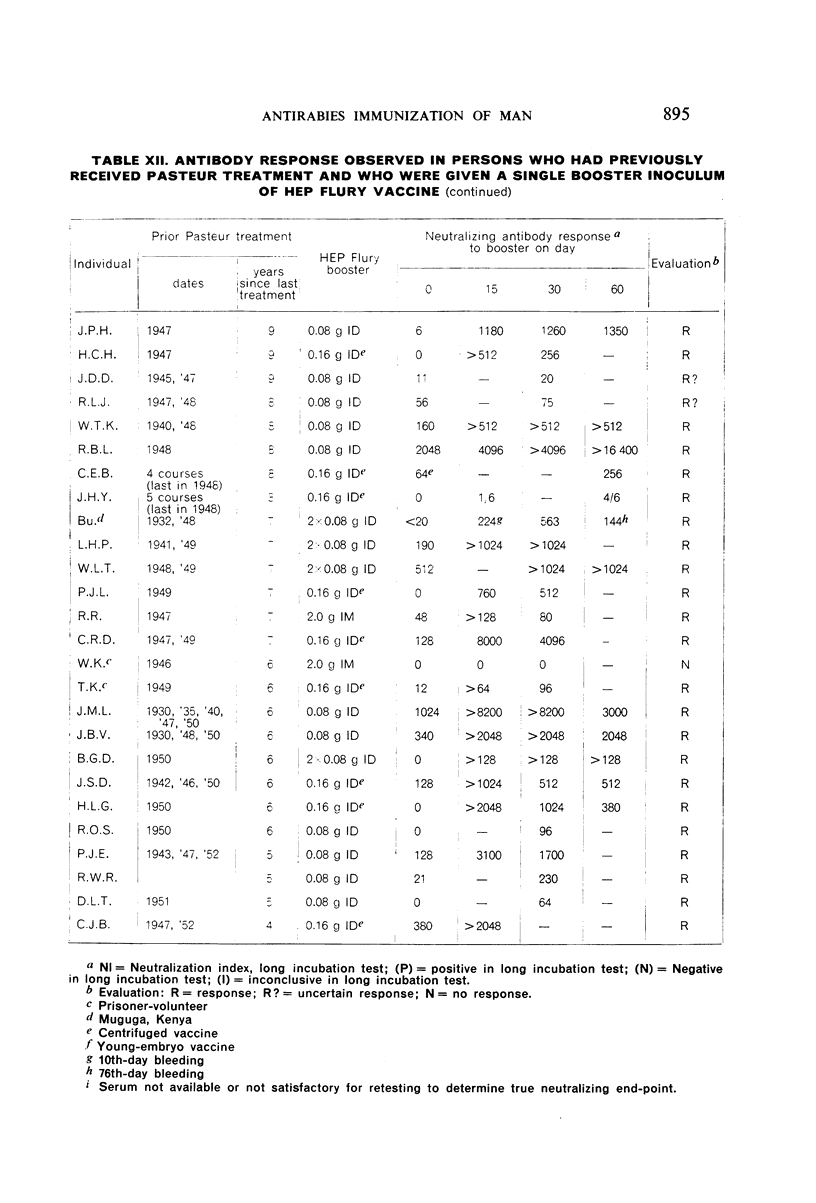
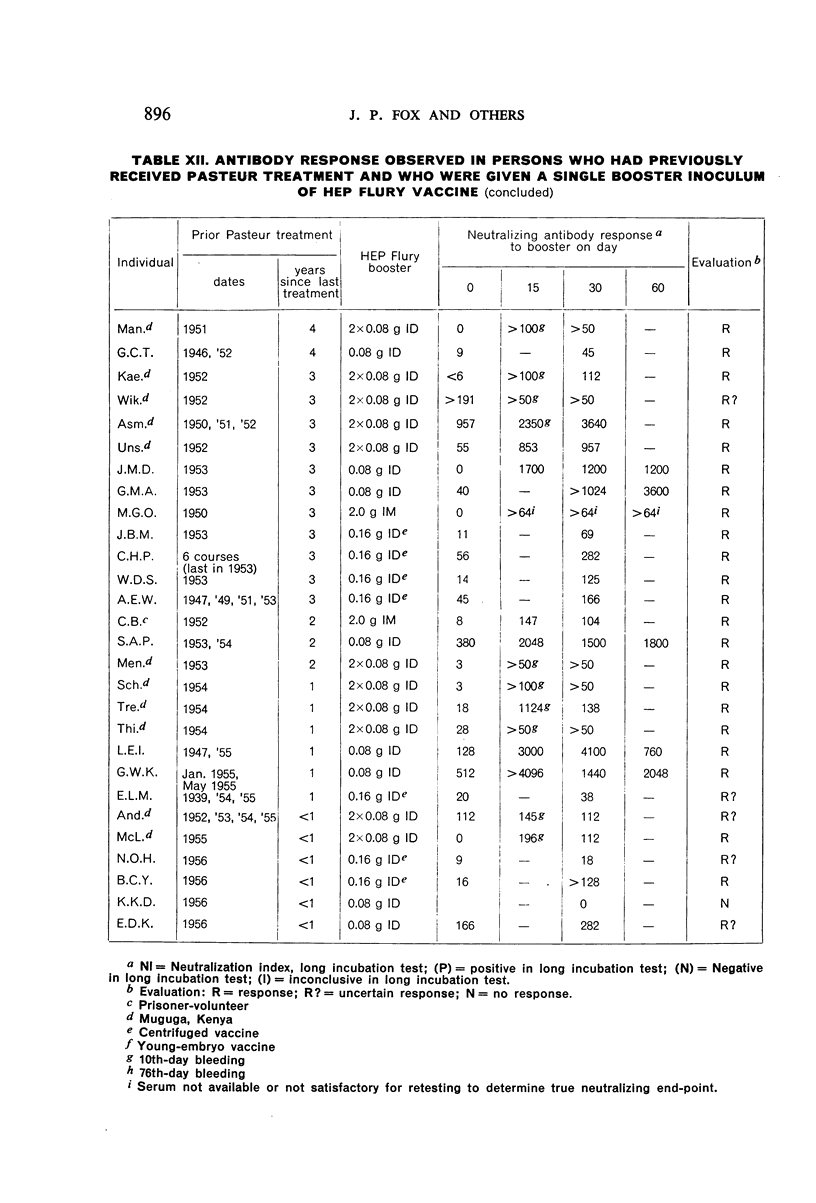
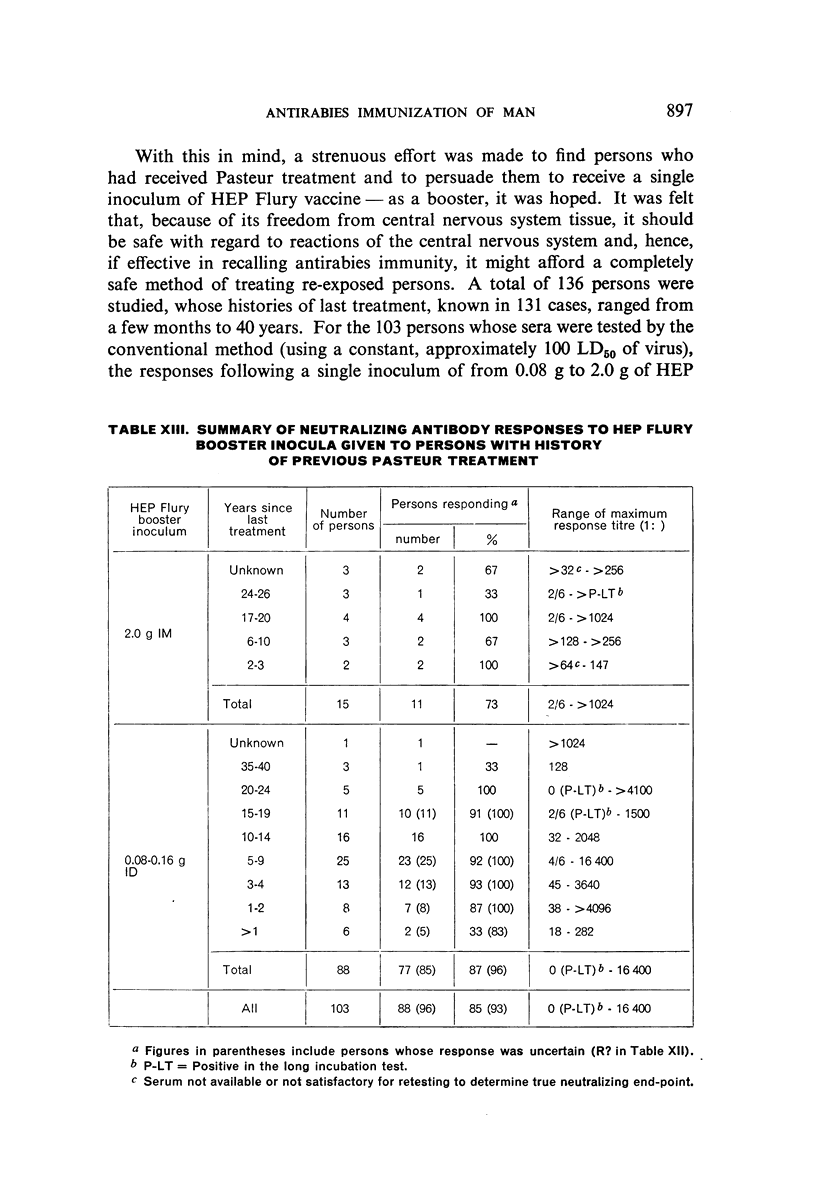
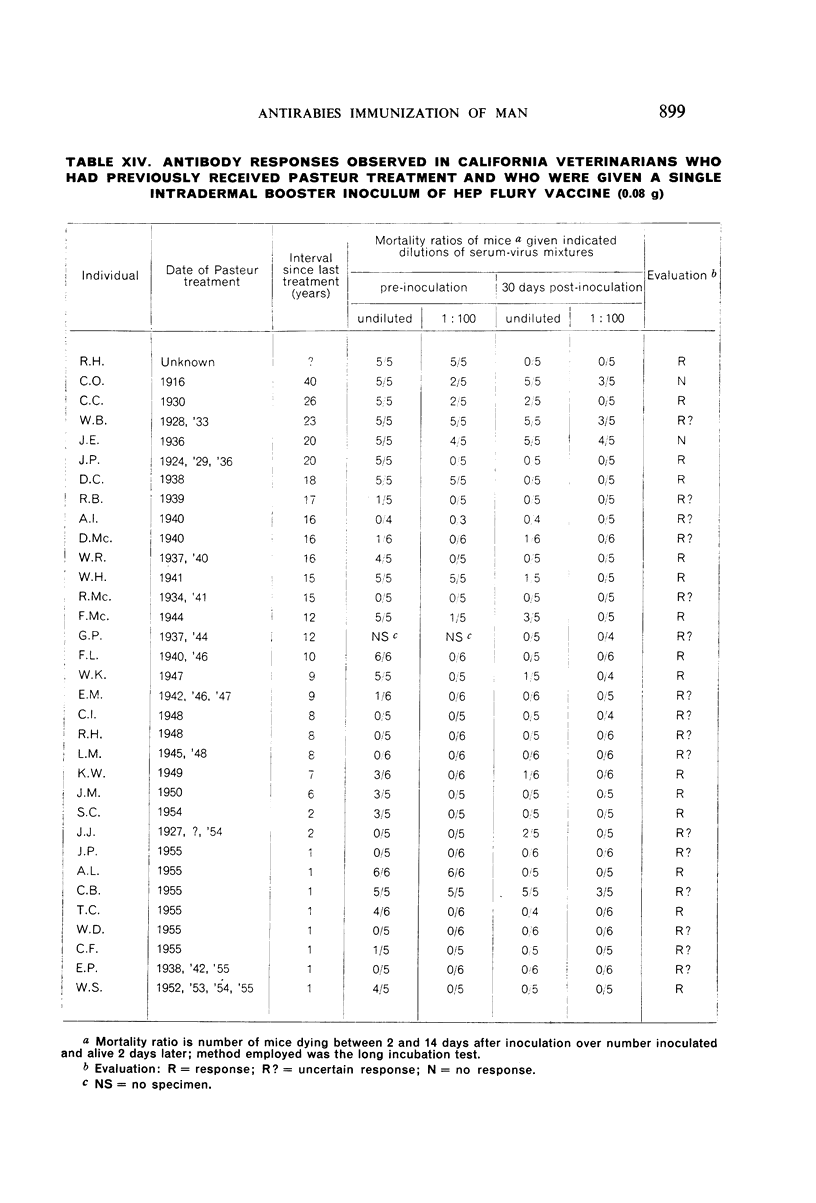
Restimulation of immunity by a booster dose of HEP Flury vaccine was studied in 64 experimentally immunized persons and in 136 persons with history of previous Pasteur treatment. In both instances small intradermal inocula were as effective as larger intramuscular inocula in recalling pre-existing immunity.
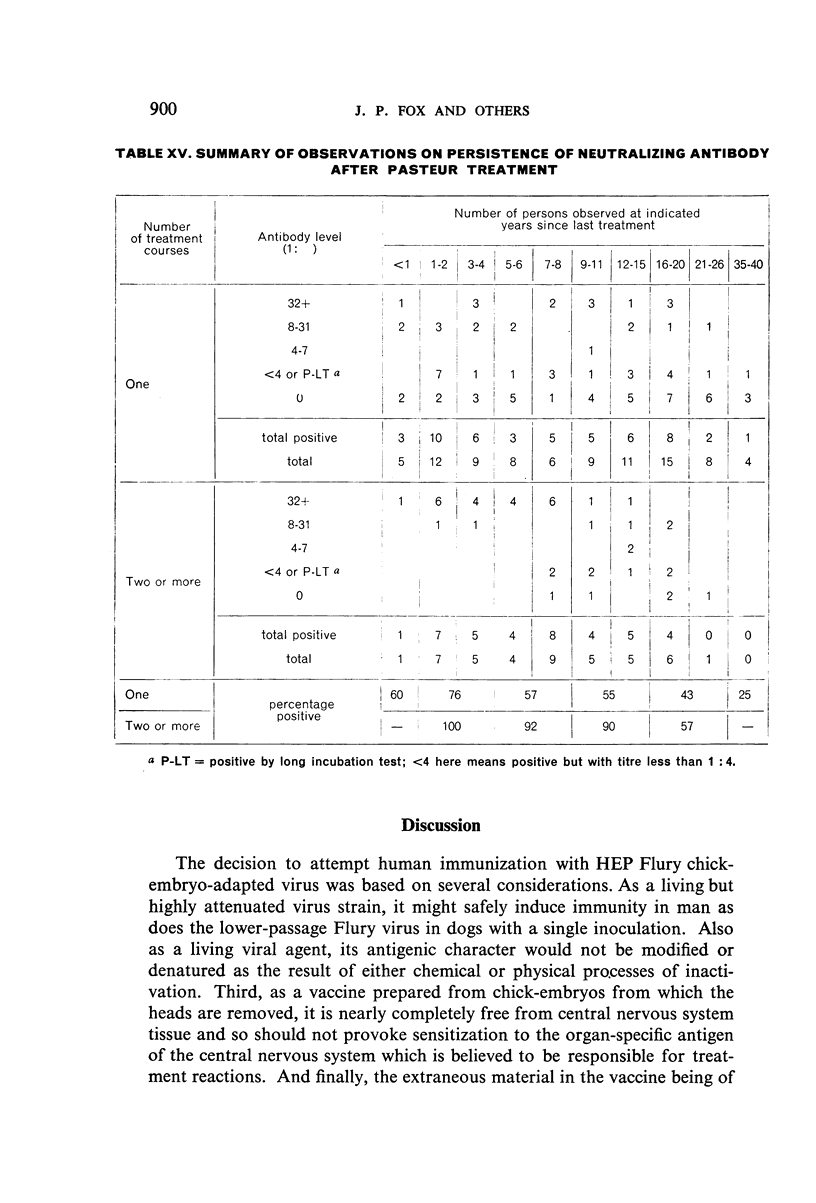
Study of recipients of Pasteur treatment indicated that antibody commonly persists for at least 5 years after a single course and for 15 or more years after re-treatment. It was also observed that the ability to respond to a booster of HEP Flury vaccine persists for at least 25 years. The response elicited by the booster is prompt and is usually at least equal to that resulting from a full primary course. The suggested conclusion is that previously treated persons need not receive more than a single booster on re-exposure, and that Pasteur treatment provides a solid basis for long-sustained immunity.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALTAZARD M., BAHMANYAR M. Essai pratique du sérum antirabique chez les mordus par loups enragés. Bull World Health Organ. 1955;13(5):747–772. [PMC free article] [PubMed] [Google Scholar]
- BODIAN D. Pathogenesis of poliomyelitis. Am J Public Health Nations Health. 1952 Nov;42(11):1388–1402. doi: 10.2105/ajph.42.11.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONWELL D. P., FOX J. P., GERHARDT P. Anti-rabies vaccination of man with HEP Flury virus. Bull Tulane Univ Med Fac. 1956 Nov;16(1):1–8. [PubMed] [Google Scholar]
- CULBERTSON C. G., PECK F. B., Jr, POWELL H. M. Duck-embryo rabies vaccine; study of fixed virus vaccine grown in embryonated duck eggs and killed with beta-propiolactone (BPL). J Am Med Assoc. 1956 Dec 8;162(15):1373–1376. [PubMed] [Google Scholar]
- HABEL K., KOPROWSKI H. Laboratory data supporting the clinical trial of anti-rabies serum in persons bitten by a rabid wolf. Bull World Health Organ. 1955;13(5):773–779. [PMC free article] [PubMed] [Google Scholar]
- KOPROWSKI H., BLACK J., NELSEN D. J. Studies on chick-embryo-adapted-rabies virus. VI. Further changes in pathogenic properties following prolonged cultivation in the developing chick embryo. J Immunol. 1954 Jan;72(1):94–106. [PubMed] [Google Scholar]
- KOPROWSKI H., BLACK J. Studies on chick embryo adapted rabies virus. III. Duration of immunity in vaccinated dogs. Proc Soc Exp Biol Med. 1952 Jul;80(3):410–415. doi: 10.3181/00379727-80-19640. [DOI] [PubMed] [Google Scholar]
- KOPROWSKI H., BLACK J. Studies on chick-embryo adapted rabies virus. II. Pathogenicity for dogs and use of egg-adapted strains for vaccination purposes. J Immunol. 1950 Mar;64(3):185–196. [PubMed] [Google Scholar]
- KOPROWSKI H., COX H. R. Studies on chick embryo adapted rabies virus; culture characteristics and pathogenicity. J Immunol. 1948 Dec;60(4):533–554. [PubMed] [Google Scholar]
- PAIT C. F., PEARSON H. E. Rabies vaccine encephalomyelitis in relation to the incidence of animal rabies in Los Angeles. Am J Public Health Nations Health. 1949 Jul;39(7):875–877. doi: 10.2105/ajph.39.7.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHINO K., KUMA N., KONDO A., KITAOKA M. Infection of the one-day old fertile hen's egg with rabies virus. I. Growth curves and serial passages. Jpn J Med Sci Biol. 1956 Dec;9(6):259–271. doi: 10.7883/yoken1952.9.259. [DOI] [PubMed] [Google Scholar]


