Abstract
Significant differences between animal and human pharmacokinetics may be responsible for the conflicting results of experimental studies. This study determined the impact of human pharmacokinetic simulation (HPS) on gentamicin activity in an Enterococcus faecalis endocarditis model. The decrease in bacterial counts was greater with HPS than with a dose-equivalent regimen without HPS.
Enterococcal organisms are a frequent cause of nosocomial infections (2, 3, 23, 25, 32, 35). While a synergistic combination involving a cell wall-active agent with an aminoglycoside is customarily recommended (6, 25), recent reports suggest a genuine activity of the latter alone (17, 30). Interspecies variations in metabolism and pharmacokinetic properties have been observed for the same drug (15, 20, 29). Thus, experimental models with animal native kinetics may not ensure reliable assessment for human therapeutics (11), and the choice of treatment schedule can have a dramatic influence, especially in the field of antibacterial agents (33). Notably, the elimination rate is usually faster in small animals than in humans (8, 13, 21, 31). This may explain the poor results obtained in experimental models. To circumvent this drawback, the dosage in the animal is often increased according to the clearance ratio, resulting in a similar area under the concentration-time curve (AUC), but at the expense of a considerable rise in the peak.
The purpose of the present study was to compare this latter dosage adjustment with a true simulation of the human pharmacokinetics (HPS), as previously described (1, 14, 22, 36), and to determine the impact of HPS on the antibacterial effect of gentamicin in an experimental model of enterococcal endocarditis.
(This work was presented in part at the 15th Annual Congress of the European Society of Intensive Care Medicine, Barcelona, Spain, 29 September to 2 October 2002.)
In vitro studies.
MICs and MBCs of gentamicin (Schering-Plough Laboratories, Paris, France) were determined by the microdilution technique in Mueller-Hinton broth (MHB) and in MHB with serum (MHBS) for the two clinical strains of Enterococcus faecalis studied, HM 1061 and JH2-2 (16, 17). For both strains, MICs and MBCs were identical, equal to 8 and 4 mg/liter in MHB and MHBS, respectively (24).
In vivo studies.
In vivo studies were carried out with New Zealand White female rabbits (CEGAV, St. Mars-d'Egrenne, France) approved by the animal study committee. Experimental endocarditis was induced as previously described (9, 26) with an inoculum of 108 CFU of either E. faecalis HM 1061 or E. faecalis JH2-2. For each strain, animals were randomly assigned to nine groups, including an untreated control group and eight therapeutic groups, according to a crossed design with doses of 16.6 or 33.2 mg/kg of body weight/day producing either native or human-like pharmacokinetics for 3 or 5 days. The doses administered corresponded to those required to reproduce an AUC in the rabbit similar to that obtained in humans with dosages of 3 or 6 mg/kg/day, respectively. Gentamicin was diluted in 9% sodium chloride for infusion in a marginal ear vein. Animals treated with native pharmacokinetics received the dose in a 30-min infusion. Animals treated by HPS received a similar dose of gentamicin by means of a computer-controlled pump regulated to reproduce in the rabbit the plasma pharmacokinetics observed in humans (4, 5). The intended serum drug concentration profile was based on a one-compartment simplification of human pharmacokinetics without reproduction of the initial distribution α phase after an intravenous bolus. Thus, it consisted of a single exponential decrease with a half-life of 2 h and an initial concentration of 18 or 35 mg/liter for the human-like doses of 3 or 6 mg/kg, respectively.
Gentamicin was assayed by an immunoenzymatic method (Emit 2000 gentamicin test; Dade Behring, Paris, France), with a detection threshold of 0.3 mg/liter. The intra-assay coefficient of variation was between 1.7 and 3.2%, and the interassay coefficient was between 3.7 and 4.3%. Pharmacokinetic parameters were calculated by nonlinear regression according to a two-compartment model for native kinetics and a one-compartment model for HPS kinetics, according to the expected simulation of a monoexponential decay. The pharmacokinetic parameters are indicated in Table 1 for each mode of gentamicin administration. Figure 1 shows the native and human-like plasma kinetics actually observed in rabbits and the theoretical human pharmacokinetics. Residual plasma drug concentrations after 3 and 5 days of treatment with gentamicin at 33 mg/kg/day in HPS were 0.65 ± 0.06 and 0.47 ± 0.12 mg/liter, respectively. The AUC was calculated by the least-squares regression method. Animals were sacrificed (100 mg of thiopental) at the end of 3 or 5 days of treatment for bacterial count in the vegetation. Quantitation was performed after a 24-h culture on Trypticase soy agar plates of serial dilutions of a homogenate of the vegetations. The limit of detection was 2 log10 CFU/g of vegetation. No significant carryover effect was expected, because the gentamicin concentrations anticipated in serum and vegetation were far less than the MIC.
TABLE 1.
Pharmacokinetic results (Cmax, Cmax/MIC, β half-life, and AUC) for the four modes of gentamicin administration in this study
| Total daily dose (mg/kg) | Pharmacokinetics | Cmax (mg/liter) | Cmax/MIC | β half-life (h) | AUC (mg/h/liter) |
|---|---|---|---|---|---|
| 16.6 | HPS (3 mg/kg/day) | 22.4 ± 6.8 | 1.4 | 2.03 ± 0.46 | 64.0 ± 17.4 |
| Native | 64.4 ± 6.6 | 4 | 0.98 ± 0.19 | 77.6 ± 5.2 | |
| 33.2 | HPS (6 mg/kg/day) | 48.7 ± 4.0 | 3 | 1.63 ± 0.29 | 115.70 ± 30.8 |
| Native | 90.1 ± 8.0 | 5.6 | 0.97 ± 0.12 | 109.2 ± 7.5 |
FIG. 1.
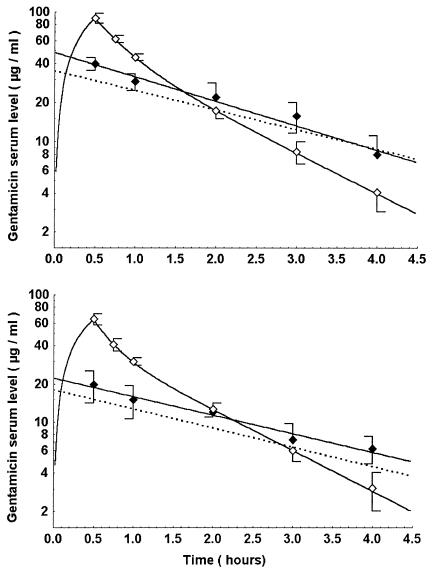
Pharmacokinetic profiles for 3 (lower panel) and 6 (upper panel) mg of gentamicin/kg/day in humans (dashed line) and the corresponding doses reproducing equivalent AUCs (16 and 33 mg/kg) in the rabbit according to a 30-min infusion with native pharmacokinetics (open symbols) or with human pharmacokinetic simulation of an intravenous bolus (solid symbols).
Experimental groups were compared by analysis of variance plus a Bonferroni test for intergroup comparisons (Statview; Abacus Concepts). A notable difference was observed for the HM 1061 strain (Table 2), depending on whether human kinetics was simulated or not. Gentamicin administration with native kinetics did not reduce the bacterial count below 7 log10 CFU/g of vegetation, regardless of the dose and treatment period. However, animals receiving gentamicin with HPS had a bacterial count of less than 4 log10 CFU/g of vegetation, regardless of the dose and treatment period.
TABLE 2.
Mean antibacterial effect of gentamicin infusion in vivo with native pharmacokinetics or HPS after 3 or 5 days of treatment against E. faecalis HM 1061 and JH2-2 in an experimental rabbit endocarditis model
| Expt group
|
Mean ± SD log10 CFU/g of vegetation (no. of animals)a
|
||||
|---|---|---|---|---|---|
| HM 1061
|
JH2-2
|
||||
| Dose (mg/kg/day) | Pharmacokinetics | 3 days | 5 days | 3 days | 5 days |
| 0 | Controls | 9.1 ± 0.4 (13) | 9.1 ± 0.4 (13) | 8.5 ± 0.5 (9) | 8.5 ± 0.5 (9) |
| 16.6 | HPS (3 mg/kg/day) | 3.8 ± 0.6 (7)*† | 3.1 ± 0.7 (5)*† | 6.0 ± 0.8 (7)*† | 3.4 ± 1.4 (6)*† |
| 16.6 | Native | 8.7 ± 0.6 (5) | 8.1 ± 0.8 (5)† | 8.2 ± 0.4 (6) | 7.1 ± 0.3 (6) |
| 33.2 | HPS (6 mg/kg/day) | 3.5 ± 0.2 (6)*† | 2.9 ± 0.1 (5)*† | 6.5 ± 0.4 (5)† | 4.8 ± 1.0 (6)† |
| 33.2 | Native | 7.7 ± 0.7 (6)† | 7.1 ± 0.6 (5)† | 7.4 ± 0.4 (5) | 6.2 ± 1.5 (6)† |
* and †, P < 0.0001 (Bonferroni's test) versus homologue native kinetics and versus controls, respectively.
For strain JH2-2 (Table 2), the antibacterial effect of gentamicin was on the whole less marked than for HM 1061 in groups receiving the antibiotic in HPS, but the effects were comparable in groups with native kinetics. However, administration of gentamicin with HPS was followed by a greater reduction of bacterial counts (0.9 to 3.7 log10 CFU/g of vegetation) than in corresponding groups receiving the same dose without simulation.
In experimental endocarditis (Table 2), a lower bacterial count was observed in animal groups receiving gentamicin with HPS versus native kinetics.
In an enterococcal endocarditis model, Vazquez et al. observed no reduction of bacteria with a daily dose of 6 mg/kg administered in two intramuscular injections during 3 days of treatment (34). In the study by Sullam et al., the bacterial count was reduced by 1 log CFU/g after 4.5 days of treatment (30), whereas in that of Lefort et al., the reduction was 1.3 log after 5 days of treatment (17). Our results are in complete agreement with these findings. Other studies in which this aminoglycoside was evaluated in the treatment of experimental endocarditis with HPS are not informative about its intrinsic activity in monotherapy, because it was associated with ampicillin (11, 18), teicoplanin (18), or penicillin (19).
In our study, pairwise comparison (same antibiotic and same treatment period) of groups with an equivalent AUC showed that the antibacterial effect was definitely improved with HPS. The comparison clearly indicates that an increase in the total daily dose (native kinetics) to compensate for faster elimination of gentamicin was not adequate for reliable prediction of the effects of the drug obtained in vivo with pharmacokinetics equivalent to that of humans (HPS). All pharmacokinetic parameters need to be taken into account.
Drug half-life is markedly shorter for small laboratory animals than for humans: the plasma half-lives of gentamicin are 22 min in the mouse (13), 0.6 h in the rat (31), 1 h in the rabbit (8), and 2 h in humans (21). Thus, it is not possible, on the basis of the native kinetic characteristics of the animal, to reproduce both an AUC and a maximum concentration of drug in serum (Cmax) equivalent to those of humans. For gentamicin, administration of the dose used in humans (3 mg/kg/day) produces an equivalent Cmax, whereas the AUC is much lower according to the clearance ratio.
The impact of these kinetic differences on the efficacy of aminoglycosides was already suggested in the studies of Potel et al. performed before and after the development of a model with HPS. Bacteriological results differed depending on the use of HPS or not (5, 27, 28). Similar discrepancies concerning aminoglycoside activity against gram-negative strains have been reported in comparisons of animal and human pharmacokinetics (7). In our study, the pharmacokinetic parameters of animals receiving gentamicin with HPS were comparable to those for humans (AUC, Cmax, and half-life) and in agreement with the results obtained by Gavalda et al. with a similar simulation model (12). Without HPS, the peak level increased and the half-life decreased. As a result, bacterial killing was greater with HPS. Most experimental studies in the past have designated maximal concentration and the AUC as good predictors for aminoglycoside activity. However, our study emphasizes the essential impact of half-life, which was obviously neglected in previous investigations based on a single species, with all animals sharing the same half-life. Furthermore, the positive impact of serum half-life on the duration of the postantibiotic effect has been previously documented (10), possibly explaining in part the results observed in this work.
In summary, this study clearly shows the importance of using an infectious animal model simulating human pharmacokinetics. This approach appears to be more reliable than others for assessment of the activity of antibiotics in the context of experimental infection and extrapolation of the results to human therapeutics.
Acknowledgments
This work was supported in part by grants from the Nantes Medical School, Nantes, France.
REFERENCES
- 1.Andes, D., and W. A. Craig. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob. Agents Chemother. 42:2375-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonten, M. J., F. H. van Tiel, S. van der Geest, E. E. Stobberingh, and C. A. Gaillard.1993. Enterococcus faecalis pneumonia complicating topical antimicrobial prophylaxis. N. Engl. J. Med. 328:209-210. [DOI] [PubMed] [Google Scholar]
- 3.Bonten, M. J. M., C. A. Gaillard, F. H. van Tiel, S. van der Geest, and E. E. Stobberingh. 1995. Colonization and infection with Enterococcus faecalis in intensive care units: the role of antimicrobial agents. Antimicrob. Agents Chemother. 39:2783-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bugnon, D., G. Potel, J. Caillon, D. Baron, H. B. Drugeon, P. Feigel, and M. F. Kergueris. 1998. In vivo simulation of human pharmacokinetics in the rabbit. Bull. Math. Biol. 60:545-567. [DOI] [PubMed] [Google Scholar]
- 5.Bugnon, D., G. Potel, Y. Q. Xiong, J. Caillon, M. F. Kergueris, P. Le Conte, D. Baron, and H. Drugeon. 1996. In vivo antibacterial effects of simulated human serum profiles of once-daily versus thrice-daily dosing of amikacin in a Serratia marcescens endocarditis experimental model. Antimicrob. Agents Chemother. 40:1164-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow, J. W. 2000. Aminoglycoside resistance in enterococci. Clin. Infect. Dis. 31:586-589. [DOI] [PubMed] [Google Scholar]
- 7.Craig, W. A., J. Redington, and S. C. Ebert. 1991. Pharmacodynamics of amikacin in vitro and in mouse thigh and lung infections. J. Antimicrob. Chemother. 27(Suppl. C):29-40. [DOI] [PubMed] [Google Scholar]
- 8.Curl, J. L., and J. S. Curl. 1988. Pharmacokinetics of gentamicin in laboratory rabbits. Am. J. Vet. Res. 49:2065-2067. [PubMed] [Google Scholar]
- 9.Durack, D. T., and P. B. Beeson. 1972. Experimental bacterial endocarditis. I. Colonization of a sterile vegetation. Br. J. Exp. Pathol. 53:44-49. [PMC free article] [PubMed] [Google Scholar]
- 10.Fantin, B., S. Ebert, J. Leggett, B. Volgeman, and W. A. Craig. 1990. Factors affecting duration of in-vivo postantibiotic effect for aminoglycosides against Gram-negative bacilli. J. Antimicrob. Chemother. 27:829-836. [DOI] [PubMed] [Google Scholar]
- 11.Gavalda, J., P. J. Cardona, B. Almirante, J. A. Capdevila, M. Laguarda, L. Pou, E. Crespo, C. Pigrau, and A. Pahissa. 1996. Treatment of experimental endocarditis due to Enterococcus faecalis using once-daily dosing regimen of gentamicin plus simulated profiles of ampicillin in human serum. Antimicrob. Agents Chemother. 40:173-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gavalda, J., P. López, T. Martin, X. Gomis, J. L. Ramirez, C. Azuaje, B. Almirante, and A. Pahissa. 2002. Efficacy of ceftriaxone and gentamicin given once a day by using human-like pharmacokinetics in treatment of experimental staphylococcal endocarditis. Antimicrob. Agents Chemother. 46:378-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerber, A. U., H. P. Brugger, C. Feller, T. Stritzko, and B. Stalder. 1986. Antibiotic therapy of infections due to Pseudomonas aeruginosa in normal and granulocytopenic mice: comparison of murine and human pharmacokinetics. J. Infect. Dis. 153:90-97. [DOI] [PubMed] [Google Scholar]
- 14.Gerber, A. U., T. Stritzko, C. Segessenmann, and B. Stalder. 1990. Simulation of human pharmacokinetic profiles in mice, and impact on antimicrobial efficacy of netilmicin, ticarcillin and ceftazidime in the peritonitis-septicemia model. Scand. J. Infect. Dis. Suppl. 74:195-203. [PubMed] [Google Scholar]
- 15.Kirkwood, J. K., and J. Merriam. 1990. Variation in plasma half life of gentamicin between species in relation to body weight and taxonomy. Res. Vet. Sci. 49:160-165. [PubMed] [Google Scholar]
- 16.Leclercq, R., and J. Duval. 1995. Activité bactéricide des associations de ticacilline-acide clavulanique ou d'autres β-lactamines (amoxicilline, piperacilline-tazobactam, ticarcilline) avec la gentamicine vis-à-vis des Enterococcus faecalis et E. faecium. Pathol. Biol. (Paris) 43:245-252. [PubMed] [Google Scholar]
- 17.Lefort, A., M. Arthur, L. Garry, C. Carbon, P. Courvalin, and B. Fantin. 2000. Bactericidal activity of gentamicin against Enterococcus faecalis in vitro and in vivo. Antimicrob. Agents Chemother. 44:2077-2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.López, P., J. Gavalda, M. T. Martin, B. Almirante, X. Gomis, C. Azuaje, N. Borrell, L. Pou, V. Falcó, C. Pigrau, and A. Pahissa. 2001. Efficacy of teicoplanin-gentamicin given once a day on the basis of pharmacokinetics in humans for treatment of enterococcal experimental endocarditis. Antimicrob. Agents Chemother. 45:1387-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marangos, M. N., D. P. Nicolau, R. Quintiliani, and C. H. Nightingale. 1997. Influence of gentamicin dosing interval on the efficacy of penicillin-containing regimens in experimental Enterococcus faecalis endocarditis. J. Antimicrob. Chemother. 39:519-522. [DOI] [PubMed] [Google Scholar]
- 20.Martin-Jimenez, T., and J. E. Riviere. 2001. Mixed effects modeling of the disposition of gentamicin across domestic animal species. J. Vet. Pharmacol. Ther. 24:321-332. [DOI] [PubMed] [Google Scholar]
- 21.Meunier, F., P. Van der Auwera, H. Schmitt, V. de Maertelaer, and J. Klastersky. 1987. Pharmacokinetics of gentamicin after i.v. infusion or i.v. bolus. J. Antimicrob. Chemother. 19:225-231. [DOI] [PubMed] [Google Scholar]
- 22.Mizen, L., G. Woodnutt, I. Kernutt, and E. J. Catherall. 1989. Simulation of human serum pharmacokinetics of ticarcillin-clavulanic acid and ceftazidime in rabbits, and efficacy against experimental Klebsiella pneumoniae meningitis. Antimicrob. Agents Chemother. 33:693-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moellering, R. C. 2000. Enterococcus species, Streptococcus bovis, and Leuconostoc species, p. 2147-2156. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases. Churchill Livingstone, New York, N.Y.
- 24.National Committee for Clinical Laboratory Standards. 1992. Methods for determining bactericidal activity of antimicrobial agents. Tentative guideline M26-T. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 25.Patterson, J. E., A. H. Sweeney, M. Simms, N. Carley, R. Mangi, J. Sabetta, and R. W. Lyons. 1995. An analysis of 110 serious enterococcal infections. Epidemiology, antibiotic susceptibility, and outcome. Medicine (Baltimore) 74:191-200. [DOI] [PubMed] [Google Scholar]
- 26.Perlman, B. B., and L. R. Freedman. 1971. Experimental endocarditis. II. Staphylococcal infection of the aortic valve following placement of a polyethylene catheter in the left side of the heart. Yale J. Biol. Med. 44:206-213. [PMC free article] [PubMed] [Google Scholar]
- 27.Potel, G., J. Caillon, B. Fantin, J. Raza, F. Le Gallou, J.-Y. Lepage, P. Le Conte, D. Bugnon, D. Baron, and H. Drugeon. 1991. Impact of dosage schedule on the efficacy of gentamicin, tobramycin, or amikacin in an experimental model of Serratia marcescens endocarditis: in vitro-in vivo correlation. Antimicrob. Agents Chemother. 35:111-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potel, G., J. Caillon, F. Le Gallou, D. Bugnon, P. Le Conte, J. Raza, J.-Y. Lepage, D. Baron, and H. Drugeon. 1992. Identification of factors affecting in vivo aminoglycoside activity in an experimental model of gram-negative endocarditis. Antimicrob. Agents Chemother. 36:744-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riviere, J. E., T. Martin-Jimenez, S. F. Sundlof, and A. L. Craigmill. 1997. Interspecies allometric analysis of the comparative pharmacokinetics of 44 drugs across veterinary and laboratory animal species. J. Vet. Pharmacol. Ther. 20:453-463. [DOI] [PubMed] [Google Scholar]
- 30.Sullam, P. M., M. G. Täuber, C. J. Hackbarth, and M. A. Sande. 1985. Antimicrobial activity of gentamicin in experimental enterococcal endocarditis. Antimicrob. Agents Chemother. 27:224-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swenson, C. E., K. A. Stewart, J. L. Hammett, W. E. Fitzsimmons, and R. S. Ginsberg. 1990. Pharmacokinetics and in vivo activity of liposome-encapsulated gentamicin. Antimicrob. Agents Chemother. 34:235-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tailor, S. A., E. M. Bailey, and M. J. Rybak. 1993. Enterococcus, an emerging pathogen. Ann. Pharmacother. 27:1231-1242. [DOI] [PubMed] [Google Scholar]
- 33.Tam, V. H., P. S. Mckinnon, D. P. Levine, S. M. Brandel, and M. J. Rybak. 2000. Once-daily aminoglycoside in the treatment of Enterococcus faecalis endocarditis: case report and review. Pharmacotherapy 20:1116-1119. [DOI] [PubMed] [Google Scholar]
- 34.Vazquez, J., M. B. Perri, L. A. Thal, S. A. Donabedian, and M. J. Zervos. 1993. Sparfloxacin and clinafloxacin alone or in combination with gentamicin for therapy of experimental ampicillin-resistant enterococcal endocarditis in rabbits. J. Antimicrob. Chemother. 32:715-721. [DOI] [PubMed] [Google Scholar]
- 35.Wiedemann, B., and H. Grimm. 1996. Susceptibility to antibiotics: species incidence and trends, p. 900-1168. In V. Lorian (ed.), Antibiotics in laboratory medicine. Williams & Wilkins, Baltimore, Md.
- 36.Woodnutt, G., V. Berry, and L. Mizen. 1992. Simulation of human serum pharmacokinetics of cefazolin, piperacillin, and BRL 42715 in rats and efficacy against experimental intraperitoneal infections. Antimicrob. Agents Chemother. 36:1427-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]


