Abstract
Arcanobacterium pyogenes, a commensal on the mucous membranes of many economically important animal species, is also a pathogen, causing abscesses of the skin, joints, and visceral organs as well as mastitis and abortion. In food animals, A. pyogenes is exposed to antimicrobial agents used for growth promotion, prophylaxis, and therapy, notably tylosin, a macrolide antibiotic used extensively for the prevention of liver abscessation in feedlot cattle in the United States. Of 48 A. pyogenes isolates, 11 (22.9%) exhibited inducible or constitutive resistance to tylosin (MIC of ≥128 μg/ml). These isolates also exhibited resistance to other macrolide and lincosamide antibiotics, suggesting a macrolide-lincosamide resistance phenotype. Of the 11 resistant isolates, genomic DNA from nine hybridized to an erm(X)-specific probe. Cloning and nucleotide sequencing of the A. pyogenes erm(X) gene indicated that it was >95% similar to erm(X) genes from Corynebacterium and Propionibacterium spp. Eight of the erm(X)-containing A. pyogenes isolates exhibited inducible tylosin resistance, which was consistent with the presence of a putative leader peptide upstream of the erm(X) open reading frame. For at least one A. pyogenes isolate, 98-4277-2, erm(X) was present on a plasmid, pAP2, and was associated with the insertion sequence IS6100. pAP2 also carried genes encoding the repressor-regulated tetracycline efflux system determinant Tet 33. The repA gene from pAP2 was nonfunctional in Escherichia coli and at least one A. pyogenes isolate, suggesting that there may be host-encoded factors required for replication of this plasmid.
The use of antimicrobial agents as feed additives for disease prophylaxis and growth promotion in the beef cattle industry is a common practice in the United States. One of the major targets is liver abscessation, which is second only to respiratory disease in terms of economic losses to cattle feedlots. Control of liver abscesses has primarily depended on the use of feed additives, such as the macrolide tylosin, chlortetracycline, and oxytetracycline (10, 26), with tylosin the most effective and commonly used feed additive (11). A study involving almost 7,000 feedlot cattle demonstrated that the use of tylosin as a feed additive reduced the incidence of liver abscessation by 73% and increased weight gain and feed conversion by 2.3 and 2.6%, respectively (G. J. Vogel and S. B. Laudert, abstract, J. Anim. Sci. 72[Supp. 1]:293, 1994). Correspondingly, tylosin use is extensive, with 42.3% of feedlot cattle receiving tylosin as a feed additive, primarily for the prevention of liver abscesses (26).
Fusobacterium necrophorum is the primary etiological agent of bovine hepatic abscessation (18), but Arcanobacterium pyogenes is a synergistic pathogen, being found in 10 (9) to 90% (12) of abscesses. Interestingly, in cattle fed tylosin, the incidence of hepatic abscesses containing A. pyogenes increased from 10 to 53% (9).
The resistance of A. pyogenes to tylosin has only recently been documented. In a survey of A. pyogenes strains conducted in our laboratory, we observed that resistance of A. pyogenes to tylosin was prevalent, with 22.9% of isolates tested (n = 48) having MICs of tylosin of ≥64 μg/ml (25). In addition, for the tylosin-resistant strains, MICs of a wide spectrum of macrolide and lincosamide antimicrobial agents were increased (25), suggesting a macrolide, lincosamide, and streptogramin B resistance phenotype. However, the mechanism(s) of resistance to tylosin in A. pyogenes was not investigated.
In this study, we report the identification and characterization of a plasmid-encoded Erm X determinant in a tylosin-resistant A. pyogenes isolate.
MATERIALS AND METHODS
Bacteria and growth conditions.
The 48 A. pyogenes strains used in this study were field isolates obtained from veterinary diagnostic laboratories or personal collections. These strains were isolated from cattle (n = 27), swine (n = 17), dogs (n = 2), a bird (n = 1), and a cat (n = 1). Specifically, strain BBR1 was isolated from a bovine abscess and strain 98-4277-2 was isolated from a case of bovine mastitis. A. pyogenes strains were grown on brain heart infusion (BHI) (Difco) agar plates, supplemented with 5% bovine blood, at 37°C and 5% CO2, or in BHI broth supplemented with 5% bovine calf serum (Omega Scientific Inc.) at 37°C with shaking. Escherichia coli DH5αMCR strains (Gibco-BRL) were grown at 37°C on Luria-Bertani (Difco) agar or in Luria-Bertani broth with shaking. Antibiotics were added as appropriate for A. pyogenes (tylosin, 15 μg/ml; kanamycin [KM], 30 μg/ml; tetracycline [TC], 1 μg/ml) and for E. coli (chloramphenicol, 30 μg/ml; erythromycin, 200 μg/ml; KM, 50, μg/ml; and TC, 2 μg/ml).
DNA techniques.
Genomic DNA from A. pyogenes was isolated by the method of Pospiech and Neumann (14). E. coli plasmid DNA extraction, transformation, DNA restriction, ligation, agarose gel electrophoresis, and Southern transfer of DNA to nylon membranes were performed essentially as described previously (2). Preparation of DNA probes using oligonucleotide primers designed to specific genes, DNA hybridization, and probe detection were performed using the digoxigenin (DIG) DNA labeling and detection kit (Roche), as recommended by the manufacturer. PCR DNA amplification was performed using TaqDNA polymerase (Promega) with the supplied reaction buffer for 35 cycles consisting of 1 min at 94°C, 1 min at 55°C, and 1 min/kb at 72°C, with a final extension step of 72°C for 5 min. The plasmids and oligonucleotide primers used in this study are shown in Tables 1 and 2, respectively.
TABLE 1.
Plasmids used in this study
| Plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| pAP2 | 9.3-kb A. pyogenes plasmid, erm(X), tetA(33) | This work |
| pBC KS | 3.4-kb, ColE1 origin, Cmr | Stratagene |
| pEP2 | 3.1-kb derivative of pNG2, Kmr, replicates in A. pyogenes | 15 |
| pJGS392 | pBC KS HindIII Ω 3.2-kb HindIII fragment of pAP2 carrying erm(X), Cmr Emr | This work |
| pJGS406 | pEP2 SalI-BamHI Ω 2.8-kb SalI-BamHI fragment of pJGS392 carrying erm(X), Emr Kmr | This work |
| pJGS446 | pBC KS SacI Ω 8.4-kb SacI fragment of pAP2, Cmr Tcr | This work |
| pJGS551 | pBC KS HindIII Ω 6.2-kb HindIII fragment of pAP2, Cmr Tcr | This work |
| pNG2 | 15.1-kb C. diphtheriae plasmid, erm(X), replicates in A. pyogenes | 19 |
TABLE 2.
Sequences of oligonucleotide primers used in this study
| Primer | Sequence (5′-3′) | Gene | Position (bp) | Size (bp) |
|---|---|---|---|---|
| ermA5 | CACCCGCCTCTAGAAATAATAG | erm(X) | −243-788 | 1,031 |
| ermA3 | CGATGGTGATCTAGAGAGGAAC | erm(X) | ||
| ermX28 | GGCTCTGTAACGTCTGCCGCGC | repA | 68-291 | 224 |
| ermX29 | TGTCCCGGCCCAAGTCCTCACC | repA | ||
| ermX12 | GATGCCGATTCTTCCGCACTGC | tetA(33) | 84-1224 | 1,089 |
| ermX19 | CCACGCATGATGAGAATCACGC | tetA(33) | ||
| ermX8 | TTGGACGGACGGAACGATGACG | tnpA | 15-736 | 722 |
| ermX2A | GCCCAATGCCAAAAGCTCTCTC | tnpA | ||
| ermX1 | GTTGCGCTCTAACCGCTAAGGC | erm(X) | −205-453 | 657 |
| ermX5 | CCATGGGGACCACTGAGCCGTC | erm(X) |
Nucleotide sequence determination.
The sequence of the erm(X) gene region was determined from pJGS392, pJGS466, and pJGS551 and their subclones, using automated DNA sequencing. Sequencing was performed on both strands, crossing all restriction sites, using KS, SK, T3, or T7 sequencing primers or oligonucleotide primers designed to the sequence of the erm(X) gene region. Sequencing reactions were performed by the Genomic Analysis and Technology Core at The University of Arizona, using a 377 DNA sequencer (Applied Biosystems Inc.).
Computer sequence analysis.
Nucleotide sequence data were compiled using the Sequencher program (GeneCodes). Database searches were performed using the BlastX and BlastP algorithms (1). Sequence analysis was performed using the suite of programs available through the Genetics Computer Group (Accelyrs). Multiple sequence alignments were performed using CLUSTAL W (24).
Determination of MICs.
Determination of MICs for A. pyogenes used National Committee for Clinical Laboratory Standards methodology (13), with the modifications described by Trinh et al. (25). The antimicrobial agents to be tested were diluted in a doubling dilution pattern over the range of 0.06 to 2,048 μg/ml in the wells of sterile, 96-well, round-bottom microtiter plates in 50-μl volumes. The MIC was read visually as the lowest concentration of the antimicrobial agent to prevent growth (turbidity), compared with the control (no antimicrobial agent added). Each isolate was tested in duplicate, on two separate occasions, and the endpoints for each antimicrobial agent did not differ. In order to determine MICs following induction, the A. pyogenes isolates were grown on BHI agar containing 5% bovine blood and 1 μg of the appropriate antimicrobial agent/ml, prior to MIC determination, as described above.
Nucleotide sequence accession number.
The sequence of pAP2 was submitted to the GenBank database under accession number AY255627.
RESULTS AND DISCUSSION
Identification and prevalence of erm(X) in A. pyogenes.
Of 48 A. pyogenes isolates previously tested, for 11 (22.9%), MICs of tylosin were ≥64 μg/ml (25). rRNA methylases, encoded by erm genes, are common determinants of macrolide resistance (16). More specifically, Erm X is a common determinant of macrolide resistance in the coryneform bacteria, to which A. pyogenes is related. A DIG-labeled probe to the erm(X) gene of the Corynebacterium diphtheriae plasmid, pNG2 (5), was generated (Table 2) and used to probe genomic DNA from 48 A. pyogenes isolates under high-stringency conditions. The DNA from 9 of the 11 tylosin-resistant, but none of the tylosin-susceptible, A. pyogenes isolates hybridized to the erm(X) probe (Fig. 1), suggesting that erm(X) is a predominant determinant of tylosin resistance in A. pyogenes.
FIG. 1.

Dot blot hybridization of A. pyogenes strains with an erm(X)-specific probe. Approximately 500 ng of genomic DNA from 48 A. pyogenes isolates was spotted onto a nylon membrane and hybridized with the erm(X)-specific probe under high-stringency conditions. The tylosin-resistant and -susceptible isolates are indicated. The two tylosin-resistant isolates which do not contain erm(X) are the first two dots on the first row.
The erm(X) gene confers inducible resistance to tylosin.
MICs of tylosin were determined for the 11 tylosin-resistant isolates, with and without induction. The erm(X)-containing strains generally exhibited inducible resistance to tylosin, with the exception of one strain, 2977, for which the uninduced MIC was already at the limit of detection (Table 3). For seven of the nine isolates, high-level uninduced MICs of tylosin were ≥128 μg/ml, which increased to >2,048 μg/ml upon induction with tylosin. For two of the erm(X)-containing strains, MICs were low at ≤8 μg/ml, which increased to 128 μg/ml following induction (Table 3). In comparison, for susceptible strains of A. pyogenes, MICs of tylosin were ≤0.06 μg/ml (25). The two tylosin-resistant, non-erm(X) containing strains, OX-1 and OX-7, did not exhibit inducible resistance to tylosin (Table 3). The resistance phenotypes observed among the erm(X)-containing strains varied considerably. This could be due to the presence of other resistance mechanisms, such as additional erm genes, genes encoding efflux pumps, or ribosomal mutations. In addition, any erm(X)-carrying plasmids could be present at variable copy number, giving rise to different MICs through a gene dosage effect.
TABLE 3.
MICs of tylosin for A. pyogenes strains with and without induction
| Strain | erm(X)a | MIC of tylosin (μg/ml) following:
|
|
|---|---|---|---|
| No induction | Induction with tylosin (1 μg/ml) | ||
| 3 | + | 256 | >2,048 |
| 4 | + | 256 | >2,048 |
| 2977 | + | >2,048 | >2,048 |
| 4759 | + | 128 | >2,048 |
| 14373-00-1 | + | 1,024 | >2,048 |
| 52789-99 | + | 8 | 128 |
| 98-4277-2 | + | 2 | 128 |
| B167 | + | 128 | >2,048 |
| E1DE | + | 128 | >2,048 |
| OX-1 | − | 128 | 128 |
| OX-7 | − | 128 | 128 |
| BBR1(pEP2) | − | ≤0.06 | NDb |
| BBR1(pJGS406) | + | 64 | 128 |
Presence and absence of the erm(X) gene is denoted by + and −, respectively.
Not determined, since BBR1(pEP2) will not grow on 1 μg of tylosin/ml.
The nine erm(X)-containing isolates were also tested to determine MICs of other macrolide and lincosamide antimicrobial agents. All the isolates showed increased MICs of erythromycin, oleandomycin, spiramycin, clindamycin, and lincomycin (data not shown), indicating that erm(X) confers a macrolide-lincosamide resistance phenotype. It is probable that the A. pyogenes erm(X) also confers resistance to the streptogramin B class of antimicrobial agents, as seen with other erm(X) genes (17). However, susceptibility to these agents was not tested.
Cloning and nucleotide sequence determination of erm(X).
A Southern blot of HindIII-digested 98-4277-2 genomic DNA was hybridized with the erm(X) probe under high-stringency conditions, and a 3.2-kb hybridizing band was identified (data not shown). A HindIII library of A. pyogenes 98-4277-2 genomic DNA was prepared in pBC KS (Stratagene) and introduced into E. coli DH5αMCR by electroporation. Clones carrying the erm(X) gene were identified by colony hybridization with the erm(X)-specific probe. One such clone, pJGS392, was selected for further analysis. pJGS392 contained a 3.2-kb HindIII insert, and sequence analysis revealed that erm(X) was indeed present in this clone. The A. pyogenes erm(X) gene had significant DNA identity with the erm(X) genes from the C. diphtheriae plasmid, pNG2 (97.7%; GenBank accession no. AF492560), the Corynebacterium striatum plasmid, pTP10 (97.5%; GenBank accession no. AF024666), and the Corynebacterium jeikeium and Propionibacterium acnes transposon, Tn5432 (94.7% and 97.5%; GenBank accession no. AF338705 and AF411029, respectively). The A. pyogenes Erm(X) protein shared 97.5% identity and 98.2% similarity with Erm(X) from pNG2. In order to confirm that erm(X) was responsible for the tylosin resistance phenotype, a 2.8-kb SalI-BamHI fragment of pJGS392, containing erm(X), was cloned into the KM resistance vector, pEP2 (15), which replicates in A. pyogenes (6). This plasmid, pJGS406, was used to transform A. pyogenes BBR1 to KM resistance, and the MIC for tylosin was determined. For BBR1(pEP2), the MIC of tylosin was ≤0.06 μg/ml, compared to that for BBR1(pJGS406) of 64 μg/ml, which increased to 128 μg/ml following induction with tylosin (Table 3). The higher uninduced MIC for BBR1(pJGS406) compared with 98-4277-2 may reflect that for BBR1(pJGS406), erm(X) is carried on a multicopy plasmid.
As with its homologues, upstream of the A. pyogenes erm(X) gene is an open reading frame (ORF) encoding a 15-amino-acid leader peptide (Fig. 2A), which has been postulated to be involved in translational attenuation similar to that seen with erm(C), leading to an inducible resistance phenotype (27). However, in strain 98-4277-2, there was an 86-bp duplication, including the ribosome binding site and the first 24 codons of erm(X), creating a small ORF, orf28, which overlaps stop codon to start codon with erm(X) (Fig. 2A). To determine whether this duplication was found in all erm(X)-containing isolates, PCR was used to amplify the region around the duplication. Primers ermX1 and ermX5 (Table 2) amplified a 657-bp product in strain 98-4277-2 (Fig. 2B). The other eight strains apparently did not possess this duplication, since the PCR products amplified were approximately 86 bp smaller (Fig. 2B). Furthermore, the PCR products from two of these strains were sequenced, confirming the lack of duplication (data not shown). The duplication in 98-4277-2 may explain the lower MIC of tylosin observed for this isolate (Table 3), since the duplication may alter any secondary structures in the leader peptide region of erm(X) which may be involved in transcriptional or translational attenuation. Similar duplications have been observed in other erm genes (7). However, these duplications relieve translational attenuation, such that gene expression is constitutive. This is not the case with the erm(X) gene from 98-4277-2, since it still retains its inducible phenotype. Additional studies will be required to determine the role of this duplication in induction and whether it acts at the transcriptional or translational level.
FIG. 2.
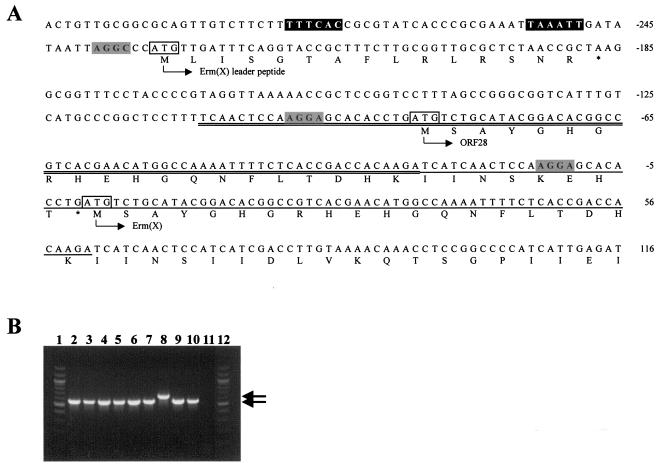
(A) The 98-4277-2 erm(X) leader peptide region. The nucleotide sequence of the erm(X) leader peptide region is shown, with ORF translations below the sequence, the start of which is denoted by the bent arrows and the ORF designations. The putative erm(X) promoter sequences are boxed in black, and the putative ribosome binding sites and start codons for each of the ORFs are boxed in gray or outlined, respectively. The 86-bp duplication is denoted by double underlining, with the corresponding sequence in erm(X) underlined. Nucleotides are numbered from the start of erm(X), as indicated to the right of the sequence. (B) PCR amplification of the leader peptide region of erm(X) using primers ermX1 and ermX5. The PCR products were visualized following electrophoresis in a 1.5% agarose gel. Lanes: 1 and 12, 100-bp ladder (Promega); 2, strain E1DE; 3, strain 4759; 4, strain 3; 5, strain 4; 6, strain 2977; 7, strain B167; 8, strain 98-4277-2; 9, strain 52785-99; 10, strain 14373-00-1; 11, no template (negative control). The 657-bp or 571-bp products are indicated by the arrows.
The A. pyogenes erm(X) gene in strain 98-4277-2 is carried on a plasmid.
Two additional overlapping clones were constructed in order to obtain DNA flanking the erm(X) gene region. pJGS466, containing an 8.4-kb SacI fragment (Fig. 3), was identified in a SacI library of 98-4277-2 genomic DNA in pBC KS by colony hybridization, using a DIG-labeled probe designed to the sequence of pJGS392 (data not shown). A HindIII library of 98-4277-2 genomic DNA in pBC KS was used to transform E. coli to TC resistance, which resulted in the identification of pJGS551, containing a 6.2-kb HindIII insert (Fig. 3). Nucleotide sequencing of pJGS466 and pJGS551 revealed that the erm(X) gene region was circularly permuted, indicating that erm(X) was carried on a plasmid. This plasmid was designated pAP2. The nucleotide sequence of the entire 9,304-bp pAP2 plasmid was determined, and the salient features of pAP2 are indicated on the restriction map in Fig. 3. In addition to erm(X) and its associated small ORFs, pAP2 contained six other ORFs, designated gcrY, repA, orf95, tetR(33), tetA(33), and tnpA, which was associated with the insertion sequence IS6100 (Fig. 3). IS6100 is found associated with integrons and antibiotic resistance genes in a variety of gram-negative and gram-positive bacteria, including Pseudomonas aeruginosa, Salmonella enterica, and Mycobacterium fortuitum, from where it was originally isolated (8).
FIG. 3.
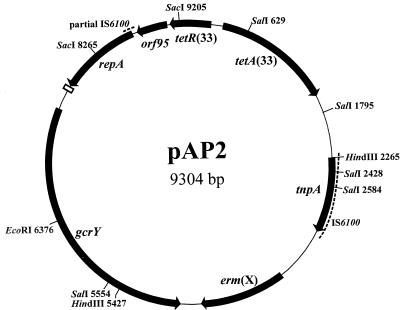
Schematic representation of pAP2. Sites for relevant restriction enzymes are shown, followed by their positions in base pairs from the zero point at the top of the map. The seven ORFs, tetR(33), tetA(33), tnpA, erm(X), gcrY, repA, and orf95, are indicated by the closed arrows. The 22-bp box element is depicted as an open rectangle. The dashed lines indicate IS6100 sequences.
A region of pAP2 from map coordinates 8585 to 3123 had 100% DNA identity with bases 21859 to 25701 of the Corynebacterium glutamicum plasmid pTET3 (GenBank accession no. NC_003227). This region encompassed the last 126 bp of the left IS6100, orf95, tetR(33), tetA(33), and the entire right IS6100 of the pTET3 IS6100-bound tet gene element, possibly suggesting defective insertion of this element into a pAP2 progenitor plasmid. orf95 was not identified in the sequence of pTET3, but the stop codon of tetR(33) and the start codon of the putative orf95 overlap, suggesting translational coupling.
Downstream of orf95 is repA, which encodes a replication protein with similarity to RepA from pNG2 (46.8% identity, 54.7% similarity) and pTET3 (39.5% identity, 54.4% similarity). The pAP2 repA gene is truncated at the 5′ end compared with that from pNG2, which may have resulted from the fusion of the truncated IS6100 sequence during insertion of the tet gene element. One hundred eighty-two base pairs downstream of repA is a copy of the 22-bp box (5′-CGTAAGCAATATACGGTTCCCC-3′) thought to be required for replication of corynebacterial rolling circle plasmids (20).
pAP2 also carries gcrY, so named because of the similarity of its translated product to GcrY, a protein of unknown function, encoded by pTP10 (71.8% identity, 87.7% similarity). The 6-bp GTATAC direct repeats downstream of gcrY in pTP10 (23) were not present in pAP2.
Given the similarity of the pAP2 repA to that of pNG2, which is broad host range (19), we tried to transform E. coli to erythromycin resistance with 98-4277-2 genomic DNA, containing pAP2, but were unsuccessful after numerous attempts (data not shown). Similar experiments were performed with A. pyogenes strain BBR1 using either 98-4277-2 genomic DNA or pJGS551, which carries an intact repA gene and its associated 22-bp box. No transformants were obtained in several attempts, indicating that pAP2 does not replicate in A. pyogenes strain BBR1 (data not shown). As a control, strain 98-4277-2 was transformed to CM resistance with pJGS551, demonstrating that pJGS551 is replication competent in the appropriate host. We conclude that pAP2 likely requires additional host-encoded factors, not present in strain BBR1, for efficient replication. One reason for this may be the apparent 5′ deletion of repA, which may have occurred during plasmid evolution.
pAP2 is not carried by all erm(X)-containing A. pyogenes isolates.
The finding that erm(X) in 98-4277-2 was plasmid borne led us to investigate whether this gene was carried on a similar plasmid in all erm(X)-containing strains. DIG-labeled probes were prepared for tnpA (IS6100), repA, and tetA(33) (Table 2) and were hybridized under high-stringency conditions against the 11 tylosin-resistant isolates and 37 tylosin susceptible isolates. IS6100 was preferentially associated with erm(X)-containing isolates, since 88.9% of erm(X) strains carried IS6100, compared with 10.3% of non-erm(X) strains (Fig. 4A). However, PCR experiments indicated that IS6100 and erm(X) were adjacent only in strain 98-4277-2 (data not shown). repA was present in only two erm(X) strains, indicating that erm(X) was not pAP2 associated in all erm(X) strains. Furthermore, the finding that only two non-erm(X) strains carried repA (Fig. 4B) indicates that pAP2-like plasmids are not widespread, correlating with the suggestion that replication of repA-containing plasmids may be strain specific. tetA(33) was present in 55.6% of erm(X)-containing strains but only in 5.1% of non-erm(X) strains (Fig. 4C). Only one other erm(X)-containing isolate in addition to 98-4277-2 carried all three ORFs. The two non-erm(X) strains carrying repA, also carried tetA(33), but only one also carried IS6100. The permuted combinations of resistance genes and insertion sequences with repA may suggest the presence of other pAP2-like plasmids.
FIG. 4.

Dot blot hybridization of A. pyogenes strains with the tnpA-specific probe (A), the repA-specific probe (B), and the tetA(33)-specific probe (C). Approximately 500 ng of genomic DNA from 48 A. pyogenes isolates was spotted onto a nylon membrane and hybridized with the respective probes under high-stringency conditions. The tylosin-resistant and -susceptible isolates are indicated. The two tylosin-resistant isolates which do not contain erm(X) are the first two dots on the first row on each blot.
erm(X) in many corynebacteria is associated with IS1249 on the transposon Tn5432 (17, 22, 23), although this is not the case with erm(X) from pNG2 (20) or pAP2. The erm(X)-containing A. pyogenes isolates were subjected to PCR with IS1249-specific primers. None of the A. pyogenes strains carried IS1249 (data not shown), suggesting that the erm(X) genes in A. pyogenes are not derived from Tn5432.
tetA(33) confers low-level TC resistance in A. pyogenes.
Sequence analysis of pAP2 indicated that like pTET3, this plasmid contained the repressor-regulated TC resistance determinant, Tet 33, which is similar to group I tetracycline efflux systems from gram-negative bacteria (21). This system was functional in E. coli, since we were able to clone the 6.2-kb HindIII fragment of pAP2 containing Tet 33 in pJGS551 by selection on TC. For C. glutamicum, tetA(33) results in an MIC of TC of 16 μg/ml (21). However, for A. pyogenes strains carrying tetA(33), this gene was only able to result in an MIC of TC of 1 μg/ml, compared with TC-susceptible strains, for which MICs of TC were ≤0.06 μg/ml (3). This is in contrast to strains carrying tet(W), the most prevalent TC resistance gene in A. pyogenes, which confers an MIC of TC of 8 μg/ml (3). For A. pyogenes strains harboring both genes, the MIC of TC is 16 μg/ml. Tauch et al. found that tetA(33) was inducible for C. glutamicum (21), but no induction was observed for A. pyogenes (data not shown).
Conclusions.
In the United States, tylosin is widely used as a feed additive to control liver abscessation in feedlot cattle (11). Resistance of the liver abscess pathogen, A. pyogenes, to macrolide antibiotics has been reported (4, 25, 28), but the mechanisms of resistance were not determined. This work is the first report of the identification and characterization of a determinant encoding tylosin resistance in A. pyogenes. Erm X is a prevalent determinant of tylosin resistance, with more than 80% of the tylosin-resistant A. pyogenes strains studied carrying this determinant. For strain 98-4277-2, erm(X) is encoded on a plasmid, pAP2, but this plasmid is not widespread among erm(X)-containing strains. pAP2 also carries a Tet 33 determinant, which is only the second Tet determinant identified for A. pyogenes. The finding that two tylosin-resistant A. pyogenes strains did not carry erm(X) suggests that there is at least one other determinant of tylosin resistance present in this organism.
Acknowledgments
Partial support for this work was provided by USDA/NRICGP award 99-35204-7818.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1994. Current protocols in molecular biology, vol 1. Greene Publishing Associates and John Wiley and Sons, Inc., New York, N.Y.
- 3.Billington, S. J., J. G. Songer, and B. H. Jost. 2002. Widespread distribution of a Tet W determinant among tetracycline-resistant isolates of the animal pathogen Arcanobacterium pyogenes. Antimicrob. Agents Chemother. 46:1281-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guérin-Faublée, V., J. P. Flandrois, E. Broye, F. Tupin, and Y. Richard. 1993. Actinomyces pyogenes: susceptibility of 103 clinical animal isolates to 22 antimicrobial agents. Vet. Res. 24:251-259. [PubMed] [Google Scholar]
- 5.Hodgson, A. L., J. Krywult, and A. J. Radford. 1990. Nucleotide sequence of the erythromycin resistance gene from the Corynebacterium plasmid pNG2. Nucleic Acids Res. 18:1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jost, B. H., S. J. Billington, and J. G. Songer. 1997. Electroporation-mediated transformation of Arcanobacterium (Actinomyces) pyogenes. Plasmid 38:135-140. [DOI] [PubMed] [Google Scholar]
- 7.Lodder, G., C. Werckenthin, S. Schwarz, and K. Dyke. 1997. Molecular analysis of naturally occurring ermC-encoding plasmids in staphylococci isolated from animals with and without previous contact with macrolide/lincosamide antibiotics. FEMS Immunol. Med. Microbiol. 18:7-15. [DOI] [PubMed] [Google Scholar]
- 8.Martin, C., J. Timm, J. Rauzier, R. Gomez-Lus, J. Davies, and B. Gicquel. 1990. Transposition of an antibiotic resistance element in mycobacteria. Nature 345:739-743. [DOI] [PubMed] [Google Scholar]
- 9.Nagaraja, T. G., A. B. Beharka, M. M. Chengappa, L. H. Carroll, A. P. Raun, S. B. Laudert, and J. C. Parrott. 1999. Bacterial flora of liver abscesses in feedlot cattle fed tylosin or no tylosin. J. Anim. Sci. 77:973-978. [DOI] [PubMed] [Google Scholar]
- 10.Nagaraja, T. G., M. L. Galyean, and N. A. Cole. 1998. Nutrition and disease. Feedlot Med. 14:257-277. [DOI] [PubMed] [Google Scholar]
- 11.Nagaraja, T. G., S. B. Laudert, and J. C. Parrott. 1996. Liver abscesses in feedlot cattle. Part II. Incidence, economic importance, and prevention. Comp. Cont. Edu. Pract. Vet. 18:S264-S273. [Google Scholar]
- 12.Narayanan, S., T. G. Nagaraja, N. Wallace, J. Staats, M. M. Chengappa, and R. D. Oberst. 1998. Biochemical and ribotypic comparison of Actinomyces pyogenes and A. pyogenes-like organisms from liver abscesses, ruminal wall, and ruminal contents of cattle. Am. J. Vet. Res. 59:271-276. [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. 1999. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; approved standard. M31-A. National Committee for Clinical and Laboratory Standards, Wayne, Pa.
- 14.Pospiech, A., and B. Neumann. 1995. A versatile quick-prep of genomic DNA from gram-positive bacteria. Trends Genet. 11:217-218. [DOI] [PubMed] [Google Scholar]
- 15.Radford, A. J., and A. L. Hodgson. 1991. Construction and characterization of a Mycobacterium-Escherichia coli shuttle vector. Plasmid 25:149-153. [DOI] [PubMed] [Google Scholar]
- 16.Roberts, M. C., J. Sutcliffe, P. Couvalin, L. B. Jensen, J. Rood, and H. Seppala. 1999. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob. Agents Chemother. 43:2823-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosato, A. E., B. S. Lee, and K. A. Nash. 2001. Inducible macrolide resistance in Corynebacterium jeikeium. Antimicrob. Agents Chemother. 45:1982-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scanlan, C. M., and T. L. Hathcock. 1983. Bovine rumenitis-liver abscess complex: a bacteriological review. Cornell Vet. 73:288-297. [PubMed] [Google Scholar]
- 19.Serwold-Davis, T., N. Groman, and M. Rabin. 1987. Transformation of Corynebacterium diphtheriae, Corynebacterium ulcerans, Corynebacterium glutamicum, and Escherichia coli with the C. diphtheriae plasmid pNG2. Proc. Natl. Acad. Sci. USA 84:4964-4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tauch, A., N. Bischoff, I. Brune, and J. Kalinowski. 2003. Insights into the genetic organization of the Corynebacterium diphtheriae erythromycin resistance plasmid pNG2 deduced from its complete nucleotide sequence. Plasmid 49:63-74. [DOI] [PubMed] [Google Scholar]
- 21.Tauch, A., S. Götker, A. Pühler, J. Kalinowski, and G. Thierbach. 2002. The 27.8-kb R-plasmid pTET3 from Corynebacterium glutamicum encodes the aminoglycoside adenyltransferase gene cassette aadA9 and the regulated tetracycline efflux system Tet 33 flanked by active copies of the widespread insertion sequence IS6100. Plasmid 48:117-129. [DOI] [PubMed] [Google Scholar]
- 22.Tauch, A., F. Kassing, J. Kalinowski, and A. Pühler. 1995. The Corynebacterium xerosis composite transposon Tn5432 consists of two identical insertion sequences, designated IS1246, flanking the erythromycin resistance gene ermCX. Plasmid 34:119-131. [DOI] [PubMed] [Google Scholar]
- 23.Tauch, A., S. Krieft, J. Kalinowski, and A. Pühler. 2000. The 51,409-bp R-plasmid pTP10 from the multiresistant clinical isolate Corynebacterium striatum M82B is composed of DNA segments initially identified in soil bacteria and in plant, animal, and human pathogens. Mol. Gen. Genet. 263:1-11. [DOI] [PubMed] [Google Scholar]
- 24.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trinh, H. T., S. J. Billington, A. C. Field, J. G. Songer, and B. H. Jost. 2002. Susceptibility of Arcanobacterium pyogenes to tetracyclines, macrolides and lincosamides. Vet. Microbiol. 85:353-359. [DOI] [PubMed] [Google Scholar]
- 26.U.S. Department of Agriculture. 2000. Part III: health management and biosecurity in U.S. feedlots, 1999, no. N336.1200. USDA:APHIS:VS, Centers for Epidemiology and Animal Health, National Animal Health Monitoring System, Fort Collins, Colo.
- 27.Weisblum, B. 1995. Insights into erythromycin action from studies of its activity as inducer of resistance. Antimicrob. Agents Chemother. 39:797-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshimura, H., A. Kojima, and M. Ishimaru. 2000. Antimicrobial susceptibility of Arcanobacterium pyogenes isolated from cattle and pigs. J. Vet. Med. B 47:139-143. [DOI] [PubMed] [Google Scholar]


