Abstract
Antimicrobial therapy of soft tissue infections in patients with sepsis sometimes lacks efficiency, despite the documented susceptibility of the causative pathogen to the administered antibiotic. In this context, impaired equilibration between the antibiotic concentrations in plasma and those in tissues in critically ill patients has been discussed. To characterize the impact of tissue penetration of anti-infective agents on antimicrobial killing, we used microdialysis to measure the concentration-versus-time profiles of levofloxacin in the interstitial space fluid of skeletal muscle in patients with sepsis. Subsequently, we applied an established dynamic in vivo pharmacokinetic-in vitro pharmacodynamic approach to simulate bacterial killing at the site of infection. The population mean areas under the concentration-time curves (AUCs) for levofloxacin showed that levofloxacin excellently penetrates soft tissues, as indicated by the ratio of the AUC from time zero to 8 h (AUC0-8) for muscle tissue (AUC0-8 muscle) to the AUC0-8 for free drug in plasma (AUC0-8 plasma free) (AUC0-8 muscle/AUC0-8 plasma free ratio) of 0.85. The individual values of tissue penetration and maximum concentration (Cmax) in muscle tissue were highly variable. No difference in bacterial killing of a select Staphylococcus aureus strain for which the MIC was 0.5 μg/ml was found between individuals after exposure to dynamically changing concentrations of levofloxacin in plasma and tissue in vitro. In contrast, the decrease in the bacterial counts of Pseudomonas aeruginosa (MIC = 2 μg/ml) varied extensively when the bacteria were exposed to levofloxacin at the concentrations determined from the individual concentration-versus-time profiles obtained in skeletal muscle. The extent of bacterial killing could be predicted by calculating individual Cmax/MIC and AUC0-8 muscle/AUC0-8 plasma free ratios (R = 0.96 and 0.93, respectively). We have therefore shown in the present study that individual differences in the tissue penetration of levofloxacin may markedly affect target site killing of bacteria for which MICs are close to 2 μg/ml.
To date, the anti-infective community relates the concentrations of antimicrobial agents in plasma to the MICs for relevant pathogens and thereby makes recommendations on antibiotic dosages and dosing intervals. Usually, these calculations are based on pharmacokinetic (PK) data for population means derived from data of healthy volunteers. However, it has been shown that equilibration between antimicrobial agent concentrations in tissue and those in plasma may differ significantly between healthy volunteers and critically ill patients (6, 22). In addition, high interindividual differences in tissue penetration properties are to be expected among patients with sepsis, since the hemodynamics and the treatment strategies used to overcome sepsis vary substantially for these patients (21, 34). Attempts to improve the tissue drug distribution and strategies to identify patients for whom the risk of not reaching sufficient levels in tissue is high are considered important steps forward in the successful antimicrobial therapy of critically ill patients.
For antimicrobial agents to become microbiologically and clinically effective, the prerequisite of reaching appropriate concentrations at the target site must be fulfilled. In several in vitro and animal studies, inappropriate concentrations at the target site have been associated with the development of bacterial resistance (3, 11, 18). Thus, high interindividual differences in PKs in tissue might explain the observation that antimicrobial therapy lacks efficiency in some patients, despite the documented in vitro susceptibility of the causative pathogen, while the same pathogen is eradicated from patients in whom tissue drug penetration is more favorable.
To test this hypothesis, we investigated the impact of interindividual differences in tissue penetration on antimicrobial killing. We used the microdialysis technique to measure the concentration-versus-time profiles of levofloxacin in skeletal muscle tissue (20, 23-25). On the basis of these data we used an in vivo PK-in vitro pharmacodynamic (PD) model to simulate the killing of select Pseudomonas aeruginosa and Staphylococcus aureus strains by levofloxacin at the target site (7, 12, 14). Levofloxacin, a broad-spectrum fluoroquinolone, was chosen in the present study because of its broad spectrum of activity against gram-positive and gram-negative bacteria. In addition, levofloxacin is considered useful for the empirical treatment of severe soft tissue infections (5, 9, 15).
MATERIALS AND METHODS
The protocol was approved by the local ethics committee and was performed in accordance with the Declaration of Helsinki (1964) and current revisions of the Good Clinical Practice Guidelines of the European Commission and the Austrian drug law (AMG). Written informed consent was obtained from all patients who were conscious at the time of the start of the study. The study met the criteria set forth by the local ethics committee for patients who were unable to give written consent because they were sedated or comatose.
Patients.
Sepsis was diagnosed as outlined by the American College of Chest Physicians-Society of Critical Care Medicine Consensus Conference Committee (4). Seven patients were enrolled in the study. The indication for levofloxacin therapy was made by an independent physician. None of the patients had serum creatinine levels higher than 3 mg/dl, none had received levofloxacin within 12 h prior to the start of the study, and none had undergone hemodialysis before the study or underwent hemodialysis during the study period. The patients' demographics, Acute Physiology and Chronic Health Evaluation (APACHE) II scores, and laboratory data are presented in Table 1.
TABLE 1.
Demographic data, hemodynamic and laboratory parameters, and APACHE II scores for the study populationa
| Parameter | Mean ± SEM | Median | Minimum | Maximum |
|---|---|---|---|---|
| Age (yr) | 59.6 ± 4.8 | 59 | 35 | 73 |
| Ht (cm) | 172.0 ± 4.0 | 178 | 154 | 180 |
| Wt (kg) | 82.0 ± 7.6 | 75 | 63 | 120 |
| BMI (kg/m2) | 27.6 ± 2.1 | 25.2 | 23.1 | 37.0 |
| CRP concn (mg/dl) | 17.6 ± 2.5 | 18.9 | 8.9 | 28.3 |
| Fibrinogen concn (mg/dl) | 612.1 ± 56.8 | 627 | 430 | 842 |
| Creatinine concn (mg/dl) | 1.2 ± 0.1 | 1.2 | 0.9 | 1.6 |
| Protein concn (g/l) | 52.1 ± 3.5 | 50.4 | 42.0 | 65.7 |
| Albumin concn (g/l) | 23.6 ± 1.1 | 22.7 | 20.0 | 28.7 |
| Blood temp (°C) | 37.3 ± 0.5 | 37.0 | 35.8 | 39.0 |
| Hematocrit (%) | 32.1 ± 1.2 | 32 | 28 | 37 |
| Hemoglobin concn (g/liter) | 10.6 ± 0.4 | 10.6 | 9.5 | 12.1 |
| Leukocyte count (G/liter) | 11.1 ± 1.2 | 11.4 | 7.2 | 14.8 |
| Arterial pH | 7.50 ± 0.01 | 7.43 | 7.34 | 7.60 |
| Arterial lactate (mM) | 1.5 ± 0.3 | 1.4 | 0.7 | 2.8 |
| SaO2 (%) | 95.3 ± 1.8 | 97 | 86 | 100 |
| HR (no. of beats min−1) | 86.7 ± 8.1 | 98 | 42 | 101 |
| MAP (mm Hg) | 78.6 ± 3.1 | 74 | 70 | 90 |
| APACHE II score | 10.4 ± 0.8 | 10 | 7 | 13 |
Seven patients were studied. Abbreviations: BMI, body mass index; CRP, C-reactive protein; SaO2, saturation of oxygen; HR, heart rate; MAP, mean arterial pressure.
Study protocol.
Arterial blood samples were obtained from a plastic cannula inserted to monitor blood levofloxacin concentrations. Microdialysis experiments were performed as described previously (21, 22, 24, 25). In brief, a commercially available microdialysis probe (CMA10; Microdialysis AB, Stockholm, Sweden) with a molecular mass cutoff of 20 kDa, an outer diameter of 0.5 mm, and a membrane length of 16 mm was inserted into the skeletal muscle tissue of the thigh by the following procedure. The surface of the skin was punctured with a 20-gauge intravenous plastic cannula. The steel trochar was removed, the appropriate site of the probe was checked by aspiration, and the dialysis probe was inserted into the plastic cannula. The microdialysis system was connected and perfused with Ringer's solution (Ringer Lösung “Leopold” Infusionslösung; Leopold Pharma Gesellschaft mbH, Graz, Austria) at a flow rate of 1.5 μl/min. This was performed with a precision pump (Precidor [Infors-AG, Basel, Switzerland] or CMA100 [Microdialysis AB]). After a 30-min baseline sampling period, 500 mg of levofloxacin (Tavanic; Aventis, Frankfurt am Main, Germany) was administered as a single intravenous dose over a 30-min period by use of an automatic infusion apparatus. Sampling of microdialysates and plasma was continued at 20-min intervals for 120 min after drug administration and thereafter at 60-min intervals for 480 min after drug administration. All samples were stored at approximately −80°C until analysis.
Sample collection for determination of interstitial space levofloxacin concentrations.
Microdialysis was performed in vivo for measurement of levofloxacin concentrations in the free interstitial space (21, 22, 24, 25). This method is based on sampling of analytes from the interstitial space with a semipermeable membrane at the tip of a microdialysis probe. The probe is constantly perfused with a physiological solution at a flow rate of 1.5 μl/min. Once the microdialysis probe is implanted into the tissue, substances present in the interstitial space fluid at a certain concentration (Ctissue) diffuse out of the extravascular fluid into the probe, resulting in a certain concentration in the perfusion medium (Cdialysate). For most analytes, equilibrium between the interstitial space fluid and the perfusion medium is incomplete; therefore, Ctissue is greater than Cdialysate. The correlating factor between these concentrations is termed “relative recovery.”
For calibration of the microdialysis probes, in vivo recovery was assessed in each experiment by the retrodialysis method. This method is based on the fact that the process of diffusion through the semipermeable membrane is equal in both directions. Therefore, levofloxacin was added to the perfusion medium at a concentration of 2 μg/ml and the disappearance rate through the membrane was subsequently calculated. In vivo recovery was assessed for each experiment. The percent recovery in vivo was calculated as 100 − [100 · (dialysate concentration out/dialysate concentration in)].
Measurement of levofloxacin concentrations.
The total levofloxacin levels in plasma and the free concentrations in the microdialysates were measured by reversed-phase high-performance liquid chromatography with fluorescence detection (27). The assays were sensitive for levofloxacin with limits of quantification of 0.02 μg/ml in plasma and 0.016 μg/ml in microdialysates. Quality control samples were used at concentrations ranging from 1 to 10 μg/ml for plasma and 0.2 to 2 μg/ml for microdialysates. The accuracies of the measurements were 90.2 to 95.1% for plasma and 88.9 to 93.7% for microdialysates. Precisions ranged from 1.70 to 2.83% for plasma and 2.43 to 2.98% for microdialysates.
For the microdialysis experiments, the absolute concentrations in the interstitial space were calculated by the following equation: 100 · (concentration in the dialysate/percent individual recovery).
PK calculations.
The level of plasma protein binding of levofloxacin has been reported to range from 20 to 40% (1, 28, 33). For the present study, free levofloxacin concentrations were calculated from the total concentrations in plasma by using a given plasma protein binding level of 35%. PK analysis was performed with a commercially available computer program (Kinetica, version 3.0; Innaphase Sarl, Paris, France). The areas under the concentration-time curves (AUCs) for plasma and the interstitium were calculated from nonfitted data by using the trapezoidal rule. The following PK parameters were determined: the maximum concentration (Cmax), the time to Cmax, and the AUC from time zero to 8 h (AUC0-8). The ratios of the AUC0-8 for muscle tissue (AUC0-8 muscle) to the AUC0-8 for total plasma (AUC0-8 total plasma) (AUC0-8 muscle/AUC0-8 total plasma ratio) and the ratio of AUC0-8 muscle to the AUC0-8 for free drug in plasma (AUC0-8 plasma free) (AUC0-8 muscle/AUC0-8 plasma free ratio) were calculated as a measure of drug penetration into peripheral compartments. All these PK data are summarized in Table 2.
TABLE 2.
Mean pharmacokinetic parameters calculated for the study populationa
| Compartment | AUC0-8 (mg · h/ml) | AUC0-8 muscle/ AUC0-8 plasma total | AUC0-8 muscle/ AUC0-8 plasma free | Cmax (μg/ml) | Tmax (h) | t1/2β (h) | CL (ml/min) | Vss (liters) |
|---|---|---|---|---|---|---|---|---|
| Plasma total | 38.3 ± 10.3 | 11.2 ± 2.2 | 0.52 ± 0.18 | 12.57 ± 5.04 | 95.2 ± 59.6 | 81.0 ± 25.3 | ||
| Plasma free | 24.9 ± 6.7 | 7.3 ± 1.5 | 0.52 ± 0.18 | 12.57 ± 5.04 | 146.5 ± 91.7 | 124.6 ± 39.0 | ||
| Muscle | 22.1 ± 13.1b | 0.55 ± 0.26 | 0.85 ± 0.39 | 3.6 ± 2.0 | 5.57 ± 1.13 | 42.11 ± 75.08 |
The data are presented as means ± standard deviations. Abbreviations: Tmax, time to reach Cmax; t1/2β, terminal elimination half-life; CL, apparent total body clearance; Vss, apparent volume of distribution at steady state.
P < 0.05 compared with the results for total concentrations in plasma.
Statistical calculations.
Mann-Whitney U tests and Spearman rank order correlations tests were performed for statistical analysis and comparison of PK parameters by using a commercially available computer program (Statistica; StatSoft, Inc., Tulsa, Okla.). All data are presented as means ± standard deviations. A P value <0.05 was considered significant.
In vitro PDs.
On the basis of the PK data obtained from the in vivo experiments, we simulated the concentration-versus-time profile of levofloxacin in plasma and in the interstitial space fluid in vitro in order to describe the antibacterial activity of levofloxacin at the target site (7, 12, 14). Therefore, 50-ml Falcon tubes with a starting volume of 10 ml of Mueller-Hinton broth (MHB; Mikrobiologie Mueller-Hinton Bouillon; Merck, Darmstadt, Germany) were kept in a water bath at 37°C and were inoculated with select clinical isolates at an approximate concentration of 5 × 105 CFU/ml. Subsequently, the levofloxacin concentration-versus-time profiles obtained in vivo from the concentrations in plasma and interstitial fluid were simulated in vitro by changing the levofloxacin concentrations in broth. This was done by adding MHB at 20-min intervals, depending on the individual PK data. The volumes were calculated by the equation V2 = (C1/C2) × V1, where C1 and V1 are the present levofloxacin concentration and the present MHB volume, respectively; C2 is the desired levofloxacin concentration; and V2 is the calculated MHB volume to be added to simulate the individual levofloxacin clearance. Increasing antibiotic concentrations were simulated by adding levofloxacin; decreasing concentrations were simulated by adding MHB at the appropriate volume over 8 h. The bacteria were counted at defined time points. After the culture tubes were vortexed, two 50-μl samples were serially diluted with 0.9% sodium chloride. After each dilution step, 20 μl was plated onto Columbia agar plates (Columbia plus 5% sheep blood; BioMerieux, Marcy l'Etoile, France). These plates were incubated for 24 h at 37°C. Afterwards, the colonies were counted and the counts were extrapolated backwards to the original volume to determine the number of CFU per milliliter. Each simulation was performed in triplicate. The growth of untreated bacteria was used as a control. The results shown in Fig. 3a and b are corrected for dilution by backward extrapolation of the number of CFU to the original volume.
FIG. 3.
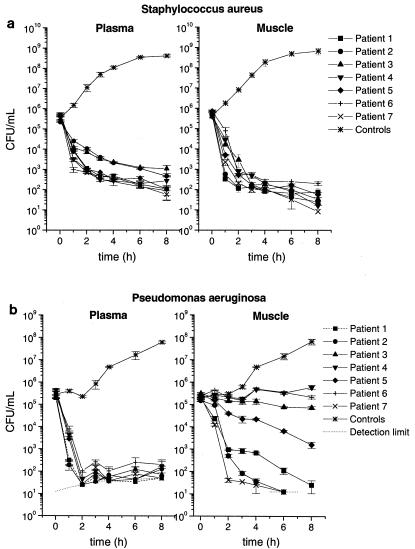
Killing-versus-time curves for clinical isolates of S. aureus (MIC = 0.5 μg/ml) (a) and P. aeruginosa (MIC = 2 μg/ml) (b) after exposure to levofloxacin at concentrations determined from individual free concentration versus-time profiles obtained in plasma and skeletal muscle tissue. Data for bacterial growth controls are also shown. All data are presented as means ± standard errors of the means (n = 3). The detection limit is represented by the dotted lines.
In vitro susceptibility tests.
The MICs for the bacterial strains used in the present study were determined by a twofold serial microdilution method with MHB, according to the criteria of the NCCLS (26). Therefore, the S. aureus and P. aeruginosa strains were precultured overnight on Columbia agar plates and then introduced at an initial inoculum of approximately 5 × 105 CFU/ml into MHB containing levofloxacin. The lowest concentration of antibiotic that inhibited visible bacterial growth after 20 h of incubation at 37°C was defined as the MIC. Conditions were controlled by using strains S. aureus ATCC 29213 and P. aeruginosa ATCC 27853.
Organisms.
All S. aureus and P. aeruginosa strains used were clinical isolates obtained at the General Hospital of Vienna. For in vivo PK-in vitro PD simulations, one S. aureus strain and one P. aeruginosa strain for which the MICs corresponded to the MIC at which 90% of isolates are inhibited (MIC90) for S. aureus (MIC90 = 0.5 μg/ml) and P. aeruginosa (MIC90 = 2 μg/ml) found in the literature were chosen (17, 31, 32). All bacteria were stored frozen in liquid nitrogen at −196°C until they were used.
RESULTS
In vivo PKs.
Figure 1 shows the mean concentration-versus-time profiles of levofloxacin in the plasma (total and free concentrations) and skeletal muscle tissue of patients with sepsis (n = 7). The mean values of the PK parameters for levofloxacin in plasma and skeletal muscle tissue are presented in Table 2. The PK profiles of free levofloxacin in plasma and skeletal muscle for individual patients are depicted in Fig. 2.
FIG. 1.
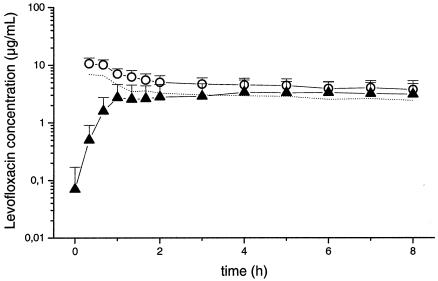
Mean concentration-versus-time profiles of levofloxacin for total (open circles) and free (dotted line) concentrations in plasma and skeletal muscle tissue (triangles) following administration of 500 mg to patients with sepsis (n = 7). Data are presented as means ± standard deviations.
FIG. 2.
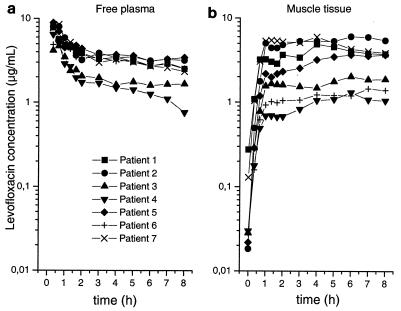
Individual pharmacokinetic concentration-versus-time profiles of free levofloxacin in plasma (a) and skeletal muscle tissue (b).
The mean AUC0-8s and Cmaxs of levofloxacin in the interstitial space of skeletal muscle tissue were significantly lower than the mean AUC0-8s and Cmaxs of total levofloxacin in plasma (P = 0.018). For the free fraction of levofloxacin, complete equilibration between the concentrations in plasma and those in tissue was observed, as indicated by an AUC0-8 muscle/AUC0-8 plasma free ratio of 0.9. The PK profiles for individual plasma samples showed relatively low levels of variability (median AUC0-8 plasma free, 26.7 mg · h/ml; range, 14.7 to 31.7 mg · h/ml). High interindividual variability was observed for the peak levofloxacin concentrations in tissue and the AUCs for levofloxacin (median AUC0-8 h muscle, 22.1 mg · h/ml; range, 7.2 to 38.7 mg · h/ml). The levels of tissue penetration, as indicated by the AUC0-8 muscle/AUC0-8 plasma free ratio, were also highly variable.
In vitro PK-PD simulations:
Figure 3a and b presents killing-versus-time curves for selected strains of S. aureus and P. aeruginosa, respectively, following exposure to levofloxacin at the concentrations determined from the individual concentration-time profiles of free levofloxacin measured in plasma and skeletal muscle tissue.
The killing kinetics of S. aureus did not differ between individuals, irrespective of whether plasma or muscle tissue PKs were simulated. Likewise, the killing kinetics of P. aeruginosa were identical for all individuals when the strain was exposed to levofloxacin at the concentrations determined from the concentration-time profiles obtained in plasma. However, when P. aeruginosa was exposed to levofloxacin at concentrations determined from the profiles measured in skeletal muscle tissue, we observed significant interindividual differences in bacterial growth inhibition.
Correlations of PK and PD parameters:
Figure 4a to c correlates the decrease in P. aeruginosa counts with the individual Cmax in muscle tissue (Cmax muscle)/MIC, AUC0-8 h muscle/MIC, and AUC0-8 muscle/AUC0-8 plasma free ratios after exposure to levofloxacin at concentrations determined from the concentration-versus-time profiles obtained with skeletal muscle tissue. The extent of bacterial eradication may be predicted by calculating individual Cmax muscle/MIC, AUC0-8 muscle/MIC, and AUC0-8 muscle/AUC0-8 plasma free ratios, as indicated by the high coefficients of correlation (R = 0.96, 0.96, and 0.93, respectively).
FIG. 4.
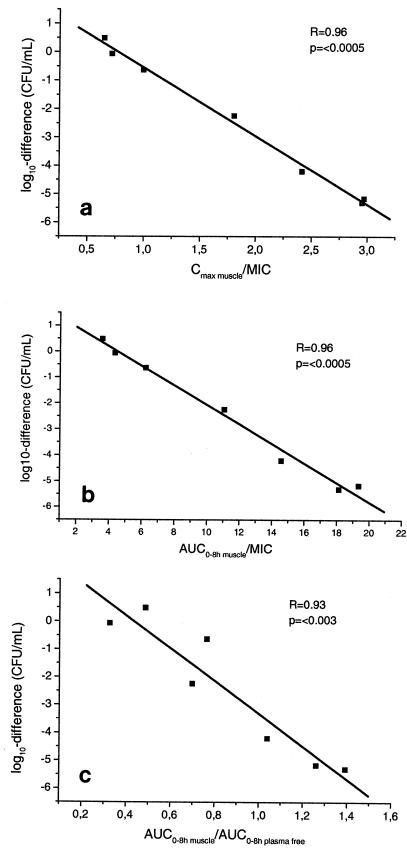
Correlations between decrease in bacterial counts of P. aeruginosa per milliliter after 8 h of exposure to levofloxacin at concentrations determined from individual concentration-time profiles of levofloxacin in muscle tissue and individual Cmax muscle/MIC ratios (a), AUC0-8 muscle/MIC ratios (b), and AUC0-8 muscle/AUC0-8 plasma free ratios (c). A P value <0.05 was considered significant.
DISCUSSION
It is recognized that effective concentrations of antibiotics must be achieved in tissues for successful clinical outcomes of antimicrobial therapy (18, 30). This is based on present knowledge indicating that it is not the central compartment but the interstitial space fluids of soft tissues in particular that mostly represent the sites of bacterial infection (29). However, the concentrations of antimicrobial agents in the tissues of critically ill patients have been reported to be substantially lower than the corresponding concentrations in plasma or the concentrations in the tissues of healthy volunteers (6, 22). This impaired equilibration between the concentrations in plasma and those in tissue has been attributed to the inflammatory systemic response of infection, hemodynamic alterations, and the administration of large amounts of fluid and vasopressive drugs to critically ill patients (6, 21, 22, 34).
The severity of the inflammatory response in critically ill patients is variable, and the subsequent treatment strategies used to overcome sepsis are multifaceted. PD breakpoints, however, are based on PK data for population means and therefore do not adequately reflect individual conditions that might markedly influence tissue drug distributions. Thus, ineffective antibiotic concentrations at the target site might help to explain the discrepancy between the in vitro susceptibility of bacteria and the clinical failure of treatments for soft tissue infections. To test this hypothesis, we carried out the present study and investigated the PKs of levofloxacin in the plasma and skeletal muscle tissue of critically ill patients. In addition, we aimed to determine the impact of interindividual variability in PKs in tissue and plasma on the bacterial killing profile.
In the present study, mean levofloxacin concentrations in skeletal muscle tissue were significantly (P = 0.018) lower than the total concentrations in plasma (Table 2). For the free fraction of levofloxacin, complete equilibration between the concentrations in tissue and those in plasma was observed after approximately 90 min, as indicated by an AUC0-8 muscle/AUC0-8 plasma free ratio of about 0.9 (Table 2 and Fig. 1). Previous studies testing the tissue penetration of beta-lactams and fosfomycin have reported AUCtissue/AUCplasma ratios ranging from approximately 0.1 to 0.7 in patients in intensive care units (14, 21, 22). Thus, an AUCtissue/AUCplasma ratio of about 0.9 for levofloxacin might be unexpectedly high in patients with sepsis but reflects present knowledge about the accumulation of levofloxacin in leukocytes and other mammalian cells, which act as drug depots (13).
While the population mean concentration-versus-time profiles of levofloxacin in tissue and plasma were identical, significant interindividual differences in PKs in tissue and plasma were observed (Fig. 2), as affirmed by AUC0-8 muscle/AUC0-8 plasma free ratios ranging from 0.33 to 1.39 (Fig. 4c). This finding confirms the hypothesis that high interindividual differences in the levels of equilibration between the concentrations in plasma and those in tissue are to be expected among patients with sepsis.
To investigate the impact of the tissue penetration of levofloxacin on antimicrobial activity at the target site, we used an in vivo PK-in vitro PD model to simulate the killing of relevant bacteria in individual patients (7, 12, 14). One strain of P. aeruginosa (MIC = 2 μg/ml) which might be considered a difficult-to-treat pathogen and one strain of S. aureus (MIC = 0.5 μg/ml) were chosen because these species are frequently isolated in intensive care units, with each species accounting for more than 12% of bacterial infections (19). However, the high frequency of P. aeruginosa isolates in intensive care units is due to respiratory tract infections, and select studies suggest that it is important to achieve appropriate antibiotic levels in the epithelial lining fluid for the successful treatment of such infections (2, 16).
Our in vitro PK-PD simulation showed no difference in bacterial killing between plasma and muscle tissue following exposure of one strain of S. aureus to levofloxacin at concentrations determined from individual concentration-versus-time profiles (Fig. 3a). This seems to be the result of the relatively low MIC of 0.5 μg/ml for S. aureus. The killing profile for P. aeruginosa (MIC = 2 μg/ml) also did not result in marked interindividual differences when the strain was exposed to levofloxacin at concentrations determined from the concentration-time profiles measured in plasma (Fig. 3b). However, when P. aeruginosa was exposed to levofloxacin at concentrations determined from the profiles obtained in skeletal muscle tissue, we observed significant interindividual differences in bacterial killing rates, ranging from killing beyond the lower limit of detection to moderate increases in bacterial counts. The extent of bacterial killing could be predicted by calculating individual Cmax muscle/MIC, AUC0-8 muscle/MIC, and AUC0-8 muscle/AUC0-8 plasma free ratios, with coefficients of correlations ranging from 0.93 to 0.96 (Fig. 4a to c). The correlation between individual AUCtissue/AUCplasma ratios and bacterial killing confirms the importance of drug penetration into tissue for the efficacy of antimicrobial therapy with fluoroquinolones.
Fluoroquinolones are antimicrobial agents that exert concentration-dependent bacterial killing (10, 33), and the Cmax/MIC ratio and the ratio of the AUC from time zero to 24 h (AUC0-24)/MIC are considered the best parameters for prediction of the clinical outcome of antimicrobial therapy with fluoroquinolones (1, 33). It has been widely documented that optimal bacterial killing in vitro and efficacious fluoroquinolone therapy are achieved when the Cmax/MIC ratio ranges from 8 to 12 and the AUC0-24/MIC ratio is greater than 100 h. As expected, such high ratios for free drug levels were not achieved in our study, but the excellent correlation between Cmax/MIC and bacterial killing confirms the predictive value of this parameter (Fig. 4a). We also observed a very good correlation between individual AUC0-8 muscle/MIC ratios and bacterial killing (Fig. 4b), although this was most likely due to the very high correlation between AUC0-8 muscle and Cmax muscle (R = 1.0) (data not shown).
On the basis of the present data, it is tempting to speculate that the levofloxacin doses used to treat critically ill patients infected with difficult-to-treat pathogens such as P. aeruginosa should be higher than 500 mg daily. This is in line with the findings of a recent study, which showed that in vitro levofloxacin at 750 mg/day in combination with another agent active against P. aeruginosa may be clinically beneficial and superior to combinations with lower doses of levofloxacin (8). A loading dose of levofloxacin at the beginning of therapy appears to be advisable for critically ill patients to ensure that the concentrations of levofloxacin at the target site are sufficiently high to kill the bacteria effectively from the start of antimicrobial therapy.
In conclusion, the present study has shown that after the administration of a single 500-mg dose of levofloxacin, the concentrations of the drug in tissue may be inadequate to eradicate less susceptible pathogens from critically ill patients, even though effective concentrations are attained in plasma. Thus, interindividual differences in the levels of equilibration between the levofloxacin concentrations in plasma and those in tissue may be responsible for differences in clinical outcomes and might explain therapeutic failures in some cases. Therefore, for PK and PD calculations for antimicrobials, more attention should be paid to individual PKs in tissue, and individual PKs in tissue should be a key consideration in the selection of the drug dosages to be used for intensive care patients.
REFERENCES
- 1.Bergogne-Berezin, E. 2002. Clinical role of protein binding of quinolones. Clin. Pharmacokinet. 41:741-750. [DOI] [PubMed] [Google Scholar]
- 2.Bergogne-Berezin, E. 1995. New concepts in the pulmonary disposition of antibiotics. Pulm. Pharmacol. 8:65-81. [DOI] [PubMed] [Google Scholar]
- 3.Blaser, J., B. B. Stone, M. C. Groner, and S. H. Zinner. 1987. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob. Agents Chemother. 31:1054-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bone, R. C., W. J. Sibbald, and C. L. Sprung. 1992. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest 101:1481-1483. [DOI] [PubMed] [Google Scholar]
- 5.Bonfiglio, G. 2001. Is levofloxacin as active as ciprofloxacin against Pseudomonas aeruginosa? Chemotherapy (Basel) 47:239-242. [DOI] [PubMed] [Google Scholar]
- 6.Brunner, M., T. Pernerstorfer, B. X. Mayer, H. G. Eichler, and M. Muller. 2000. Surgery and intensive care procedures affect the target site distribution of piperacillin. Crit. Care Med. 28:1754-1759. [DOI] [PubMed] [Google Scholar]
- 7.Brunner, M., H. Stabeta, J. G. Moller, C. Schrolnberger, B. Erovic, U. Hollenstein, M. Zeitlinger, H. G. Eichler, and M. Muller. 2002. Target site concentrations of ciprofloxacin after single intravenous and oral doses. Antimicrob. Agents Chemother. 46:3724-3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgess, D. S., R. G. Hall, and T. C. Hardin. 2003. In vitro evaluation of the activity of two doses of levofloxacin alone and in combination with other agents against Pseudomonas aeruginosa. Diagn. Microbiol. Infect. Dis. 46:131-137. [DOI] [PubMed] [Google Scholar]
- 9.Cafiso, V., C. Messina, M. Santagati, F. Campanile, G. Bonfiglio, and S. Stefani. 2001. In vitro activity of levofloxacin against coagulase-positive and -negative staphylococci. Drugs Exp. Clin. Res. 27:107-111. [PubMed] [Google Scholar]
- 10.Craig, W. A. 1998. Choosing an antibiotic on the basis of pharmacodynamics. Ear Nose Throat J. 77:7-11. [PubMed] [Google Scholar]
- 11.Crokaert, F. 2001. Pharmacodynamics, a tool for a better use of antibiotics? Intensive Care Med. 27:340-343. [DOI] [PubMed] [Google Scholar]
- 12.Delacher, S., H. Derendorf, U. Hollenstein, M. Brunner, C. Joukhadar, S. Hofmann, A. Georgopoulos, H. G. Eichler, and M. Muller. 2000. A combined in vivo pharmacokinetic-in vitro pharmacodynamic approach to simulate target site pharmacodynamics of antibiotics in humans. J. Antimicrob. Chemother. 46:733-739. [DOI] [PubMed] [Google Scholar]
- 13.Fish, D. N., and A. T. Chow. 1997. The clinical pharmacokinetics of levofloxacin. Clin. Pharmacokinet. 32:101-119. [DOI] [PubMed] [Google Scholar]
- 14.Frossard, M., C. Joukhadar, B. M. Erovic, P. Dittrich, P. E. Mrass, M. Van Houte, H. Burgmann, A. Georgopoulos, and M. Muller. 2000. Distribution and antimicrobial activity of fosfomycin in the interstitial fluid of human soft tissues. Antimicrob. Agents Chemother. 44:2728-2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geddes, A., M. Thaler, S. Schonwald, M. Harkonen, F. Jacobs, and I. Nowotny. 1999. Levofloxacin in the empirical treatment of patients with suspected bacteraemia/sepsis: comparison with imipenem/cilastatin in an open, randomized trial. J. Antimicrob. Chemother. 44:799-810. [DOI] [PubMed] [Google Scholar]
- 16.Honeybourne, D. 1997. Antibiotic penetration in the respiratory tract and implications for the selection of antimicrobial therapy. Curr. Opin. Pulm. Med. 3:170-174. [DOI] [PubMed] [Google Scholar]
- 17.Hoogkamp-Korstanje, J. A. 1997. In-vitro activities of ciprofloxacin, levofloxacin, lomefloxacin, ofloxacin, pefloxacin, sparfloxacin and trovafloxacin against gram-positive and gram-negative pathogens from respiratory tract infections. J. Antimicrob. Chemother. 40:427-431. [DOI] [PubMed] [Google Scholar]
- 18.Hyatt, J. M., P. S. McKinnon, G. S. Zimmer, and J. J. Schentag. 1995. The importance of pharmacokinetic/pharmacodynamic surrogate markers to outcome. Focus on antibacterial agents. Clin. Pharmacokinet. 28:143-160. [DOI] [PubMed] [Google Scholar]
- 19.Jarvis, W. R., and W. J. Martone. 1992. Predominant pathogens in hospital infections. J. Antimicrob. Chemother. 29(Suppl. A):19-24. [DOI] [PubMed] [Google Scholar]
- 20.Joukhadar, C., H. Derendorf, and M. Muller. 2001. Microdialysis. A novel tool for clinical studies of anti-infective agents. Eur. J. Clin. Pharmacol. 57:211-219. [DOI] [PubMed] [Google Scholar]
- 21.Joukhadar, C., M. Frossard, B. X. Mayer, M. Brunner, N. Klein, P. Siostrzonek, H. G. Eichler, and M. Muller. 2001. Impaired target site penetration of beta-lactams may account for therapeutic failure in patients with septic shock. Crit. Care Med. 29:385-391. [DOI] [PubMed] [Google Scholar]
- 22.Joukhadar, C., N. Klein, B. X. Mayer, N. Kreischitz, G. Delle-Karth, P. Palkovits, G. Heinz, and M. Muller. 2002. Plasma and tissue pharmacokinetics of cefpirome in patients with sepsis. Crit. Care Med. 30:1478-1482. [DOI] [PubMed] [Google Scholar]
- 23.Lonnroth, P., P. A. Jansson, and U. Smith. 1987. A microdialysis method allowing characterization of intercellular water space in humans. Am. J. Physiol. 253:E228-E231. [DOI] [PubMed] [Google Scholar]
- 24.Muller, M., O. Haag, T. Burgdorff, A. Georgopoulos, W. Weninger, B. Jansen, G. Stanek, H. Pehamberger, E. Agneter, and H. G. Eichler. 1996. Characterization of peripheral-compartment kinetics of antibiotics by in vivo microdialysis in humans. Antimicrob. Agents Chemother. 40:2703-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller, M., R. Schmid, A. Georgopoulos, A. Buxbaum, C. Wasicek, and H. G. Eichler. 1995. Application of microdialysis to clinical pharmacokinetics in humans. Clin. Pharmacol. Ther. 57:371-380. [DOI] [PubMed] [Google Scholar]
- 26.National Committee for Clinical Laboratory Standards. 2001. MIC testing. Supplemental tables. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 27.Neckel, U., C. Joukhadar, M. Frossard, W. Jäger, M. Muller, and B. X. Mayer. 2002. Simultaneous determination of levofloxacin and ciprofloxacin in microdialysates and plasma by high-performance liquid chromatography. Anal. Chim. Acta 463:199-206. [Google Scholar]
- 28.Norrby, S. R. 1999. Levofloxacin. Expert Opin. Pharmacother. 1:109-119. [DOI] [PubMed] [Google Scholar]
- 29.Ryan, D. M. 1993. Pharmacokinetics of antibiotics in natural and experimental superficial compartments in animals and humans. J. Antimicrob. Chemother. 31(Suppl. D):1-16. [DOI] [PubMed] [Google Scholar]
- 30.Schentag, J. J., D. E. Nix, and M. H. Adelman. 1991. Mathematical examination of dual individualization principles. I. Relationships between AUC above MIC and area under the inhibitory curve for cefmenoxime, ciprofloxacin, and tobramycin. DICP 25:1050-1057. [DOI] [PubMed] [Google Scholar]
- 31.Soussy, C. J., M. Cluzel, M. C. Ploy, M. D. Kitzis, C. Morel, A. Bryskier, and P. Courvalin. 1999. In-vitro antibacterial activity of levofloxacin against hospital isolates: a multicentre study. J. Antimicrob. Chemother. 43(Suppl. C):43-50. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka, M., E. Yamazaki, M. Chiba, K. Yoshihara, T. Akasaka, M. Takemura, and K. Sato. 2002. In vitro antibacterial activities of DQ-113, a potent quinolone, against clinical isolates. Antimicrob. Agents Chemother. 46:904-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turnidge, J. 1999. Pharmacokinetics and pharmacodynamics of fluoroquinolones. Drugs 58(Suppl. 2):29-36. [DOI] [PubMed] [Google Scholar]
- 34.van Dalen, R., and T. B. Vree. 1990. Pharmacokinetics of antibiotics in critically ill patients. Intensive Care Med. 16(Suppl. 3):S235-238. [DOI] [PubMed] [Google Scholar]


