Abstract
The emergence of multidrug-resistant tuberculosis calls for new, rapid drug susceptibility tests. We have tested 150 Mycobacterium tuberculosis isolates against the second-line drugs ethionamide, kanamycin, capreomycin, ofloxacin, and para-aminosalicylic acid by the colorimetric resazurin microtiter assay and the proportion method. By visual reading, MICs were obtained after 8 days. A very good correlation between results by the colorimetric resazurin microtiter assay and the proportion method was obtained. The colorimetric resazurin microtiter assay is inexpensive, rapid, and simple to perform, and implementation of the assay is feasible for low-resource countries.
The global situation with respect to tuberculosis (TB) has worsened with the emergence of multidrug-resistant (MDR) TB worldwide (5, 36). MDR TB, defined by resistance to at least isoniazid and rifampin, is ubiquitous (12) and is most prevalent in some countries of Eastern Europe as well as China and India (14, 47). Immigration from areas where TB is endemic, human immunodeficiency virus infection (2, 16, 23, 28), homelessness, poor socioeconomic situations (6, 21), and prison populations (10, 39, 41) are among the responsible factors. Some patients with MDR TB do not respond to treatment with first-line drugs (isoniazid, rifampin, ethambutol, pyrazinamide, and streptomycin) (17). Several studies have shown that MDR TB can be cured by a combination of second-line drugs under DOTS-Plus, the treatment strategy proposed by the World Health Organization to address the management of MDR TB in settings with good control programs (15, 18, 22, 30, 35, 48). However, these drugs are expensive, have to be taken for long periods, and can cause adverse reactions (48). Drug susceptibility testing (DST) with Löwenstein-Jensen (LJ) medium or Middlebrook agar requires 3 to 6 weeks to obtain results (7, 8). Critical drug concentrations for second-line drugs have not been completely established. The development of new, rapid DST which is easy to use and inexpensive is thus an urgent priority for determining the susceptibility to second-line drugs (29, 32). A rapid and inexpensive colorimetric method based on the oxidation-reduction indicators Alamar blue and MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium] have been successfully used for determining MICs of first-line drugs in DST of Mycobacterium tuberculosis (1, 9, 20, 31, 38, 49). Since resazurin has been recently identified as the main component of Alamar blue (34, 43), we recently developed a resazurin microtiter assay (REMA) plate (37) for detecting MDR TB and demonstrated a very good correlation between results by this method and the proportion method (PM). In this study we evaluated the second-line drugs ethionamide (ETH), kanamycin monosulfate (KAN), capreomycin sulfate (CAP), ofloxacin (OFX), and para-aminosalicylic acid (PAS) with clinical isolates of M. tuberculosis by the colorimetric method using the REMA plate, and we compared the results with those of the PM.
One hundred fifty clinical isolates from Bolivia, Peru, and countries in Eastern Europe were studied. Fifty percent of the strains were MDR TB, 14% were polyresistant to first-line drugs, and 36% were susceptible. M. tuberculosis H37Rv (ATCC 27294) was used as the susceptible control.
ETH, OFX, and CAP were obtained from Sigma-Aldrich (St. Louis, Mo.); KAN was obtained from ICN Biomedicals, Inc. (Aurora, Ohio); and PAS (4-aminosalicylic acid sodium salt hydrate, 98%) was obtained from Acros Organic NV (Geel, Belgium). Stock solutions at 1 mg/ml were filter sterilized and stored at −20°C. Working solutions were prepared at four times the final higher concentration in 7H9-S broth (Middlebrook 7H9 supplemented with 0.1% Casitone, 0.5% glycerol, and 10% OADC [oleic acid, albumin, dextrose, and catalase]; Becton-Dickinson). The final drug concentrations tested were as follows: for PAS and OFX, 8 μg/ml; for ETH and KAN, 20 μg/ml; and for CAP, 10 μg/ml. Resazurin sodium salt powder from Acros Organic NV was prepared at 0.02% (wt/vol) in distilled water, sterilized by filtration, and stored at 4°C for up to 1 week.
Isolates were freshly subcultured on LJ medium. The inoculum was prepared in 7H9-S broth, adjusted spectrophotometrically to a no. 1 McFarland tube standard, and further diluted 1:10 in 7H9-S broth for the test.
The REMA plate assay was carried out as described by Palomino et al. (37). Briefly, 100 μl of 7H9-S broth was dispensed in each well of a sterile flat-bottom 96-well plate, and serial twofold dilutions of each drug were prepared directly in the plate. One hundred microliters of inoculum was added to each well. A growth control and a sterile control were also included for each isolate. Sterile water was added to all perimeter wells to avoid evaporation during the incubation. The plate was covered, sealed in a plastic bag, and incubated at 37°C under a normal atmosphere. After 7 days of incubation, 30 μl of resazurin solution was added to each well, and the plate was reincubated overnight. A change in color from blue to pink indicated the growth of bacteria, and the MIC was defined as the lowest concentration of drug that prevented this change in color. The drug concentration ranges used were as follows: for ETH and KAN, 0.62 to 20 μg/ml; for OFX and PAS, 0.25 to 8 μg/ml; and for CAP, 0.3 to 10 μg/ml.
The PM was performed with 7H11 agar or LJ medium (for PAS) (7, 8) with the following recommended critical drug concentrations: for PAS, 0.5 μg/ml; for ETH and CAP, 10 μg/ml; for OFX, 2 μg/ml; and for KAN, 6 μg/ml (3, 7, 8, 33).
To study the sensitivity of resistance detection by the REMA plate method and assuming similar growth kinetics, mixed populations of resistant and susceptible strains were prepared and tested. For each drug and in separate experiments, an increasing proportion (0 to 16%) of the resistant strain was added to the susceptible H37Rv strain, keeping the number of bacteria to a no. 1 McFarland tube standard. We determined the percentage of resistance by visual reading of the MICs.
The tentative breakpoints for each drug were based on receiver operating characteristic curve analysis (50) and calculated by using MedCalc (Mariakerke, Belgium) software, which gives the corresponding cutoff point with the best separation by the dot plot diagram. To evaluate the performance of the test, sensitivity, specificity, and accuracy values were calculated.
By visual reading of the assay plates, the MICs of the second-line drugs OFX, KAN, CAP, ETH, and PAS for 150 isolates of M. tuberculosis were determined, and the results were obtained after 8 days of incubation. Results of testing by the PM were available after 3 weeks. Isolates with discordant results were reevaluated by both methods. Table 1 shows the results of DST obtained by the PM and the REMA plate method. Among the 150 isolates, 31 were resistant to ETH, 29 were resistant to KAN, 9 were resistant to PAS, 8 were resistant to OFX, and 19 were resistant to CAP by the PM.
TABLE 1.
Susceptibility testing of 150 isolates of M. tuberculosis by the PM and the REMA plate method
| Drug | MIC (μg/ml) by REMA plate method | PM
|
|
|---|---|---|---|
| No. of resistant strains | No. of susceptible strains | ||
| ETH | <0.62 | 109 | |
| 1.25 | 1 | 10 | |
| 2.5a | |||
| 5.0 | 4 | ||
| 10.0 | 7 | ||
| >20.0 | 19 | ||
| KAN | <0.62 | 70 | |
| 1.25 | 34 | ||
| 2.5a | 17 | ||
| 5.0 | 4 | ||
| 10.0 | 3 | ||
| >20.0 | 22 | ||
| PAS | <0.25 | 13.3 | |
| 0.5 | 5 | ||
| 1.0 | 2 | ||
| 2.0a | 1 | ||
| 4.0 | |||
| >8.0 | 9 | ||
| OFX | <0.25 | 14.1 | |
| 0.5 | 1 | ||
| 1.0 | |||
| 2.0a | |||
| 4.0 | 4 | ||
| >8.0 | 4 | ||
| CAP | <0.30 | 29 | |
| 0.62 | 40 | ||
| 1.25 | 1 | 53 | |
| 2.5a | 2 | 9 | |
| 5.0 | 5 | ||
| 10.0 | 11 | ||
Tentative breakpoint concentration.
Complete agreement in results was found for KAN, PAS, and OFX by the two methods. Tentative breakpoint concentrations were defined as follows: for PAS and OFX, 2.0 μg/ml; and for KAN, 2.5 μg/ml. The tentative breakpoint concentration of ETH was 2.5 μg/ml. One discordant result was a finding of resistance by the PM and a finding of susceptibility by the REMA plate method at drug MICs of 1.25 μg/ml. The strain with these results was MDR. For CAP we propose a tentative breakpoint concentration of 2.5 μg/ml. Three isolates gave discordant results, as they were resistant by the PM and susceptible by the REMA plate method at drug MICs of 1.25 and 2.5 μg/ml, respectively. These strains were also MDR. The overall performance of the REMA plate method for the five antimicrobial agents in comparison with that of the PM was very good. The specificity was 100% for the five drugs, and the sensitivity ranged from 96.8 to 100%; for CAP the sensitivity was 84.2%. The accuracy of the REMA plate method was very good.
To determine if the REMA plate method could detect small populations of drug-resistant strains in the presence of a large proportion of susceptible strains by visual reading, we mixed increasing concentrations of the resistant strain (0 to 16%) with the susceptible H37Rv strain and tested it with the relevant drug. Figure 1 shows that the REMA plate method was able to detect small populations of each resistant strain. Bacterial populations containing 1% or more of drug-resistant bacilli could easily be detected by visual reading of MICs of KAN, ETH, OFX, and CAP that were higher than their cutoff values. When PAS was used, we were able to detect 4% of the resistant bacteria.
FIG. 1.
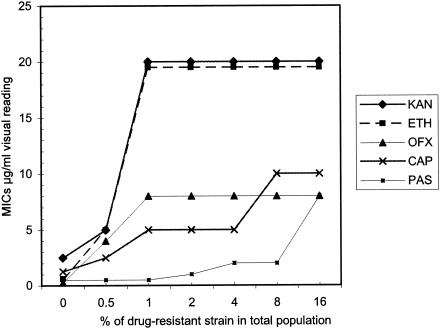
Sensitivity of detection of resistant strains by visual reading. For each resistant strain (ETH, strain 99-1877; PAS, 99-3304; CAP, 00-1071; OFX, 01-2613; and KAN, 01-2614), a culture of H37Rv containing various proportions of the resistant strain (0 to 16%) was tested by the REMA plate method for MIC determination.
In this study, for the first time, DST of 150 M. tuberculosis isolates has been performed with five second-line drugs by the REMA plate method. With the spread of MDR TB, there is an increasing demand for DST for second-line drugs. The development of better and faster diagnostic assays is an urgent priority for the management of MDR TB (32). The turnaround time is important for the patient to receive an early start of appropriate treatment. Standard laboratory methods are slow, with results not available before 4 to 8 weeks after testing. An inexpensive and rapid method for MDR TB detection and for DST of second-line drugs is desirable for low-resource countries. One of the few studies of MDR TB detection was a multicenter study by Pfyffer et al. (40), who examined second-line drugs and other antibiotics by using the BACTEC 460 system. This system is rapid and reliable (45, 46), but it requires heavy equipment and the use of radioisotopes, which limits its application. Preliminary studies of DST of MDR TB isolates using MTT, Alamar blue, or resazurin showed a good correlation with the conventional PM (1, 13, 19, 20, 31, 37, 49). The limitation of some proprietary dyes is the lack of knowledge of their compositions (27). Recently, resazurin, a nonproprietary product, has been identified as the main component of Alamar blue (34, 43). The REMA plate method has proven to be, in recent experience, a reliable method for the detection of MDR TB (37). In this study, the REMA plate method has also shown a high level of agreement with the conventional PM. Results are easily determined visually by reading the change to a stable color from blue to pink. The results can be also determined by spectophotometry or fluorometry, but the cost of equipment is high and would limit its application in low-resource countries. By using populations of each resistant strain mixed with the susceptible strain H37Rv, the REMA plate method could detect the presence of the resistant population at a level as low as 1%. Our proposed breakpoint values fully agree with the drug MICs of previous studies (Table 2). An advantage of the microtiter format is that it allows the screening of several isolates in a short period of time. One disadvantage, however, is biosafety, since the plates require the use of liquid medium and could generate aerosols. It has recently been shown that this format can be adapted for screw-cap tubes to avoid this situation (1, 19, 31). Cost is an important factor for low-resource countries. Resazurin greatly reduces the cost of this method, to approximately $3 for each strain; it is even cheaper than MTT. Therefore, it would be feasible to implement this method in laboratories with limited resources. Tests like the REMA plate method described here have the potential in the future to be the method of choice for DST of M. tuberculosis and other mycobacteria. Our study helps to optimize the critical concentrations of second-line drugs for establishing this type of test.
TABLE 2.
Comparison of MICs obtained by different studies with M. tuberculosis
| Method [reference(s)] | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|
| ETH | KAN | OFX | CAP | PAS | |
| REMA plate (this study) | 2.5 | 2.5 | 2.0 | 2.5 | 2.0 |
| BACTEC 460 (40) | 1.25-2.5 | 2.5-5.0 | 1.0-2.0 | 1.25-2.5 | —a |
| BACTEC 460 (44) | 0.25-5.0 | 2.0-4.0 | 0.5-1.0 | 1.0-2.0 | — |
| BACTEC 460 (11, 24, 25, 26) | 0.3-1.25 | — | 0.5-2.0 | 1.5-3.0 | — |
| Alamar blue (4) | — | 2.5 | — | — | — |
| Alamar blue (42) | — | — | 1.0-2.0 | ||
—, not tested.
Acknowledgments
This study was supported by the European Commission RDG (INCO-DEV Programme), project No. ICA4-CT-2001-10087, and by the Damien Foundation, Brussels, Belgium.
REFERENCES
- 1.Abate, G., R. N. Mshana, and H. Miorner. 1998. Evaluation of a colorimetric assay based on 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) for rapid detection of rifampicin resistance in Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 2:1011-1016. [PubMed] [Google Scholar]
- 2.Barnes, P. F., D. L. Lakey, and W. J. Burman. 2002. Tuberculosis in patients with HIV infection. Infect. Dis. Clin. N. Am. 16:107-126. [DOI] [PubMed] [Google Scholar]
- 3.Bastian, I., and R. Colebunders. 1999. Treatment and prevention of multidrug-resistant tuberculosis. Drugs 58:633-661. [DOI] [PubMed] [Google Scholar]
- 4.Bastian, I., L. Rigouts, J. C. Palomino, and F. Portaels. 2001. Kanamycin susceptibility testing of Mycobacterium tuberculosis using Mycobacterium Growth Indicator Tube and a colorimetric method. Antimicrob. Agents Chemother. 45:1934-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloch, A. B., G. M. Cauthen, I. M. Onorato, K. G. Dansbury, G. D. Kelly, C. R. Driver, and D. E. Snider, Jr. 1994. Nationwide survey of drug-resistant tuberculosis in the United States. JAMA 271:665-671. [PubMed] [Google Scholar]
- 6.Brudney, K., and J. Dobkin. 1991. Resurgent tuberculosis in New York City. Human immunodeficiency virus, homelessness, and the decline of tuberculosis control programs. Am. Rev. Respir. Dis. 144:745-749. [DOI] [PubMed] [Google Scholar]
- 7.Canetti, G., F. Froman, J. Grosset, P. Hauduroy, M. Langerova, H. T. Mahler, G. Meissner, D. A. Mitchison, and L. Sula. 1963. Mycobacteria: laboratory methods for testing drug sensitivity and resistance. Bull. W. H. O. 29:565-578. [PMC free article] [PubMed] [Google Scholar]
- 8.Canetti, G., W. Fox, A. Khomenko, H. T. Mahler, N. K. Menon, D. A. Mitchison, N. Rist, and N. A. Smelev. 1969. Advances in techniques of testing mycobacterial drug sensitivity, and the use of sensitivity tests in tuberculosis control programmes. Bull. W. H. O. 41:21-43. [PMC free article] [PubMed] [Google Scholar]
- 9.Caviedes, L., J. Delgado, and R. H. Gilman. 2002. Tetrazolium microplate assay as a rapid and inexpensive colorimetric method for determination of antibiotic susceptibility of Mycobacterium tuberculosis. J. Clin. Microbiol. 40:1873-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaves, F., F. Dronda, M. D. Cave, M. Alonso-Sanz, A. Gonzalez-Lopez, K. D. Eisenach, A. Ortega, L. Lopez-Cubero, I. Fernandez-Martin, S. Catalan, and J. H. Bates. 1997. A longitudinal study of transmission of tuberculosis in a large prison population. Am. J. Respir. Crit. Care Med. 155:719-725. [DOI] [PubMed] [Google Scholar]
- 11.Chen, C. H., J. F. Shih, P. J. Lindholm-Levy, and L. B. Heifets. 1989. Minimal inhibitory concentrations of rifabutin, ciprofloxacin, and ofloxacin against Mycobacterium tuberculosis isolated before treatment of patients in Taiwan. Am. Rev. Respir. Dis. 140:987-989. [DOI] [PubMed] [Google Scholar]
- 12.Cohn, D. L., F. Bustreo, M. C. Raviglione, et al. 1997. Drug-resistant tuberculosis: review of the worldwide situation and the WHO/IUATLD Global Surveillance Project. Clin. Infect. Dis. 24(Suppl. 1):S121-S130. [DOI] [PubMed] [Google Scholar]
- 13.Collins, L. A., and S. G. Franzblau. 1997. Microplate Alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob. Agents Chemother. 41:1004-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Espinal, M. A., A. Laszlo, L. Simonsen, F. Boulahbal, S. J. Kim, A. Reniero, S. Hoffner, H. L. Rieder, N. Binkin, C. Dye, R. Williams, M. C. Raviglione, et al. 2001. Global trends in resistance to antituberculosis drugs. N. Engl. J. Med. 344:1294-1303. [DOI] [PubMed] [Google Scholar]
- 15.Espinal, M. A., C. Dye, M. Raviglione, and A. Kochi. 1999. Rational ‘DOTS plus’ for the control of MDR-TB. Int. J. Tuberc. Lung Dis. 3:561-563. [PubMed] [Google Scholar]
- 16.Espinal, M. A., K. Laserson, M. Camacho, Z. Fusheng, S. J. Kim, R. E. Tlali, I. Smith, P. Suarez, M. L. Antunes, A. G. George, N. Martin-Casabona, P. Simelane, K. Weyer, N. Binkin, and M. C. Raviglione. 2001. Determinants of drug-resistant tuberculosis: analysis of 11 countries. Int. J. Tuberc. Lung Dis. 5:887-893. [PubMed] [Google Scholar]
- 17.Espinal, M. A., S. J. Kim, P. G. Suarez, K. M. Kam, A. G. Khomenko, G. B. Migliori, J. Baez, A. Kochi, C. Dye, and M. C. Raviglione. 2000. Standard short-course chemotherapy for drug-resistant tuberculosis: treatment outcomes in 6 countries. JAMA 283:2537-2545. [DOI] [PubMed] [Google Scholar]
- 18.Farmer, P., and J. Y. Kim. 1998. Community based approaches to the control of multidrug resistant tuberculosis: introducing “DOTS-plus.” BMJ 317:671-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foongladda, S., D. Roengsanthia, W. Arjrattanakool, C. Chuchottaworn, A. Chaiprasert, and S. G. Franzblau. 2002. Rapid and simple MTT method for rifampicin and isoniazid susceptibility testing of Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 6:1118-1122. [PubMed] [Google Scholar]
- 20.Franzblau, S. G., R. S. Witzig, J. C. McLaughlin, P. Torres, G. Madico, A. Hernandez, M. T. Degnan, M. B Cook, V. K. Quenzer, R. M. Ferguson, and R. H. Gilman. 1998. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J. Clin. Microbiol. 36:362-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frieden, T. R., T. Sterling, A. Pablos-Mendez, J. O. Kilburn, G. M. Cauthen, and S. W. Dooley. 1993. The emergence of drug-resistant tuberculosis in New York City. N. Engl. J. Med. 328:521-526. [DOI] [PubMed] [Google Scholar]
- 22.Gupta, R., M. C. Raviglione, and M. A. Espinal. 2001. Should tuberculosis programmes invest in second-line treatments for multidrug-resistant tuberculosis (MDR-TB)? Int. J. Tuberc. Lung Dis. 5:1078-1079. [PubMed] [Google Scholar]
- 23.Harries, A. D., D. Maher, B. Mvula, and D. Nyangulu. 1995. An audit of HIV testing and HIV serostatus in tuberculosis patients, Blantyre, Malawi. Tuber. Lung Dis. 76:413-417. [DOI] [PubMed] [Google Scholar]
- 24.Heifets, L. B., and P. J. Lindholm-Levy. 1987. Bacteriostatic and bactericidal activity of ciprofloxacin and ofloxacin against Mycobacterium tuberculosis and Mycobacterium avium complex. Tubercle 68:267-276. [DOI] [PubMed] [Google Scholar]
- 25.Heifets, L. B., P. J. Lindholm-Levy, and M. Flory. 1991. Comparison of bacteriostatic and bactericidal activity of isoniazid and ethionamide against Mycobacterium avium and Mycobacterium tuberculosis. Am. Rev. Respir. Dis. 143:268-270. [DOI] [PubMed] [Google Scholar]
- 26.Heifets, L., and P. Lindholm-Levy. 1989. Comparison of bactericidal activities of streptomycin, amikacin, kanamycin, and capreomycin against Mycobacterium avium and M. tuberculosis. Antimicrob. Agents Chemother. 33:1298-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horobin, R. W. 2001. The problem of proprietary dyes, with special reference to Alamar blue, a proprietary dye revealed. Biotech. Histochem. 76:163-164. [PubMed] [Google Scholar]
- 28.Kazionny, B., C. D. Wells, H. Lue, N. Gusseynova, and V. Molotilov. 2002. Implications of the growing HIV-1 epidemic for tuberculosis control in Russia. Lancet 358:1513-1514. [DOI] [PubMed] [Google Scholar]
- 29.Loddenkemper, R., D. Sagebiel, and A. Brendel. 2002. Strategies against multidrug-resistant tuberculosis. Eur. Respir. J. Suppl. 36:66s-77s. [DOI] [PubMed] [Google Scholar]
- 30.Mitnick, C., J. Bayona, E. Palacios, S. Shin, J. Furin, F. Alcantara, E. Sanchez, M. Sarria, M. Becerra, M. C. Fawzi, S. Kapiga, D. Neuberg, J. H. Maguire, J. Y. Kim, and P. Farmer. 2003. Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru. N. Engl. J. Med. 348:119-128. [DOI] [PubMed] [Google Scholar]
- 31.Mshana, R. N., G. Tadesse, G. Abate, and H. Miörner. 1998. Use of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide for rapid detection of rifampin-resistant Mycobacterium tuberculosis. J. Clin. Microbiol. 36:1214-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nachega, J. B., and R. E. Chaisson. 2003. Tuberculosis drug resistance: a global threat. Clin. Infect. Dis. 36:S24-S30. [DOI] [PubMed] [Google Scholar]
- 33.NCCLS. 2000. Susceptibility testing of Mycobacteria, Nocardia, and other aerobic actinomycetes; tentative standard, 2nd ed. Document M24-T2. NCCLS, Wayne, Pa. [PubMed]
- 34.O'Brien, J., I. Wilson, T. Orton, and F. Pognan. 2000. Investigation of the Alamar blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 267:5421-5426. [DOI] [PubMed] [Google Scholar]
- 35.Pablos-Mendez, A., D. K. Gowda, and T. R. Frieden. 2002. Controlling multidrug-resistant tuberculosis and access to expensive drugs: a rational framework. Bull. W. H. O. 80:489-495. [PMC free article] [PubMed] [Google Scholar]
- 36.Pablos-Mendez, A., M. C. Raviglione, A. Laszlo, N. Binkin, H. L. Rieder, F. Bustreo, D. L. Cohn, C. S. Lambregts-van Weezenbeek, S. J. Kim, P. Chaulet, P. Nunn, et al. 1998. Global surveillance for antituberculosis-drug resistance, 1994-1997. N. Engl. J. Med. 338:1641-1649. [DOI] [PubMed] [Google Scholar]
- 37.Palomino, J.-C., A. Martin, M. Camacho, H. Guerra, J. Swings, and F. Portaels. 2002. Resazurin microtiter assay plate: simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 46:2720-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palomino, J. C., and F. Portaels. 1999. Simple procedure for drug susceptibility testing of Mycobacterium tuberculosis using a commercial colorimetic assay. Eur. J. Clin. Microbiol. Infect. Dis. 18:380-383. [DOI] [PubMed] [Google Scholar]
- 39.Pfyffer, G. E., A. Strassle, T. Van Gorkum, F. Portaels, L. Rigouts, C. Mathieu, F. Mirzoyev, H. Traore, and J. D. Van Embden. 2001. Multidrug-resistant tuberculosis in prison inmates, Azerbaijan. Emerg. Infect. Dis. 7:855-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfyffer, G. E., D. A. Bonato, A. Ebrahimzadeh, W. Gross, J. Hotaling, J. Kornblum, A. Laszlo, G. Roberts, M. Salfinger, F. Wittwer, and S. Siddiqi. 1999. Multicenter laboratory validation of susceptibility testing of Mycobacterium tuberculosis against classical second-line and newer antimicrobial drugs by using the radiometric BACTEC 460 technique and the proportion method with solid media. J. Clin. Microbiol. 37:3179-3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Portaels, F., L. Rigouts, and I. Bastian. 1999. Addressing multidrug-resistant tuberculosis in penitentiary hospitals and in the general population of the former Soviet Union. Int. J. Tuberc. Lung Dis. 3:582-588. [PubMed] [Google Scholar]
- 42.Pracharktam, R., K. Angkananukool, and A. Vibhagool. 2001. In vitro susceptibility testing of levofloxacin and ofloxacin by microtiter plate Alamar blue against multidrug and non multidrug resistant Mycobacterium tuberculosis in Thailand. J. Med. Assoc. Thail. 84:1241-1245. [PubMed] [Google Scholar]
- 43.Rasmussen, E. V. 1999. Use of fluorescent redox indicators to evaluate cell proliferation and viability. In Vitro Mol. Toxicol. 12:47-58. [Google Scholar]
- 44.Rastogi, N., V. Labrousse, and K. S. Goh. 1996. In vitro activities of fourteen antimicrobial agents against drug susceptible and resistant clinical isolates of Mycobacterium tuberculosis and comparative intracellular activities against the virulent H37Rv strain in human macrophages. Curr. Microbiol. 33:167-175. [DOI] [PubMed] [Google Scholar]
- 45.Roberts, G. D., N. L. Goodman, L. Heifets, H. W. Larsh, T. H. Lindner, J. K. McClatchy, M. R. McGinnis, S. H. Siddiqi, and P. Wright. 1983. Evaluation of the BACTEC radiometric method for recovery of mycobacteria and drug susceptibility testing of Mycobacterium tuberculosis from acid-fast smear-positive specimens. J. Clin. Microbiol. 18:689-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siddiqi, S. H., J. P. Libonati, and G. Middlebrook. 1981. Evaluation of rapid radiometric method for drug susceptibility testing of Mycobacterium tuberculosis. J. Clin. Microbiol. 13:908-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organization. 2001. Global tuberculosis control. WHO. report WHO/CDS/B2001.287. World Health Organization, Geneva, Switzerland.
- 48.World Health Organization. 2000. Guidelines for establishing DOTS-Plus pilot projects for the management of multidrug resistant tuberculosis (MDRTB). WHO report WHO/ODS/TB/2000.279. World Health Organization, Geneva, Switzerland.
- 49.Yajko, D. M., J. J. Madej, M. V. Lancaster, C. A. Sanders, V. L. Cawthon, B. Gee, A. Babst, and W. K. Hadley. 1995. Colorimetric method for determining MICs of antimicrobial agents for Mycobacterium tuberculosis. J. Clin. Microbiol. 33:2324-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeig, M. H., and G. Campbell. 1993. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin. Chem. 39:561-577. [PubMed] [Google Scholar]


