Abstract
Candida biofilms display increased resistance to most antifungal agents. We have evaluated the efficacy of combinations of fluconazole (FLC), amphotericin B, and caspofungin (CSP) against Candida albicans biofilms in vitro. Indifference was observed for all the combinations of paired antifungal agents when a checkerboard titration method was used. Time-kill experiments revealed an antagonistic effect of high FLC doses with CSP.
Candida albicans readily forms biofilms, consortia of cells that coexist as an organized community, attached to a solid substratum that is enveloped within an exopolysaccharide matrix (4, 12). Biofilms are a well-described phenomenon which have gained notoriety from their ability to resist antimicrobials and immune cell challenge (4, 12).
In this study, we initially assessed the effects of antifungal combinations on biofilms by a checkerboard microdilution method with biofilms formed on the wells of microtiter plates and an XTT-based colorimetric assay (10). From these experiments we calculated the SMIC80 for each drug alone and in combination, the fractionary inhibitory concentration (FIC), and the FIC indices of the paired combinations of antifungal agents. By use of the interpretation of FICs recommended by Hindler (6), indifference (FIC index of >0.5 to ≤4) was observed for all combinations of paired antifungal agents. Fluconazole (FLC) did not alter amphotericin B (AMB) activity, resulting in an FIC index of 1.00. The combination of AMB and caspofungin (CSP) showed an FIC index of 0.56, indicating indifference with a trend towards additivism. A calculated FIC index of 2.00 for the FLC-CSP combination also indicated indifference but with a trend towards antagonism which was evident at high FLC concentrations.
The interactions observed in the checkerboard microtiter plate testing combining the different antifungal agents were confirmed in time-kill curve experiments according to the methodology described before by our group (11). Log plots of decreased biofilm viability versus time for the different combinations used are presented in Fig. 1. When FLC at a concentration of 16 μg/ml was combined with 2 μg of AMB/ml, we found a nearly identical killing curve compared to that of AMB alone at 2 μg/ml. FLC at a dose of 64 μg/ml slightly inhibited AMB at 2 μg/ml. FLC at either concentration slightly decreased the effect of AMB at 0.5 μg/ml (Fig. 1A). There was no difference when AMB was used at a concentration of 2 μg/ml alone or in combination with CSP at concentrations of 0.125 or 0.5 μg/ml, and only a slight, nonsignificant improvement of AMB at 0.5 μg/ml in combination with either CSP concentration was observed (Fig. 1B). The time-kill kinetics of the combination of FLC and CSP showed a clear inhibitory effect compared to that for CSP alone with nearly identical killing curves for all tested concentration combinations (Fig. 1C).
FIG. 1.
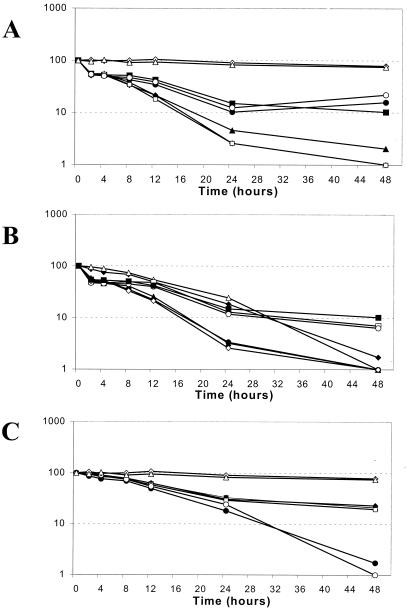
(A) Log plots of killing kinetics of FLC-AMB combinations against preformed biofilms of C. albicans 3153A. Symbols: ▵, FLC (16 μg/ml); ◊, FLC (64 μg/ml); ○, FLC (16 μg/ml)-AMB (0.5 μg/ml); •, FLC (64 μg/ml)-AMB (0.5 μg/ml); ⧫, FLC (16 μg/ml)-AMB (2 μg/ml); ▴, FLC (64 μg/ml)-AMB (2 μg/ml); ▪, AMB (0.5 μg/ml); □, AMB (2 μg/ml). (B) Log plots of killing kinetics of AMB-CSP combinations against preformed biofilms of C. albicans 3153A. Symbols: ▪, AMB (0.5 μg/ml); ◊, AMB (2 μg/ml); □, AMB (0.5 μg/ml)-CSP (0.125 μg/ml); ○, AMB (0.5 μg/ml)-CSP (0.5 μg/ml); ▴, AMB (2 μg/ml)-CSP (0.125 μg/ml); •, AMB (2 μg/ml)-CSP (0.5 μg/ml); ⧫, CSP (0.125 μg/ml); ▵, CSP (0.5 μg/ml). (C) Log plots of killing kinetics of FLC-CSP combinations against preformed biofilms of C. albicans 3153A. Symbols: ▵, FLC (16 μg/ml); ⋄, FLC (64 μg/ml); ▴, FLC (16 μg/ml)-CSP (0.125 μg/ml); ⧫, FLC (64 μg/ml)-CSP (0.125 μg/ml); □, FLC (16 μg/ml)-CSP (0.5 μg/ml); ▪, FLC (64 μg/ml)-CSP (0.5 μg/ml); •, CSP (0.125 μg/ml); ○, CSP (0.5 μg/ml). Results are mean values for each condition tested and are expressed as percentages of reduction in absorbance by the XTT assay. Standard deviations were within 5% of the mean values and are not depicted in the graphs.
Previous studies by our group and others have demonstrated lack of activity of FLC against C. albicans biofilms, increased resistance to AMB, and efficacy of CSP against C. albicans biofilms (1-5, 7, 9-12). The expanding armamentarium of antifungal drugs including agents with different molecular targets should also open new possibilities for exploring the usefulness of combination therapy. In the present study we have examined the effects and interactions of AMB, FLC, and CSP used in combination for the treatment of C. albicans biofilms in vitro. A checkerboard broth microdilution method was used to examine the effects of antifungal combinations against C. albicans biofilms. In general, these experiments pointed towards indifference for all antifungal combinations tested. Results of the effects of antifungal combinations were confirmed using time-kill methods. Because the FLC concentrations used in these experiments were high, the antagonistic effects of high FLC concentrations, particularly in combination with CSP, were evident. It was also observed that, even though combinations of AMB and CSP showed in general an indifferent effect, the use of these two agents in combination against C. albicans biofilms may still benefit from the rapid killing by high concentrations of AMB and the more sustained effect of physiological concentrations of CSP. This approach to therapy could be appealing in a clinical setting, particularly if biofilm resistance is due to the presence of a few “persister” cells able to withstand antimicrobial treatment, as suggested by other authors for bacterial biofilms (8).
Acknowledgments
This work was supported by grant ATP 3659-0080 from the Texas Higher Education Coordinating Board (Advance Technology Program, Biomedicine) (to J.L.L.-R. and B.L.W.) and Public Health Service grant 5 R01 DE11381-04 from the National Institute of Dental and Craniofacial Research (to T.F.P.). J.L.L.-R. is the recipient of a New Investigator Award in Molecular Pathogenic Mycology from the Burroughs Wellcome Fund.
We thank Merck & Co., Inc., for providing CSP for this study.
S. P. Bachmann and G. Ramage contributed equally to the work.
REFERENCES
- 1.Bachmann, S. P., K. VandeWalle, G. Ramage, T. F. Patterson, B. L. Wickes, J. R. Graybill, and J. L. López-Ribot. 2002. In vitro activity of caspofungin against Candida albicans biofilms. Antimicrob. Agents Chemother. 46:3591-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baillie, G. S., and L. J. Douglas. 1999. Candida biofilms and their susceptibility to antifungal agents. Methods Enzymol. 310:644-656. [DOI] [PubMed] [Google Scholar]
- 3.Chandra, J., P. K. Mukherjee, S. D. Leidich, F. F. Faddoul, L. L. Hoyer, L. J. Douglas, and M. A. Ghannoum. 2001. Antifungal resistance of candidal biofilms formed on denture acrylic in vitro. J. Dent. Res. 80:903-908. [DOI] [PubMed] [Google Scholar]
- 4.Douglas, L. J. 2003. Candida biofilms and their role in infection. Trends Microbiol. 11:30-36. [DOI] [PubMed] [Google Scholar]
- 5.Hawser, S. P., and L. J. Douglas. 1995. Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob. Agents Chemother. 39:2128-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hindler, J. 1995. Antimicrobial susceptibility testing, p. 5.18.11-15.18.20. In H. D. Isenberg (ed.), Clinical microbiology procedures handbook. American Society for Microbiology, Washington, D.C.
- 7.Kuhn, D. M., T. George, J. Chandra, P. K. Mukherjee, and M. A. Ghannoum. 2002. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob. Agents Chemother. 46:1773-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis, K. 2001. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 45:999-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramage, G., S. Bachmann, T. F. Patterson, B. L. Wickes, and J. L. López-Ribot. 2002. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J. Antimicrob. Chemother. 49:973-980. [DOI] [PubMed] [Google Scholar]
- 10.Ramage, G., K. Vande Walle, B. L. Wickes, and J. L. López-Ribot. 2001. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 45:2475-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramage, G., K. VandeWalle, S. P. Bachmann, B. L. Wickes, and J. L. López-Ribot. 2002. In vitro pharmacodynamic properties of three antifungal agents against preformed Candida albicans biofilms determined by time-kill studies. Antimicrob. Agents Chemother. 46:3634-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramage, G., B. L. Wickes, and J. L. López-Ribot. 2001. Biofilms of Candida albicans and their associated resistance to antifungal agents. Am. Clin. Lab. 20:42-44. [PubMed] [Google Scholar]


