Abstract
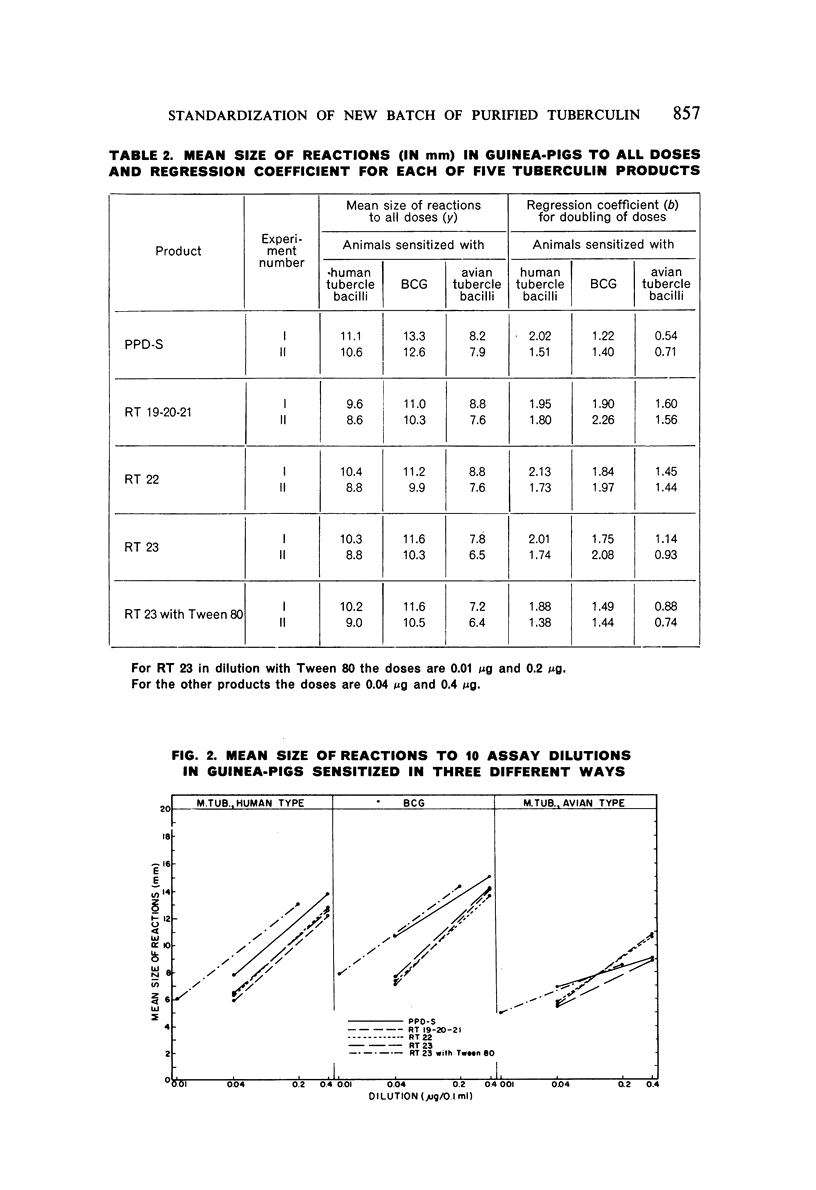
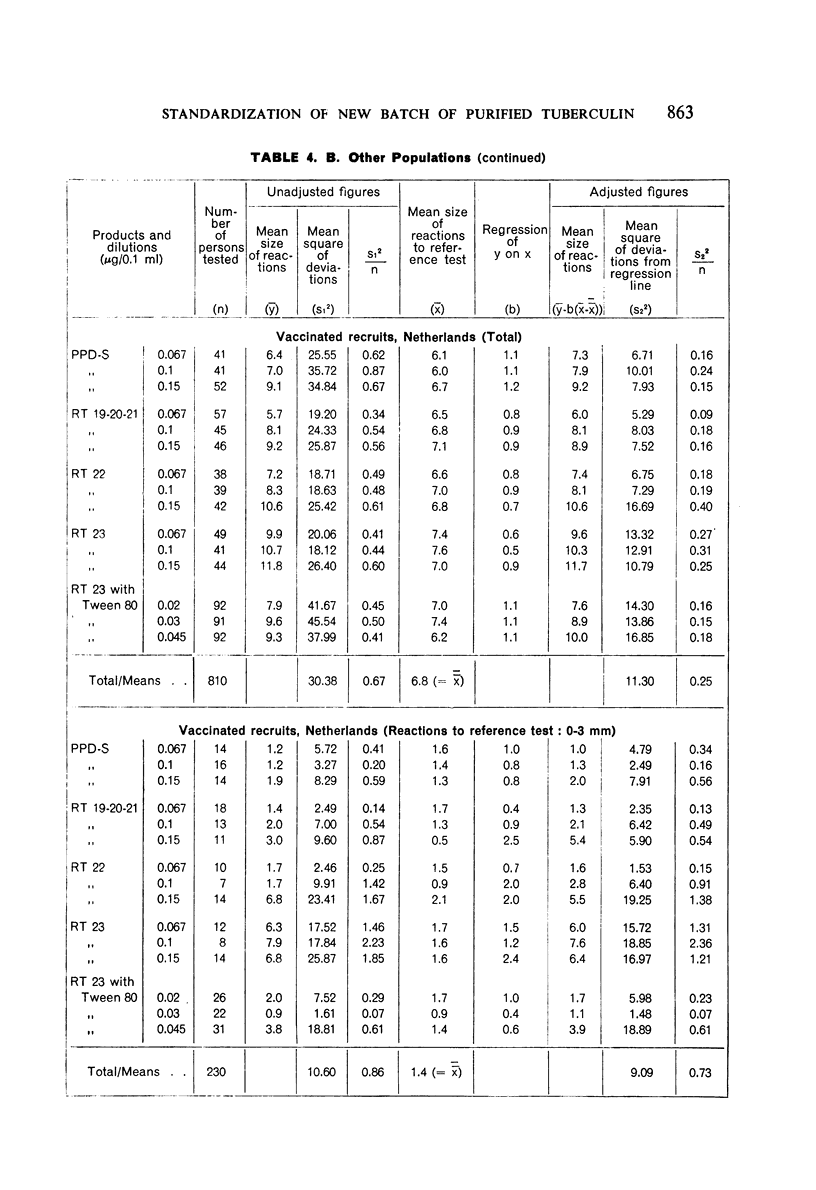
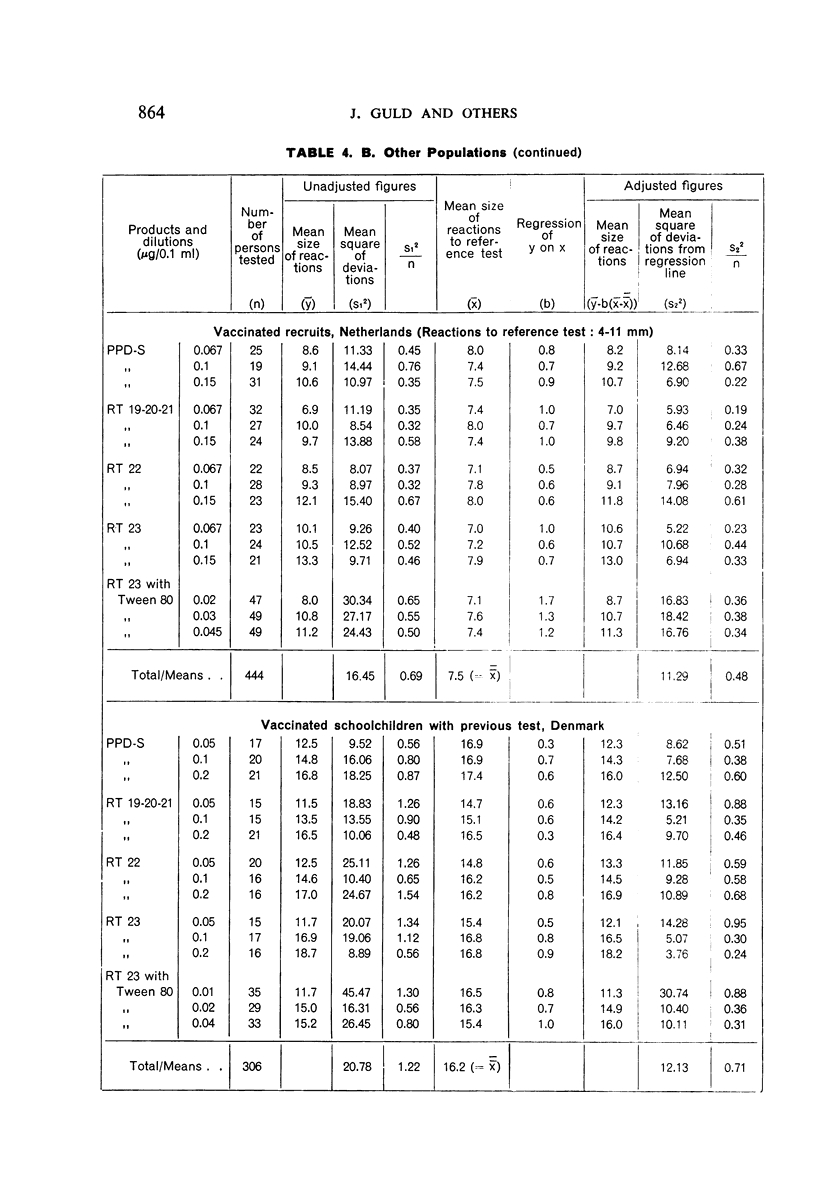
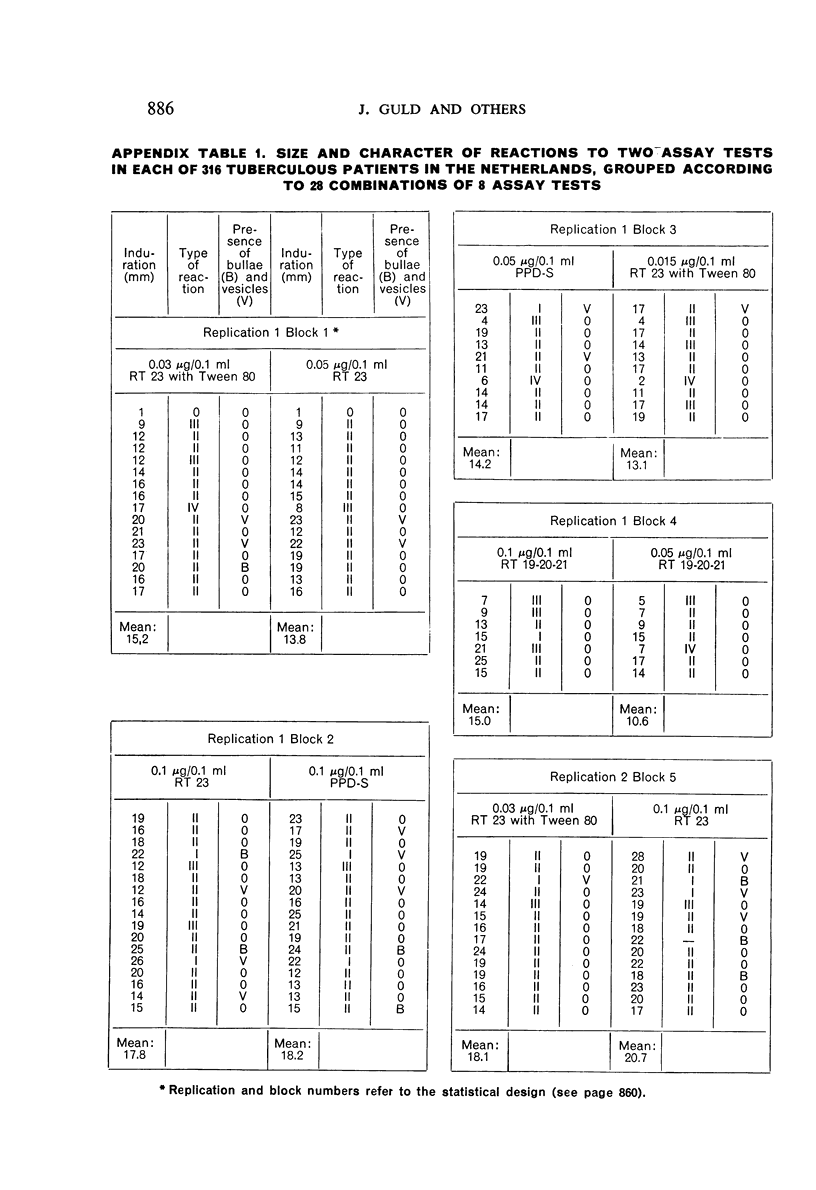
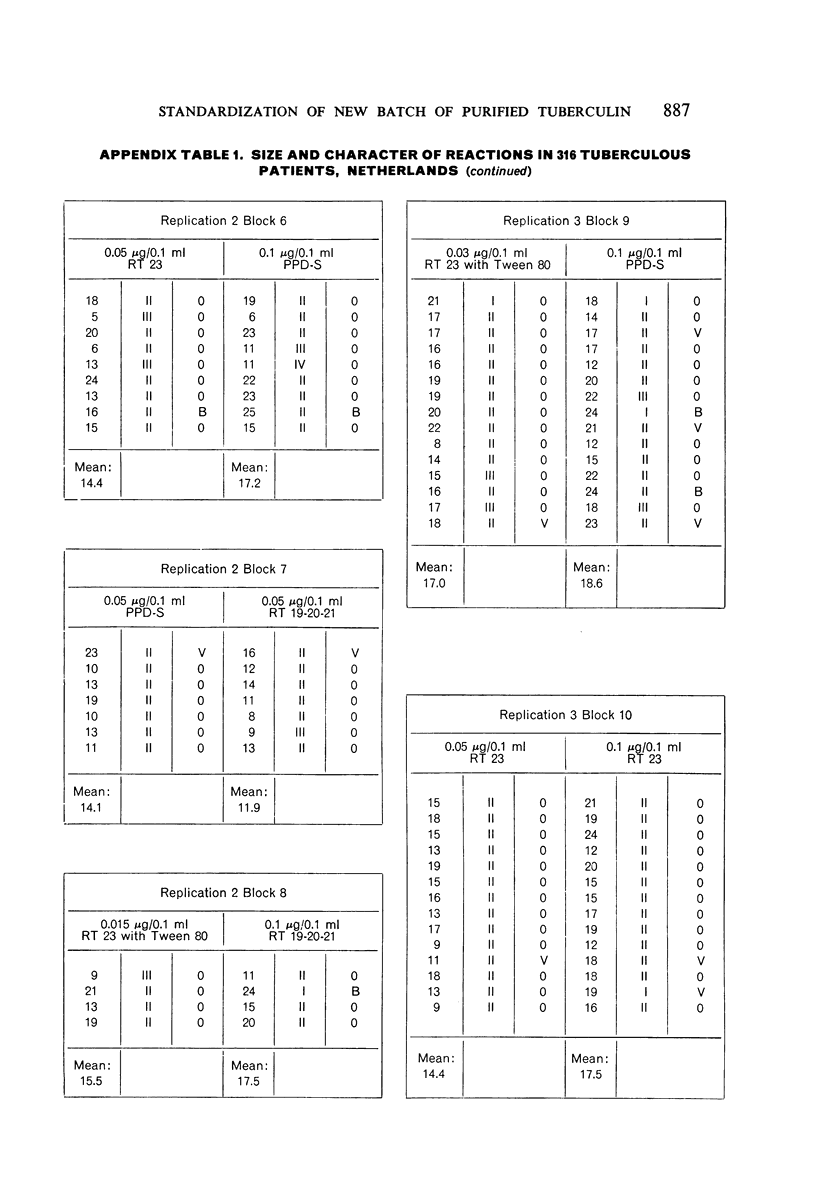
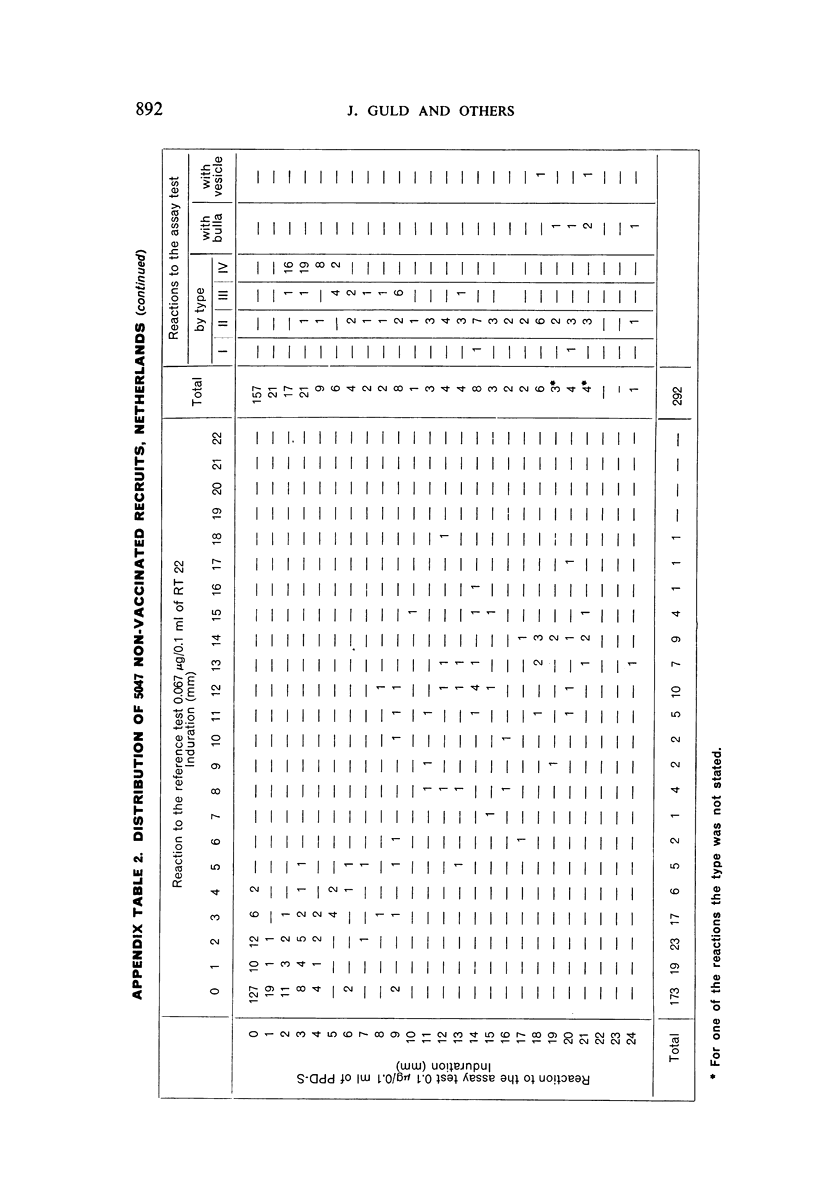
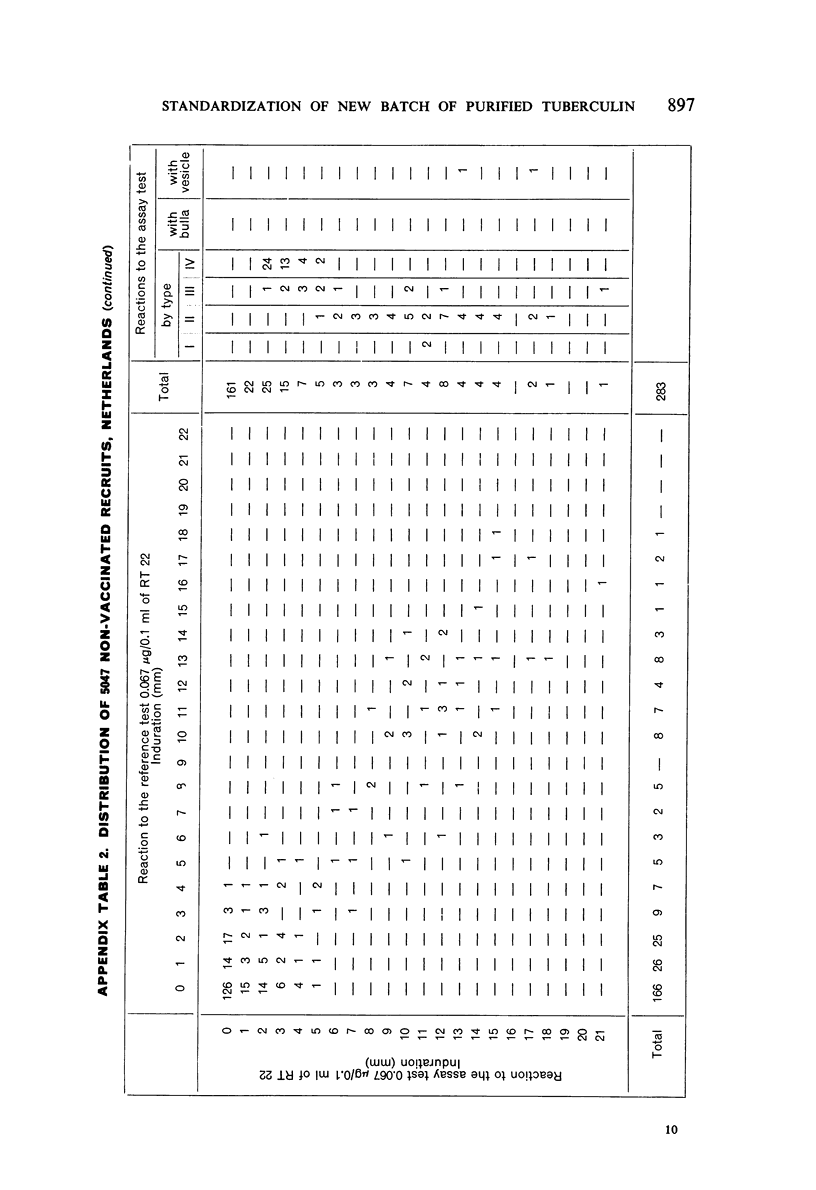
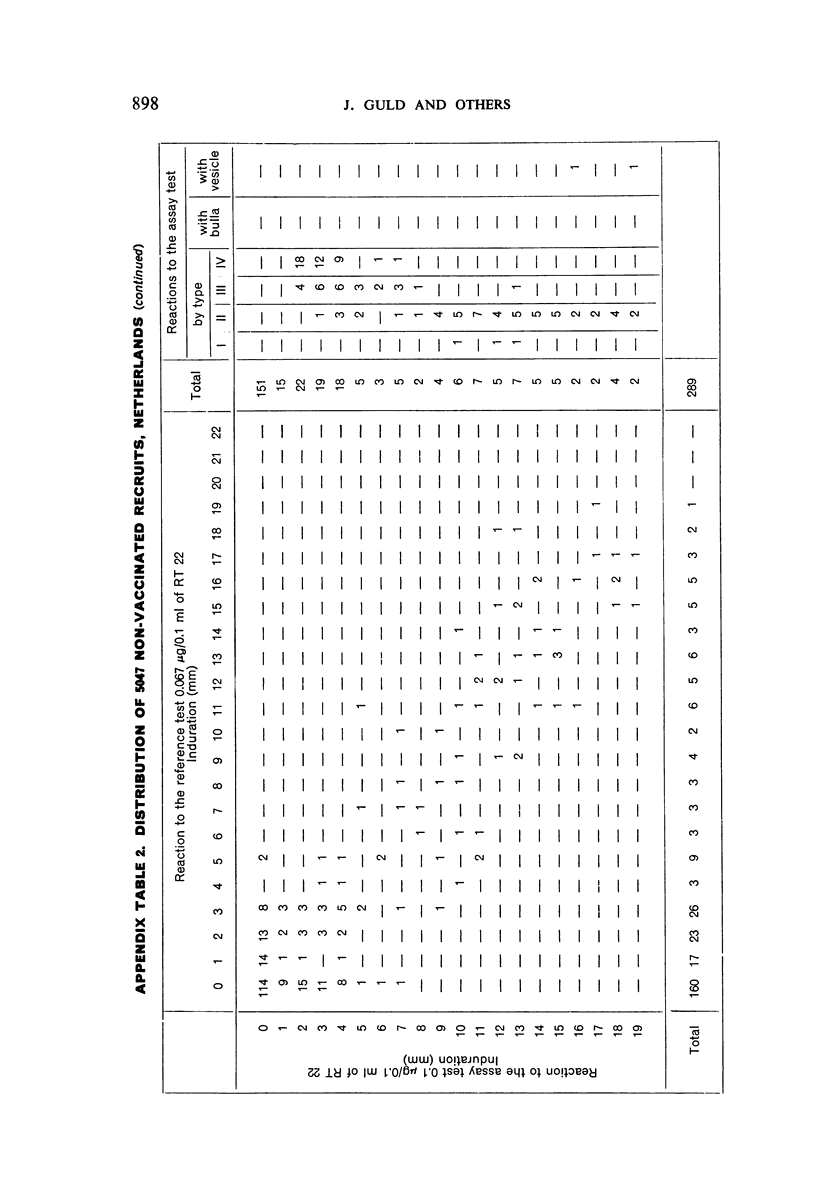
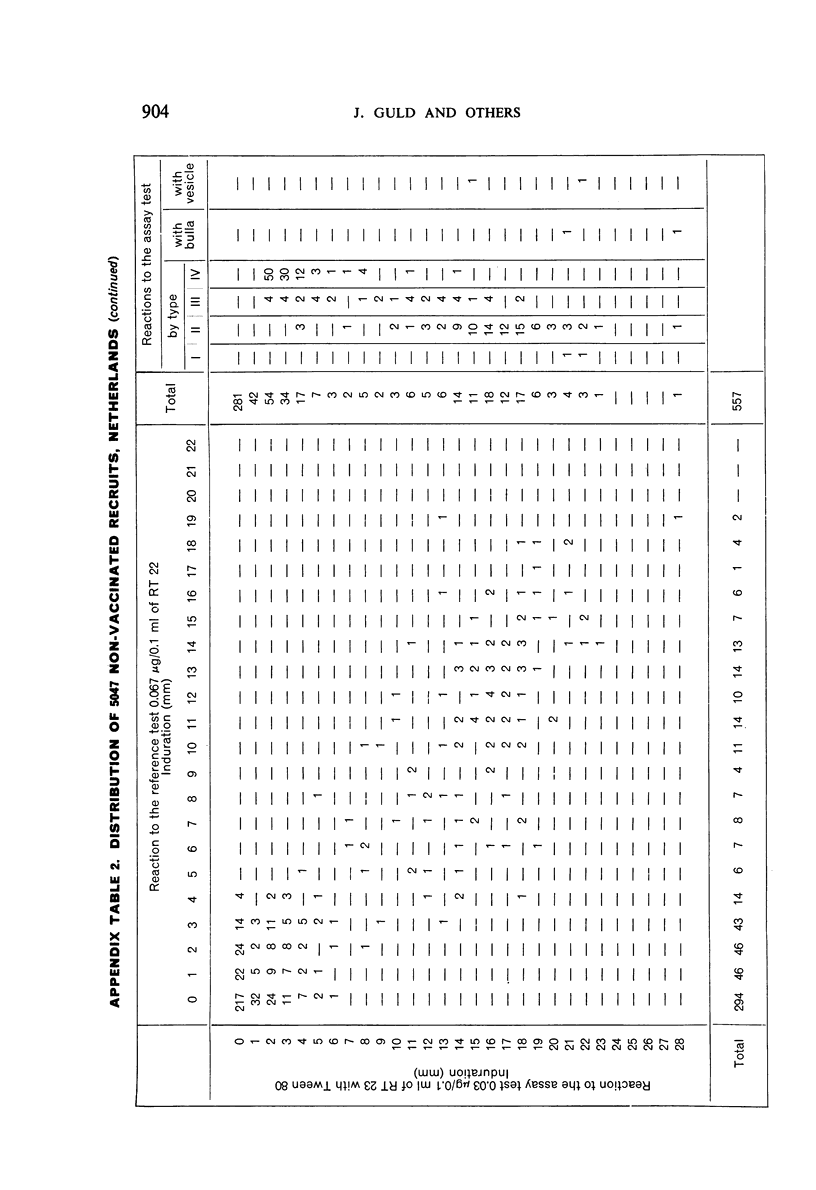
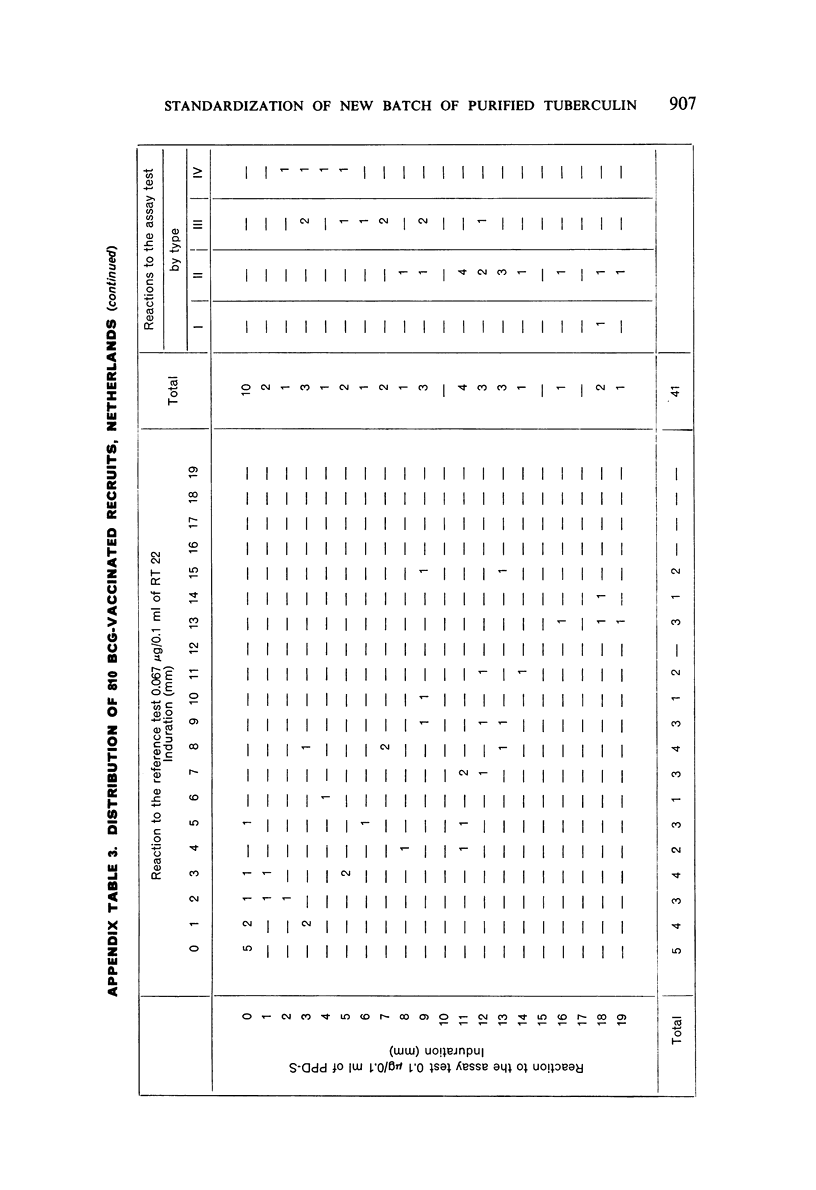
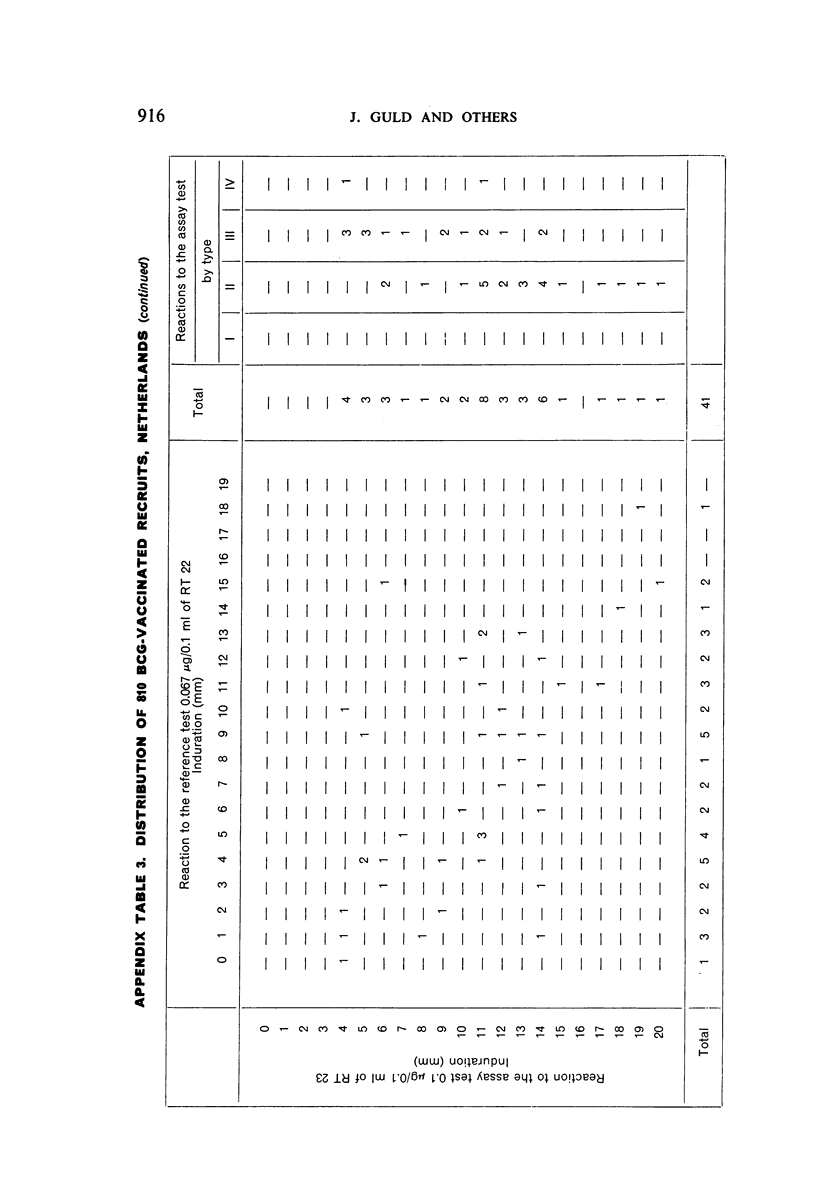
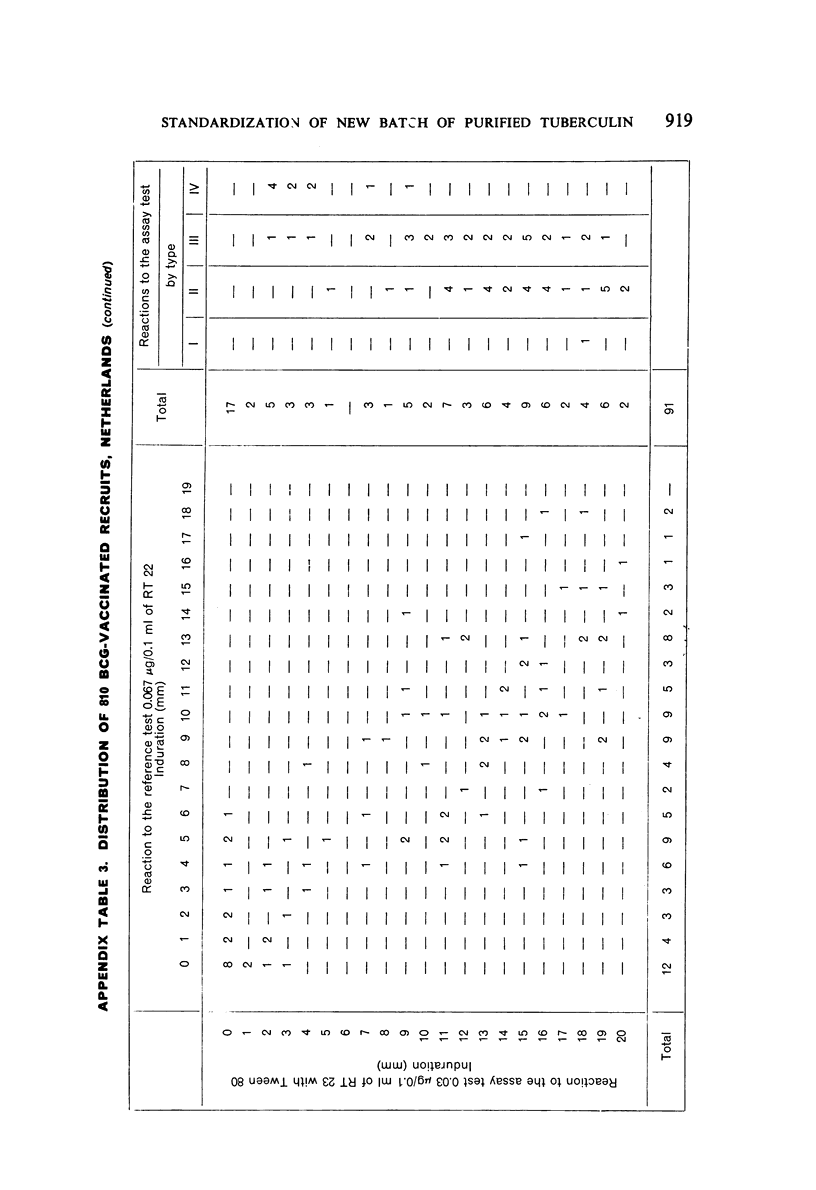
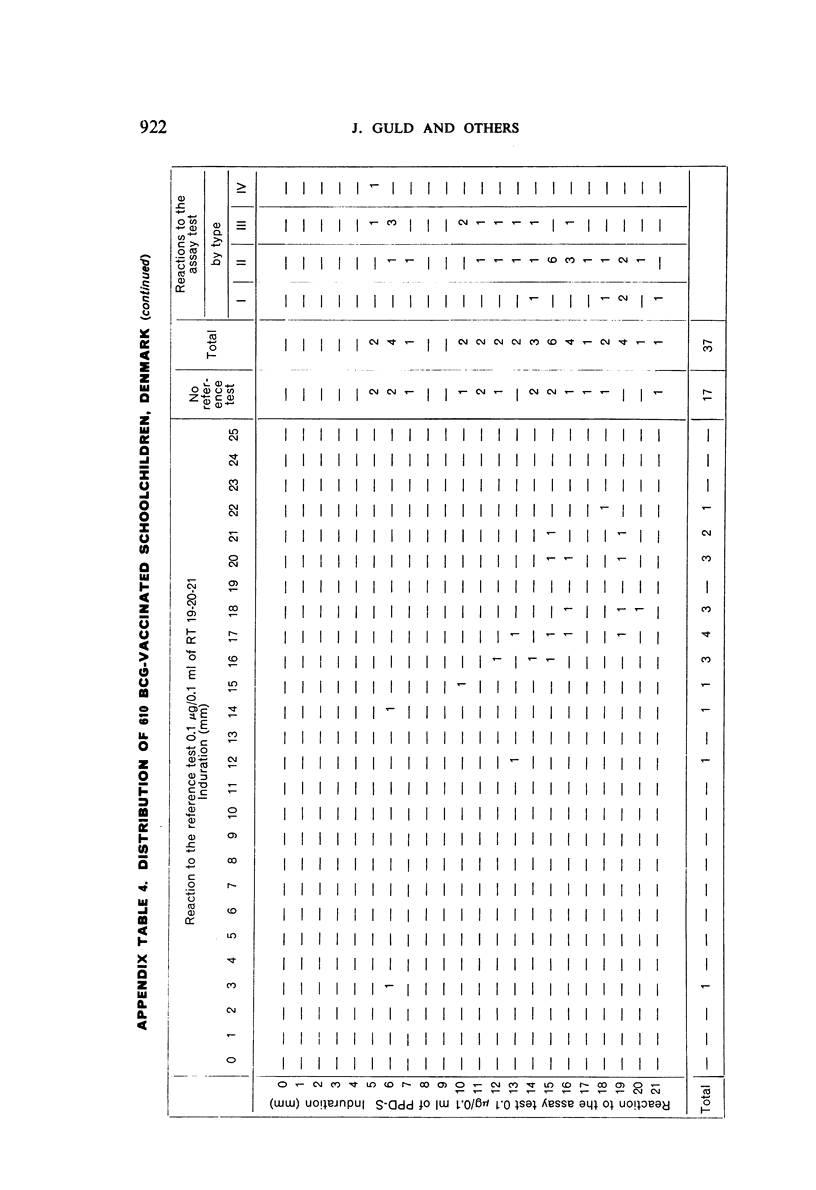
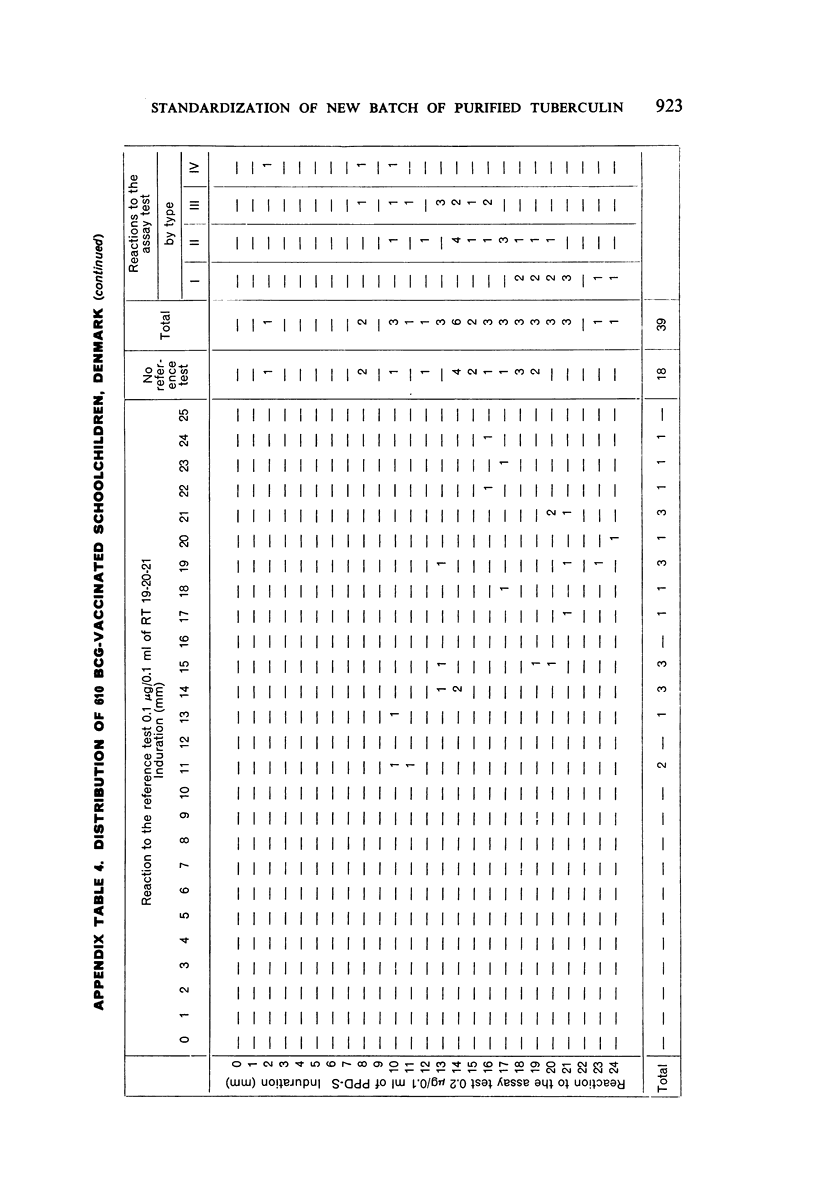
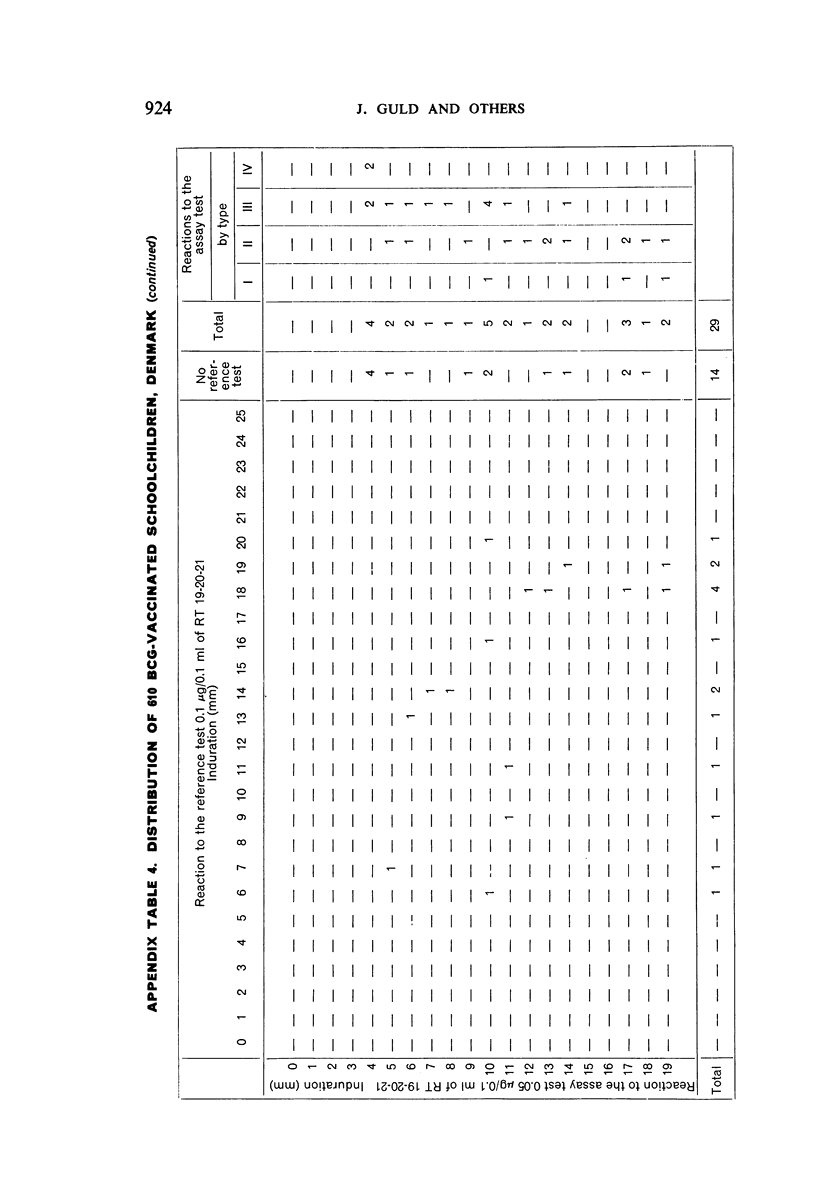
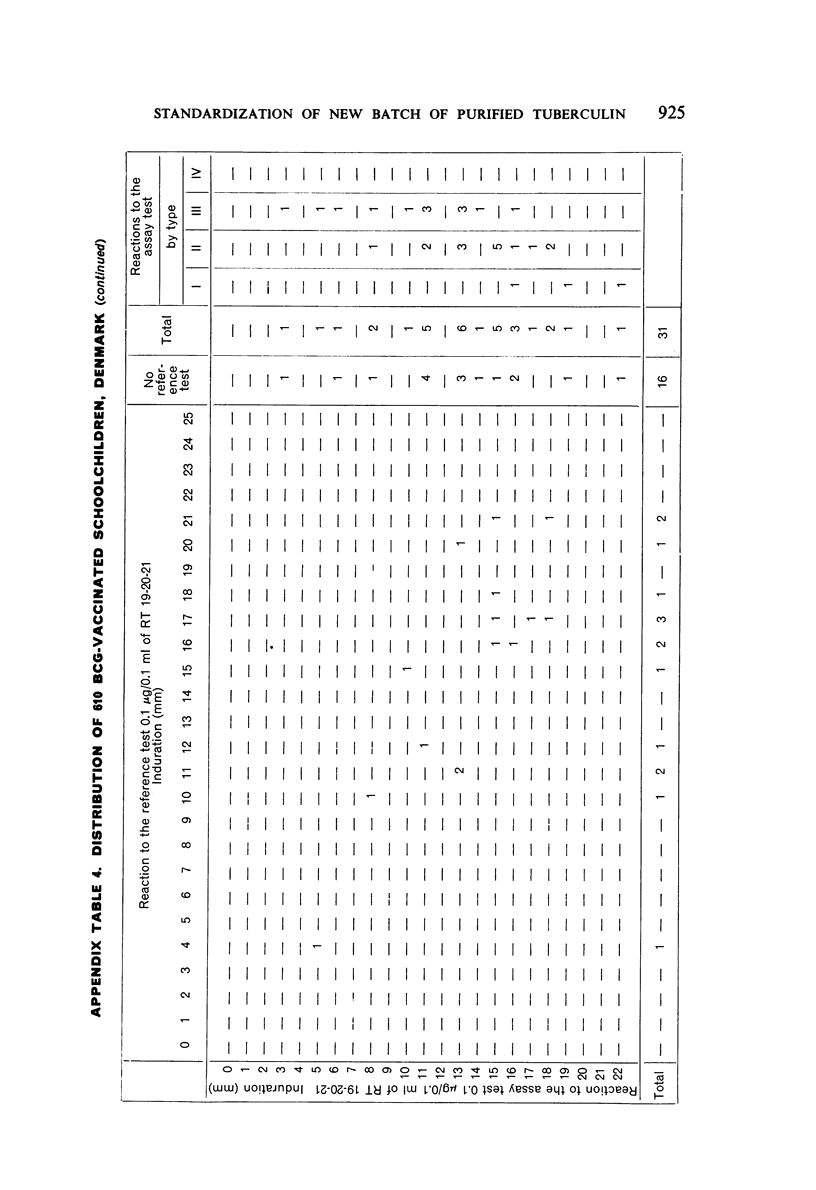
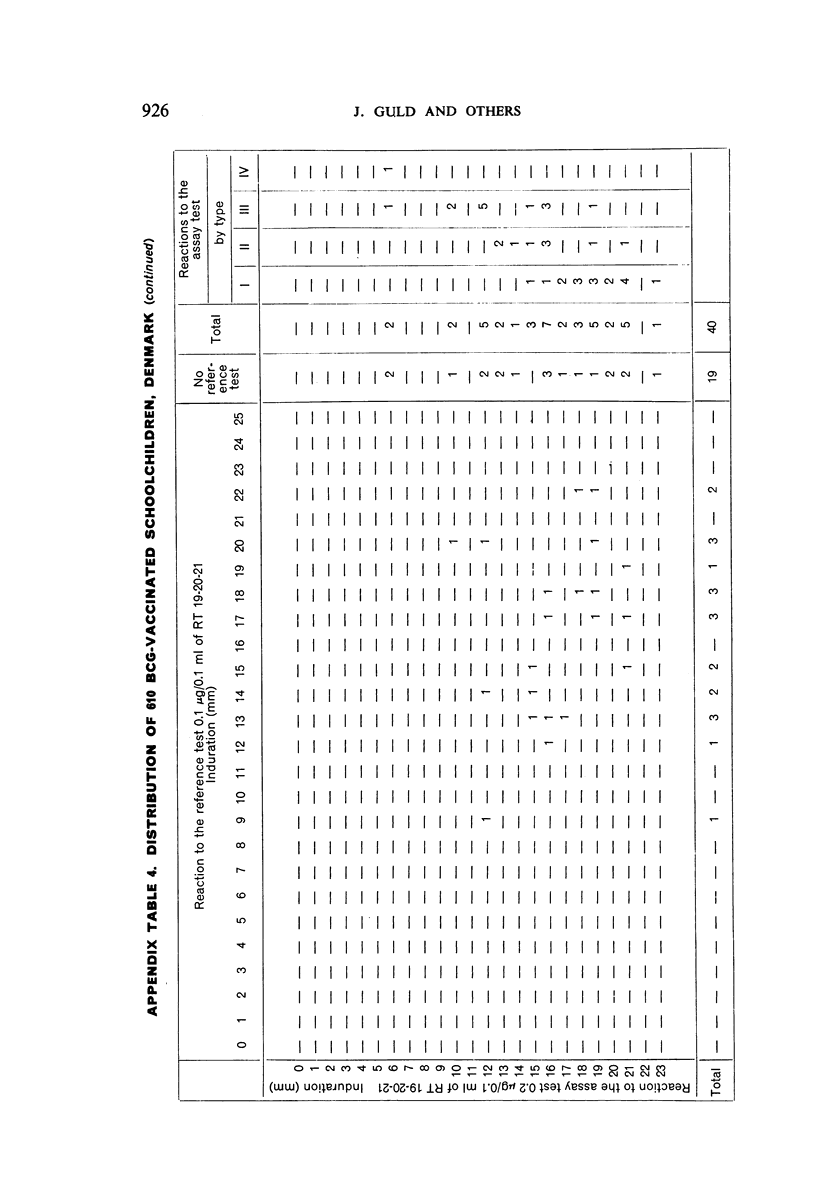
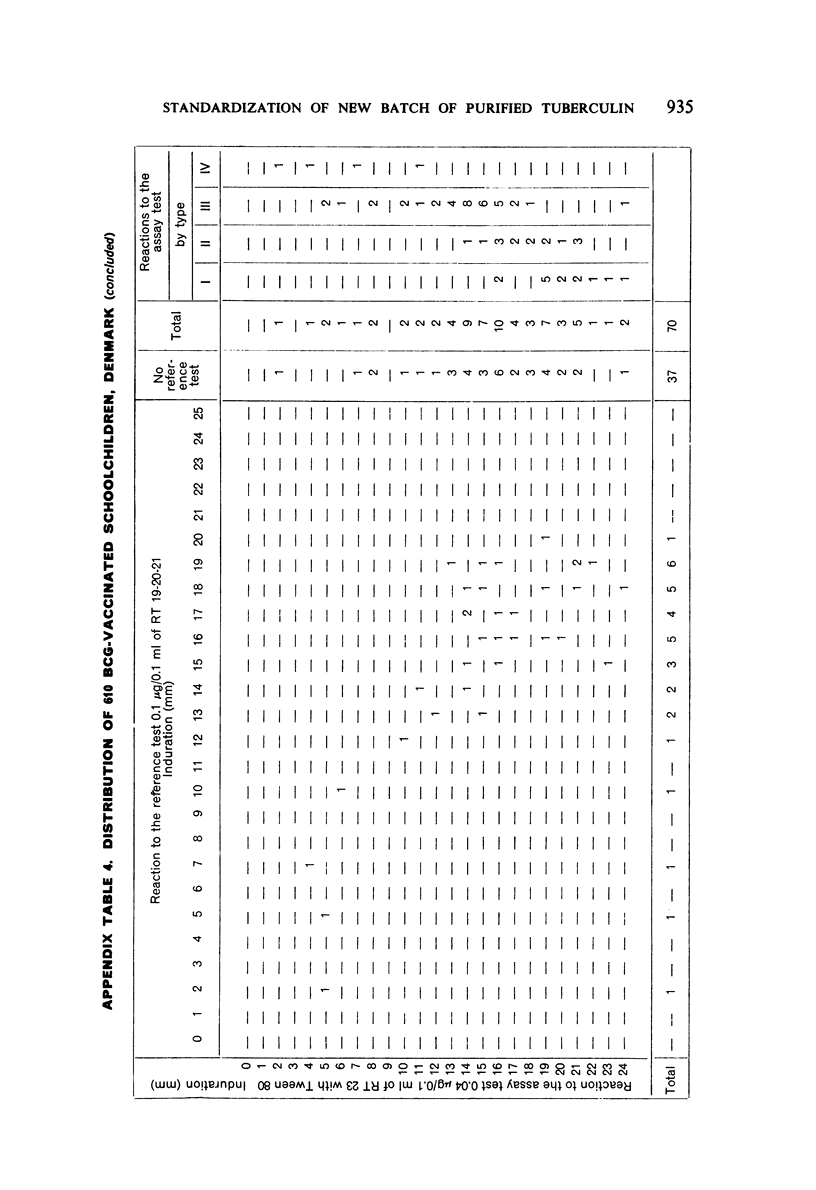
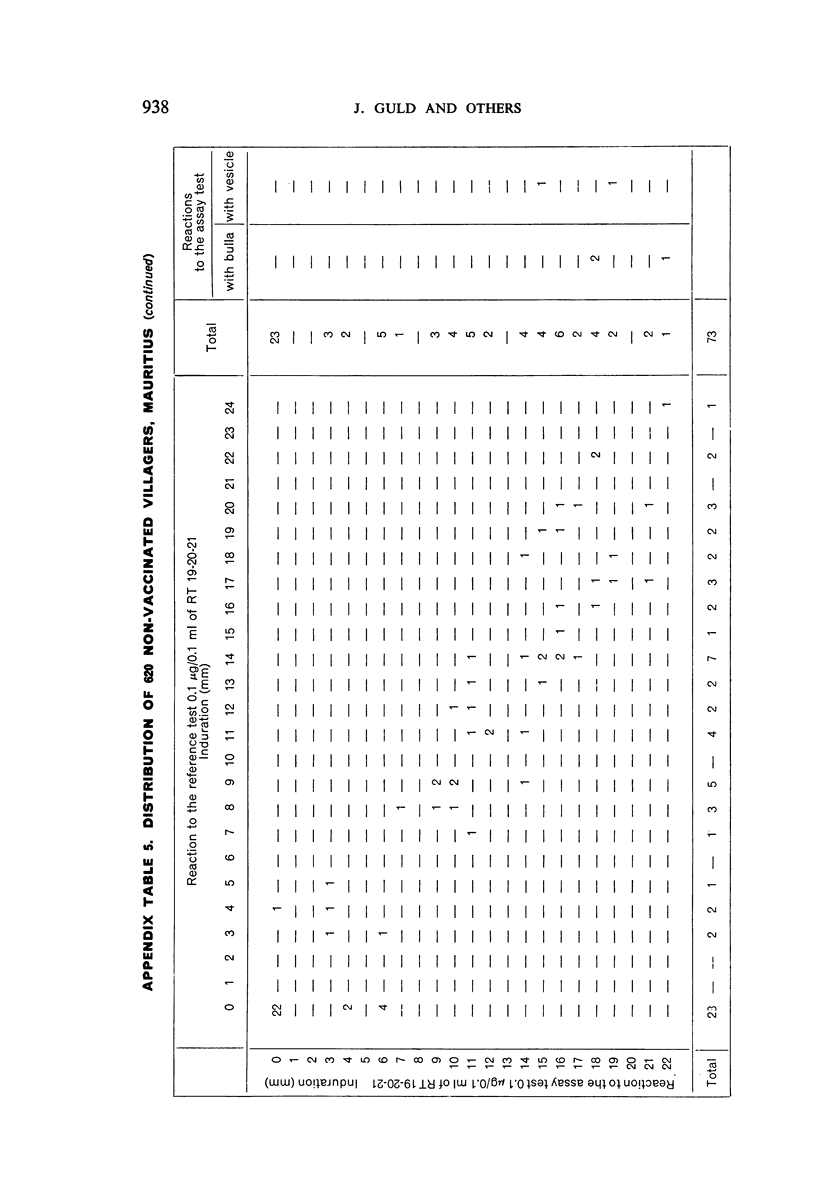
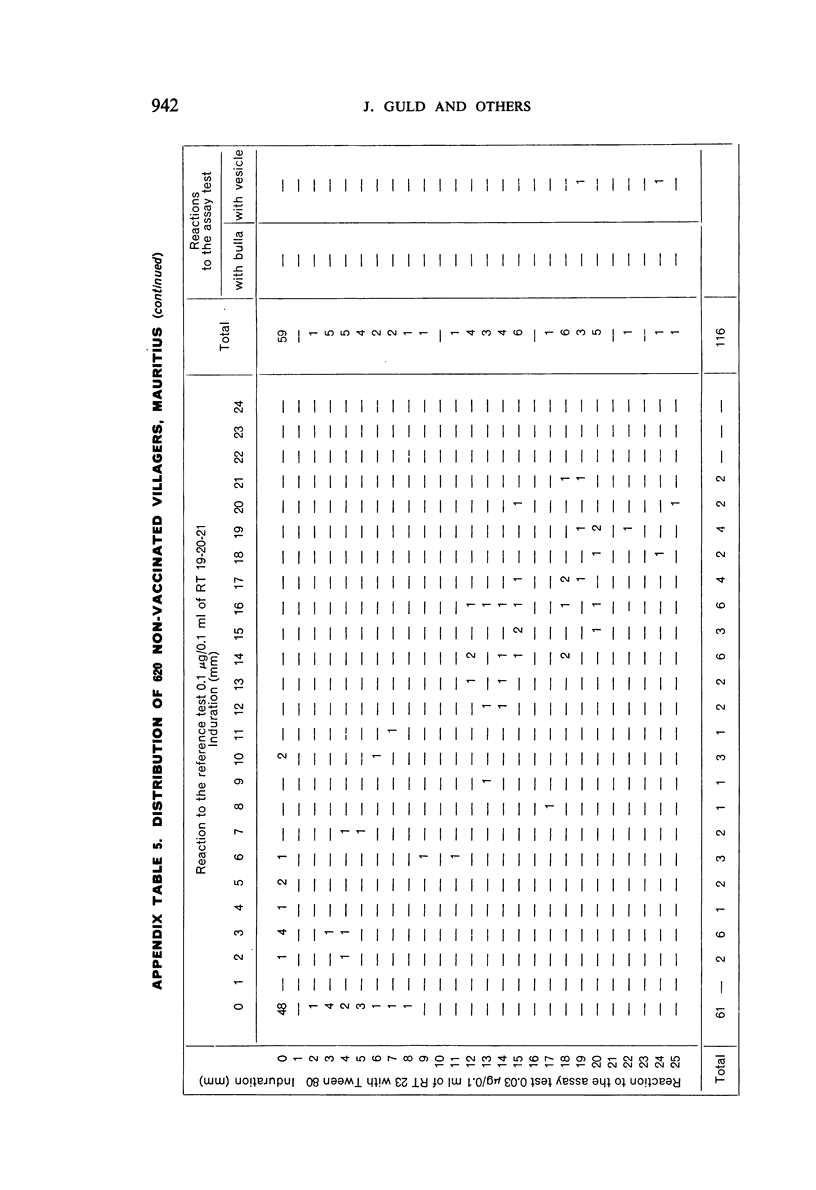
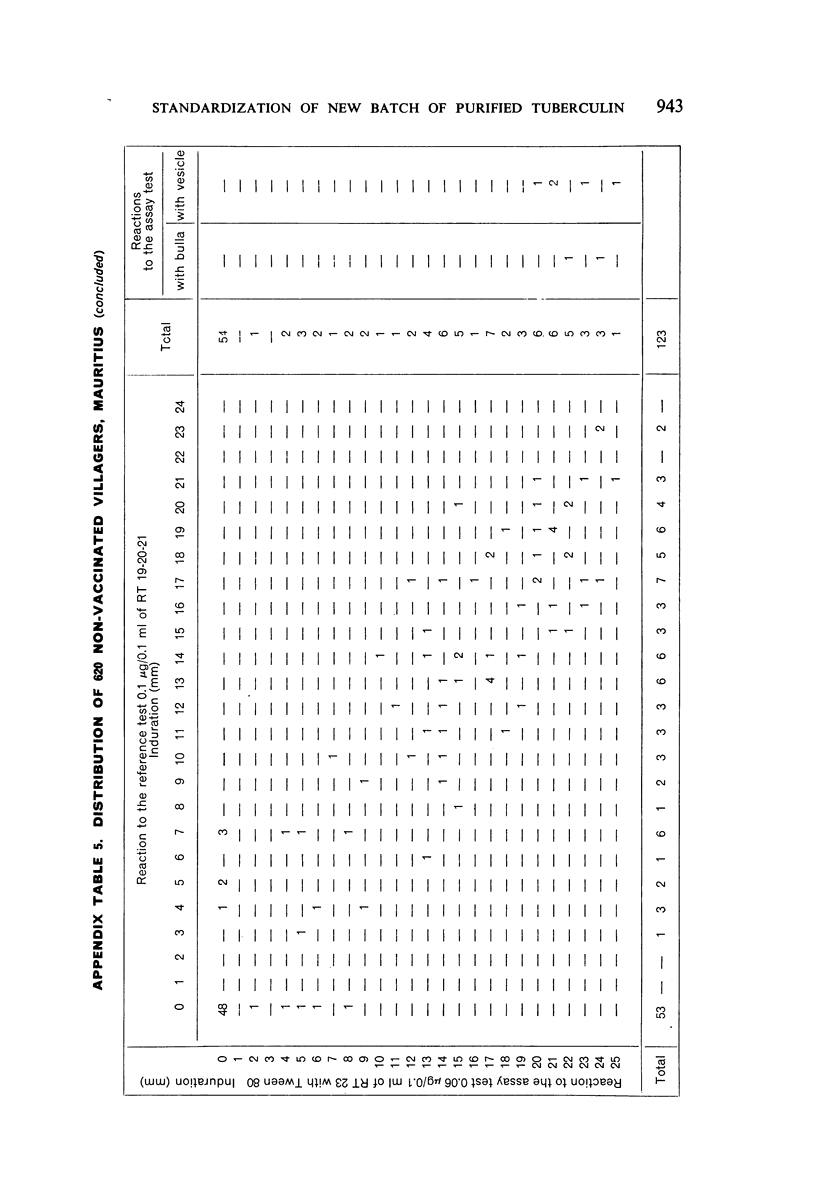
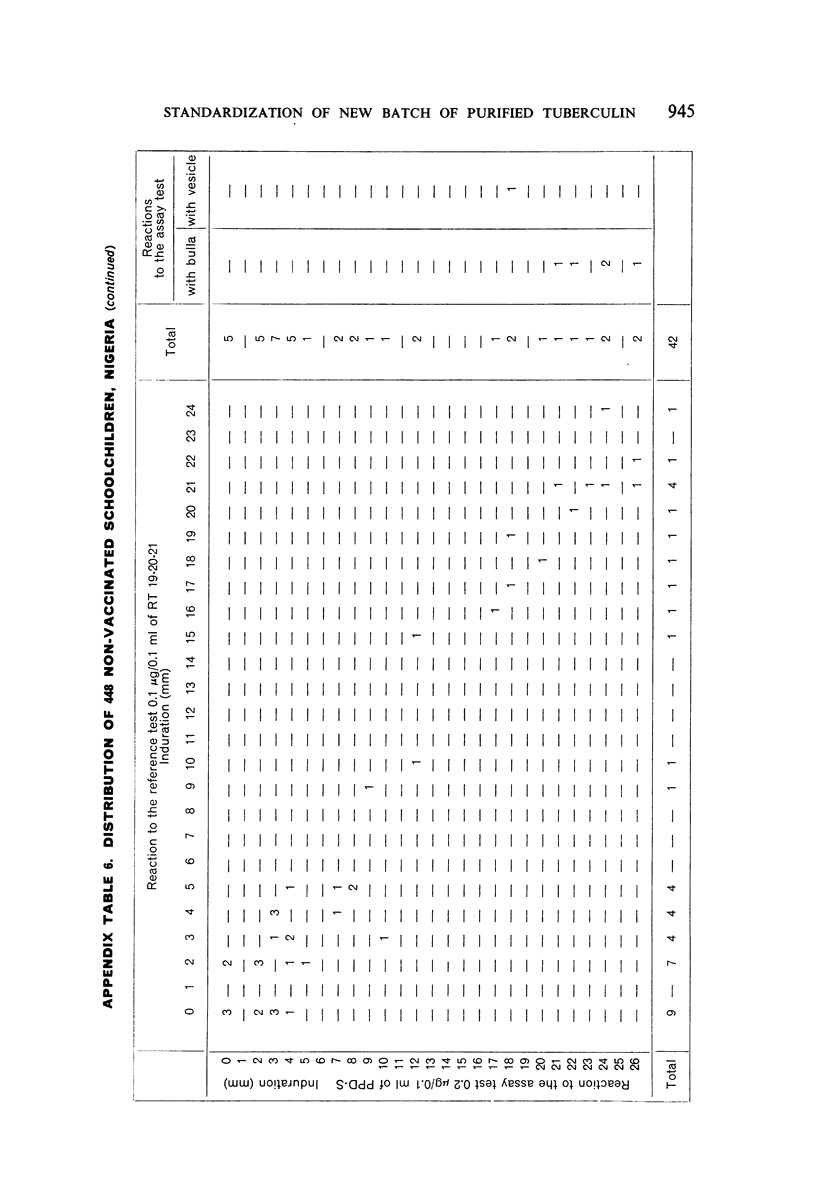
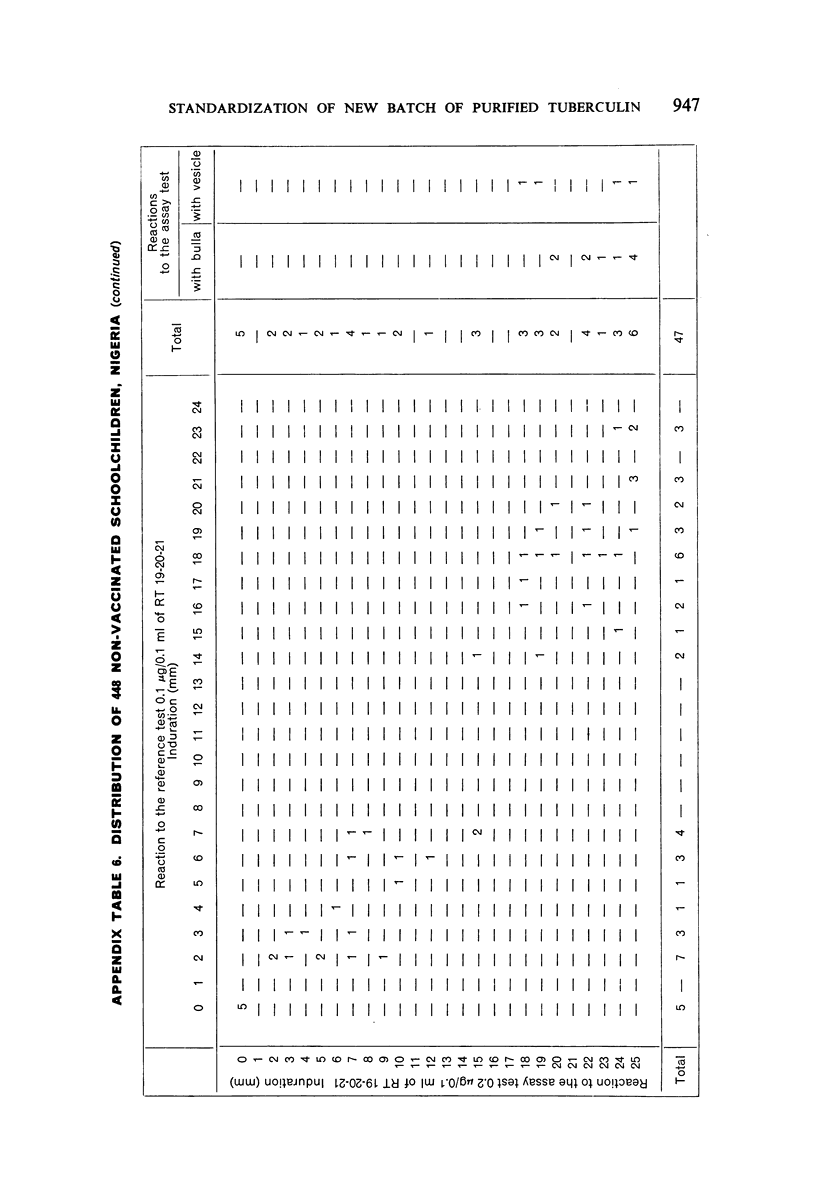
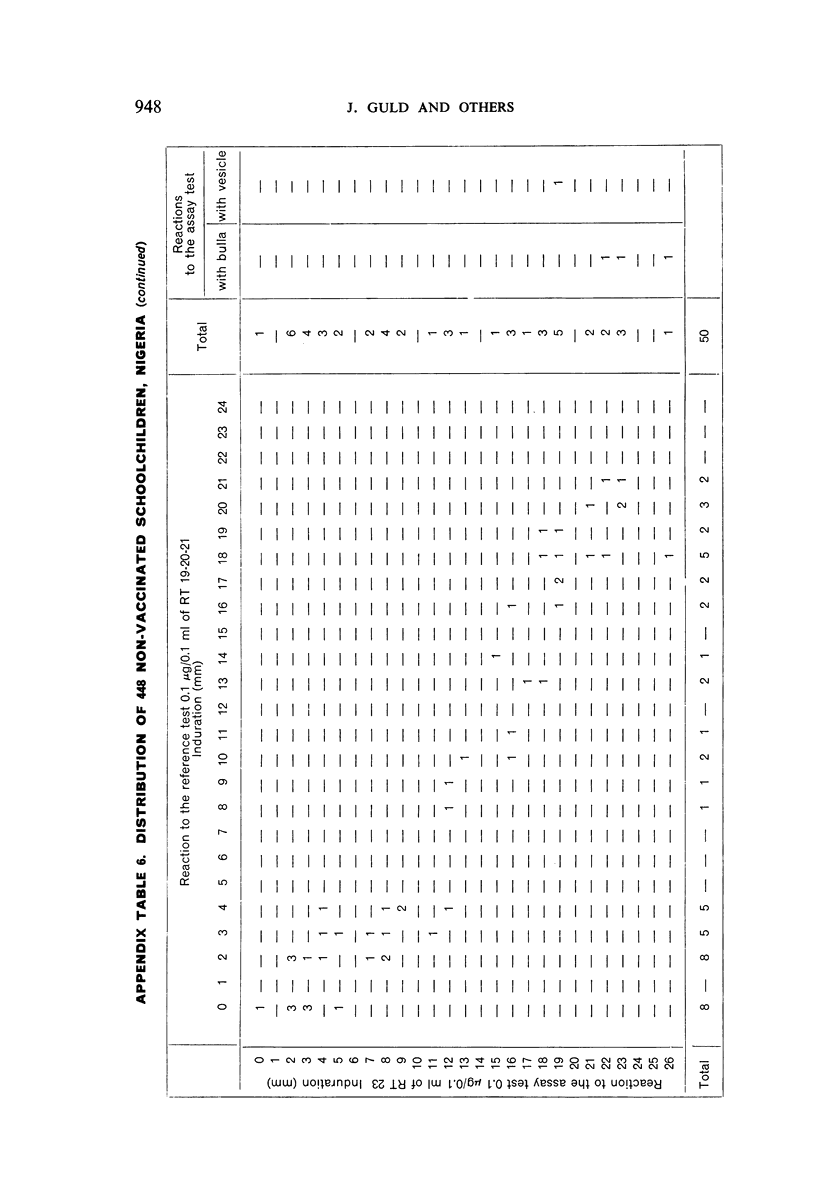
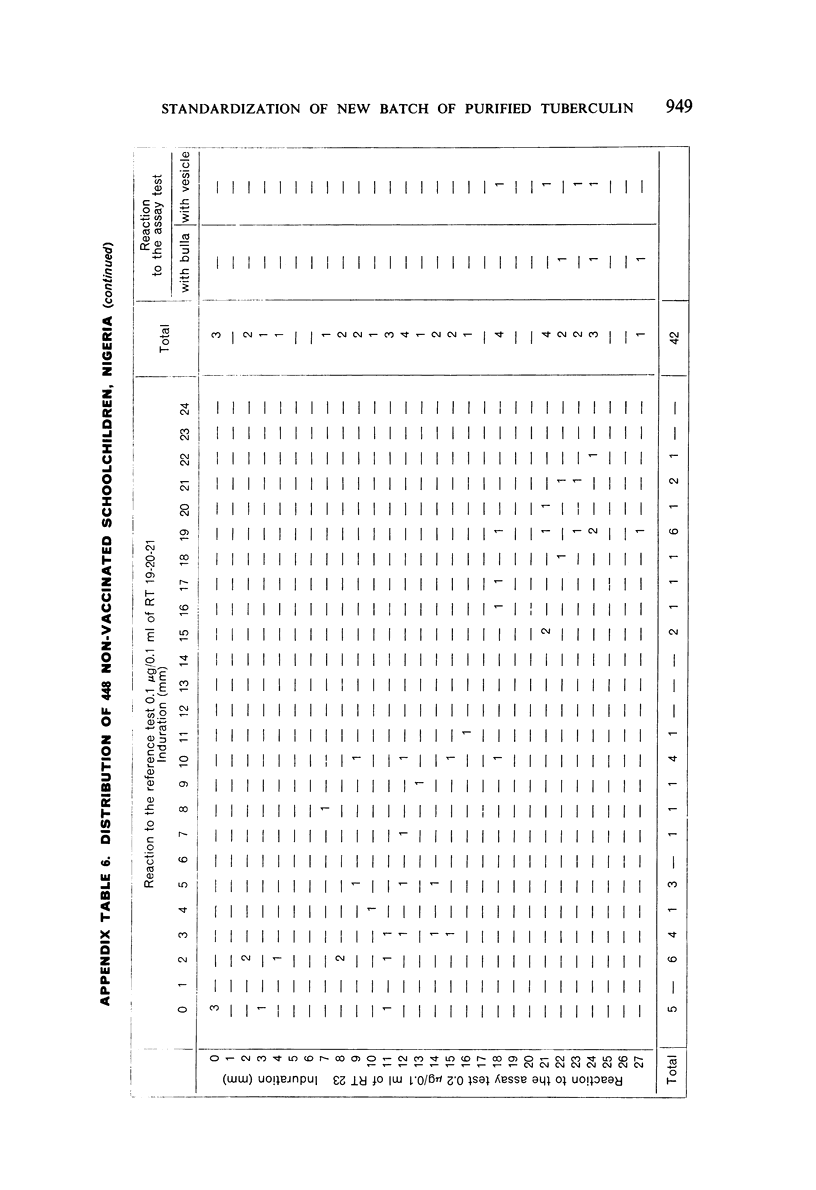
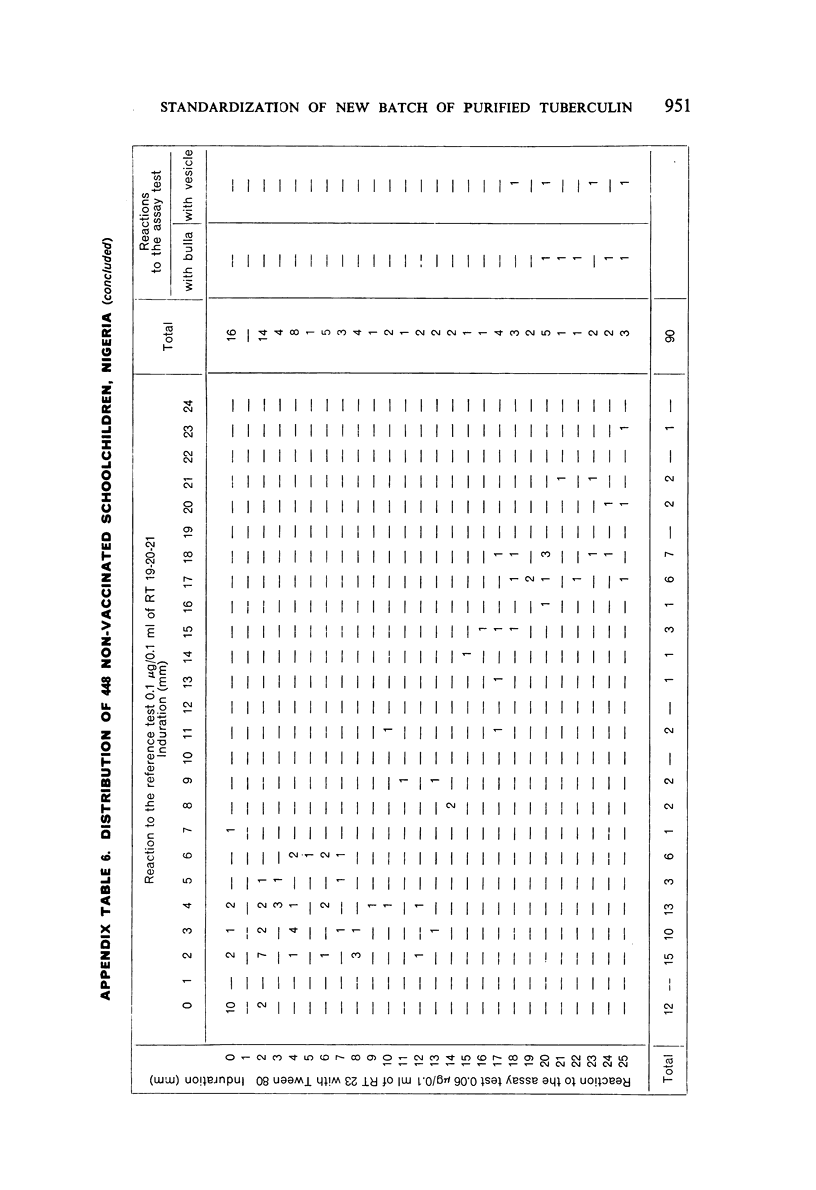
A new batch of PPD tuberculin, RT 23, prepared at the request of UNICEF by the Statens Seruminstitut, Copenhagen, has been compared with previous batches of tuberculin from the Statens Seruminstitut and with the International Standard for the Purified Protein Derivative of Mammalian Tuberculin. The comparisons were made by intradermal tuberculin testing. The majority of tests were carried out in human subjects with various types and levels of tuberculin sensitivity—namely, tuberculous patients in the Netherlands; BCG-vaccinated as well as spontaneously tuberculin-sensitive army recruits in the Netherlands; BCG-vaccinated schoolchildren in Denmark; population groups in the tropics (Mauritius and Nigeria) including a high proportion of persons with low-grade sensitivity. In addition, comparisons were made in guinea-pigs sensitized in different ways.
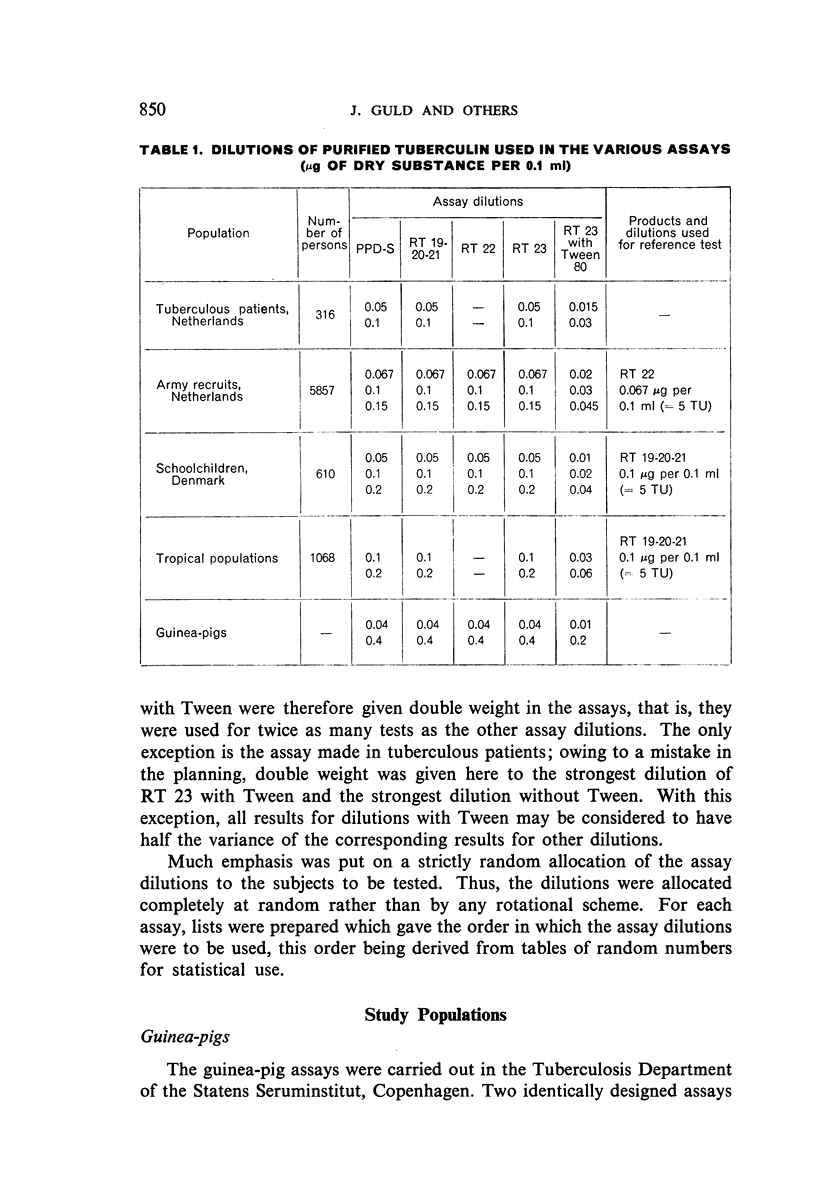
For these comparisons, RT 23 was diluted both with phosphate-buffered saline alone (as were the other preparations) and with phosphate-buffered saline containing 0.05‰ Tween 80 as a stabilizing agent.
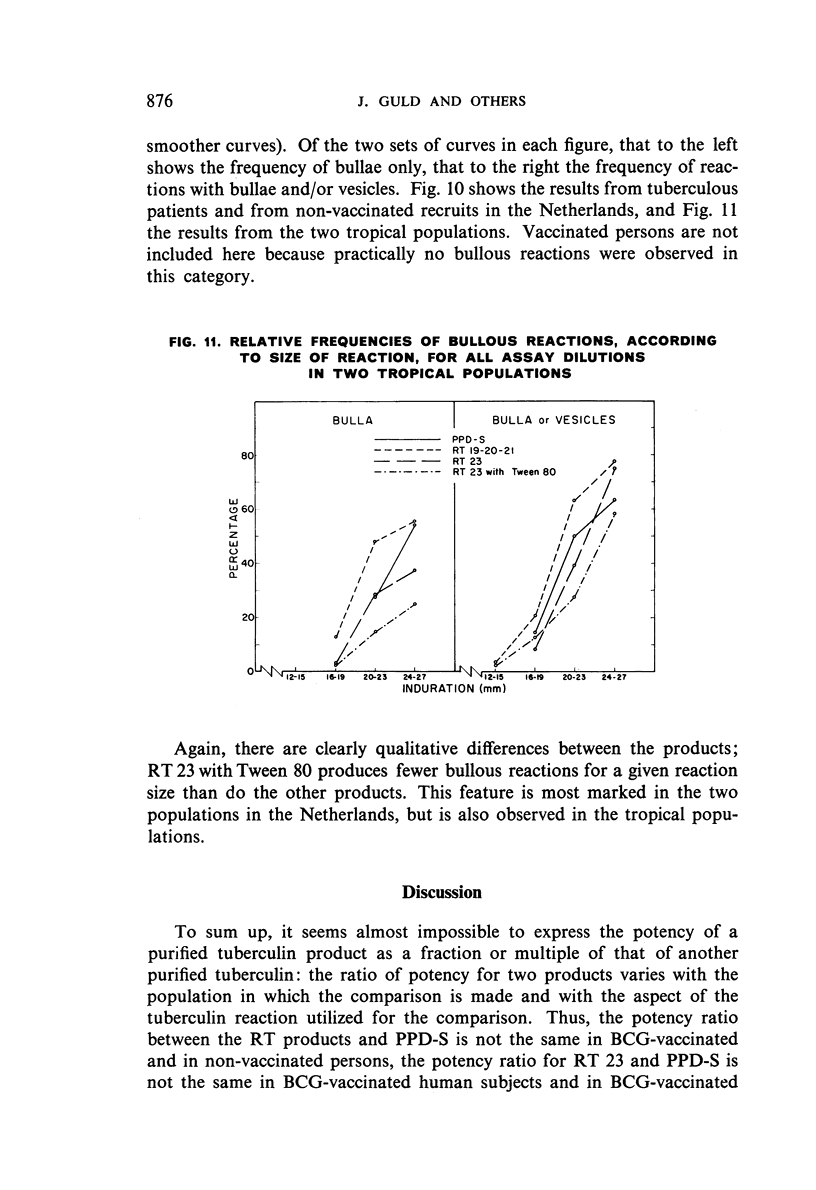
The results were found to differe significantly with the type and level of sensitivity of the groups tested. Thus, while the potency ratio of RT 23 and the International Standard is about 2:1 in BCG-vaccinated persons, it is about 1:1 in persons with naturally acquired tuberculin sensitivity. RT 23 in diluent containing Tween 80 elicited larger reactions than did RT 23 in ordinary diluent; however, when reactions of the same size were compared, a difference in character was observed: the reactions to RT 23 diluted with Tween 80 were softer and less frequently bullous.
On the basis of the results—in terms of reaction size—obtained in patients and in non-vaccinated recruits, it is suggested that one unit of RT 23 should be defined as 0.02 μg of the dry substance; for use as a first or single dose in routine testing, 0.02 μg of RT 23 in diluent stabilized with Tween 80, or 0.06 μg in diluent without Tween 80, is recommended.
Full text
PDF

















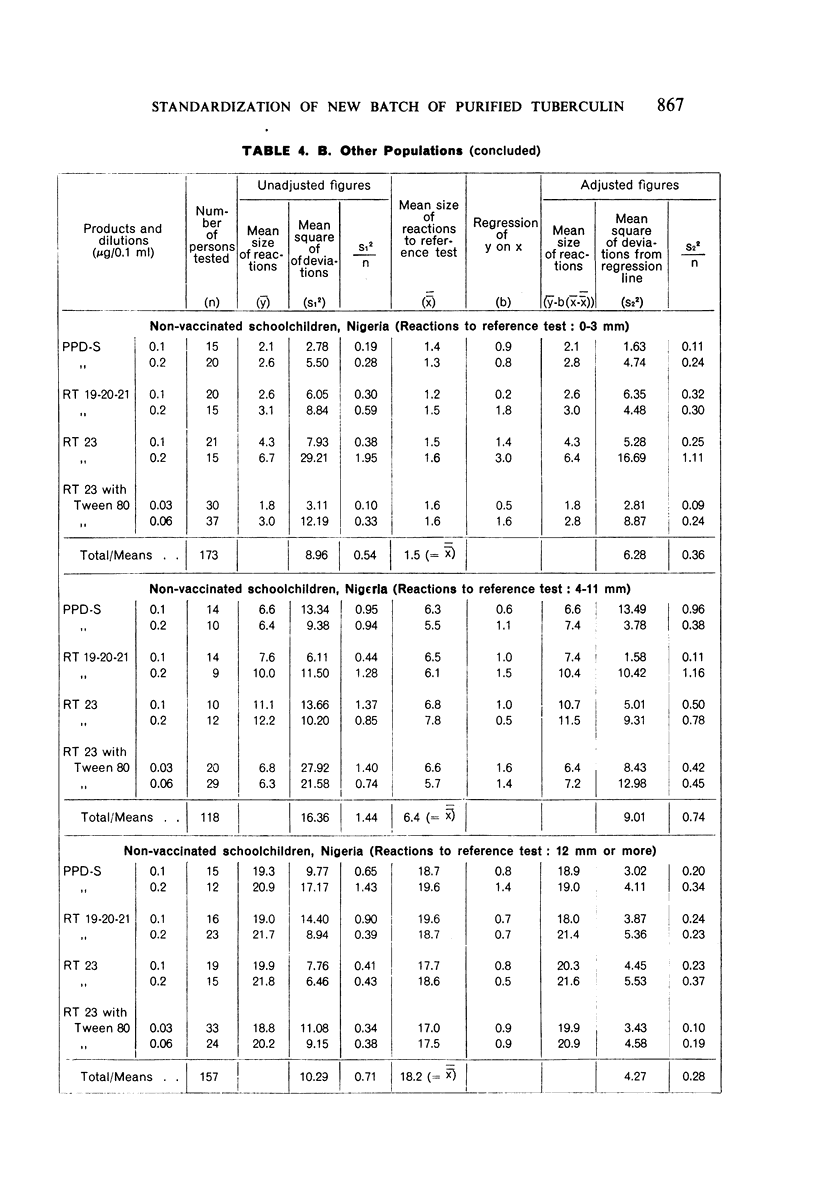
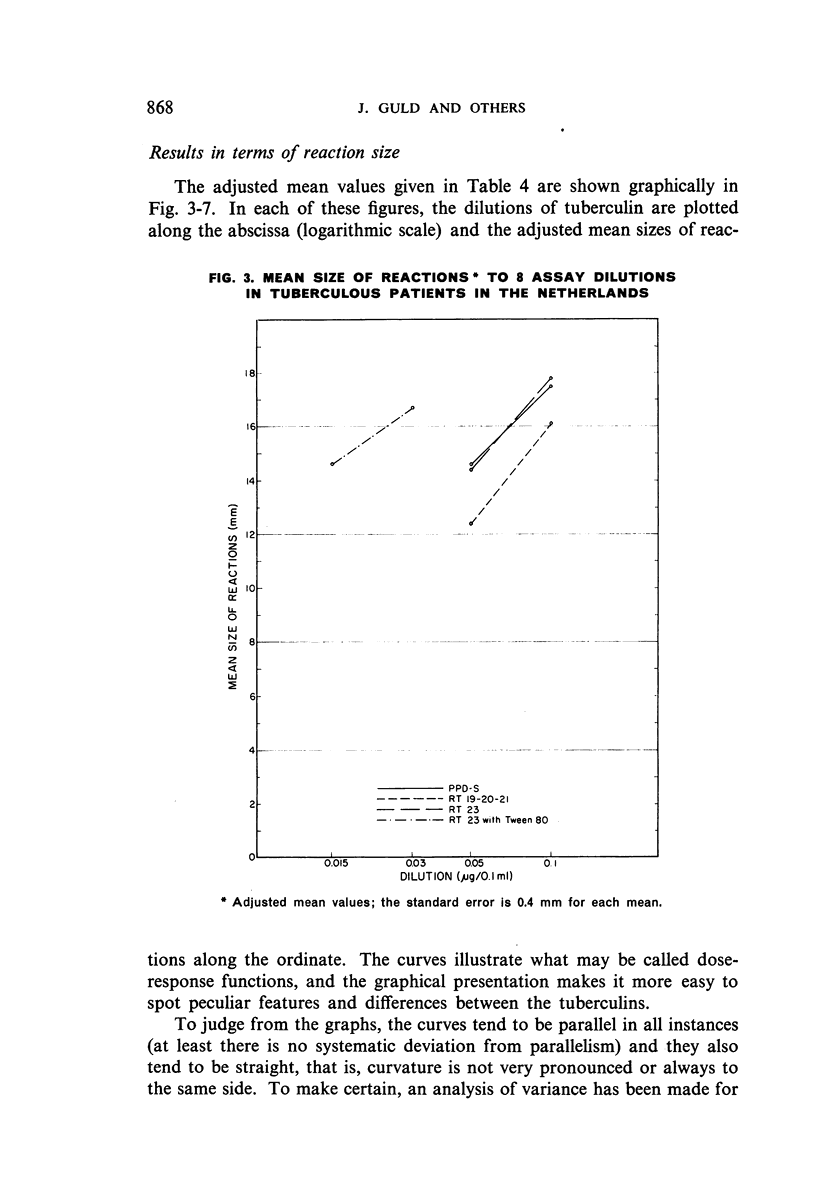
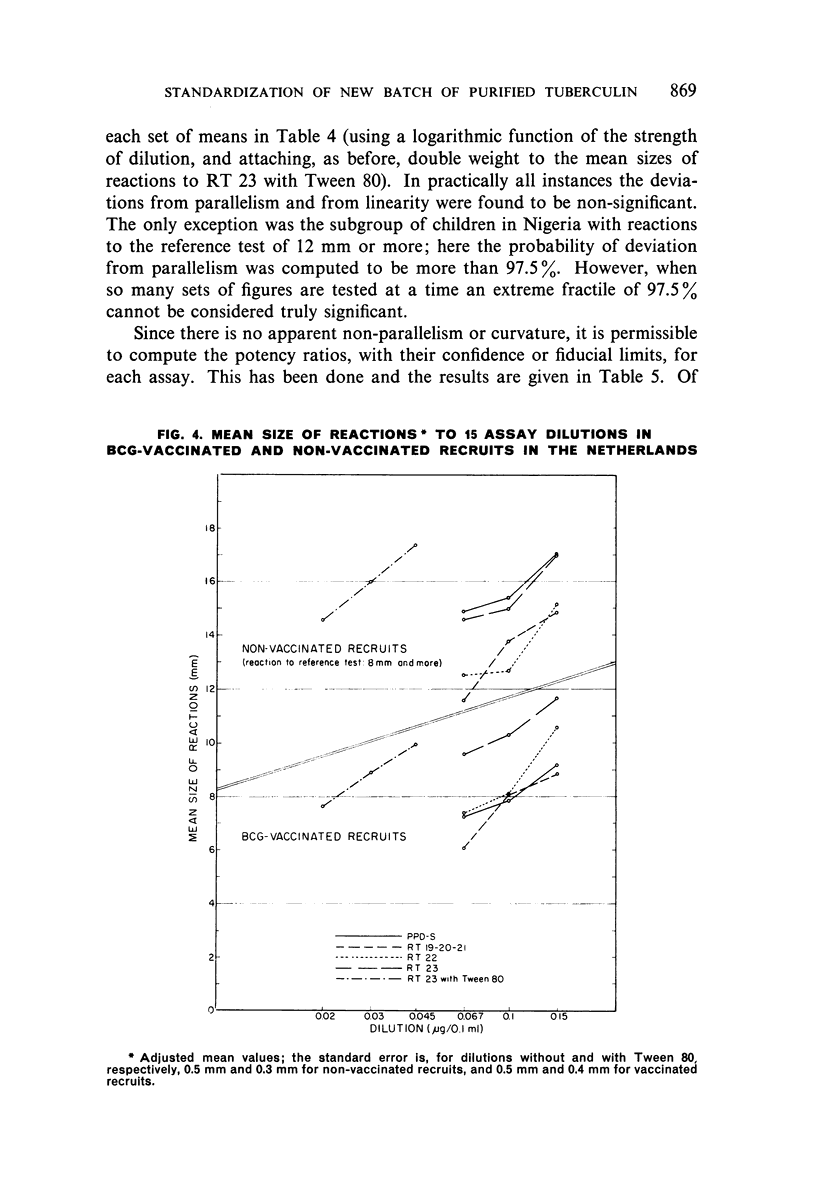

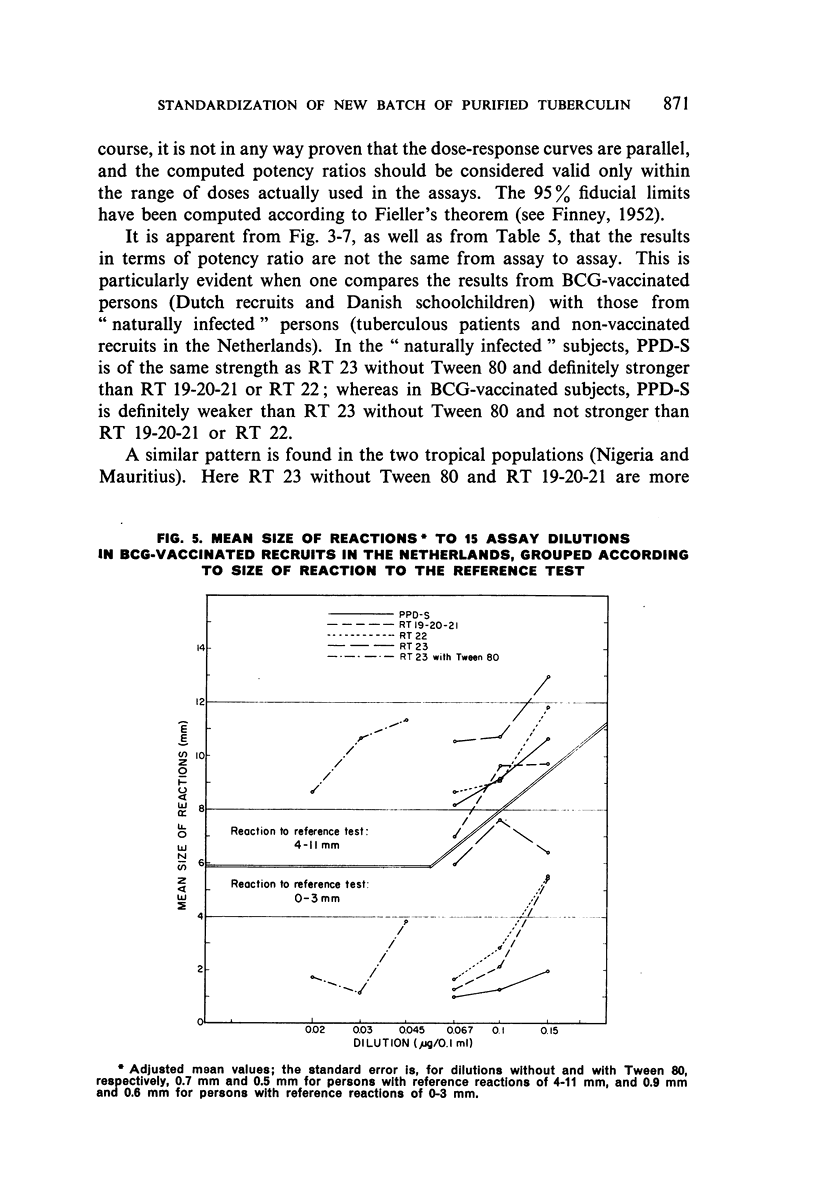























































Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GRIEP W. A., BLEIKER M. A. De reactie van Mantoux. Ned Tijdschr Geneeskd. 1957 Dec 28;101(52):2452–2471. [PubMed] [Google Scholar]
- NISSEN MEYER S. A method for standardization of tuberculin preparations by intracutaneous reactions in humans; comparison of two purified tuberculins. Am Rev Tuberc. 1952 Sep;66(3):292–313. [PubMed] [Google Scholar]
- PALMER C. E., FEREBEE S. H., PETERSEN O. S. Studies of pulmonary findings and antigen sensitivity among student nurses; geographic differences in sensitivity to tuberculin as evidence of nonspecific allergy. Public Health Rep. 1950 Sep 1;65(35):1111–1131. [PMC free article] [PubMed] [Google Scholar]


