Abstract
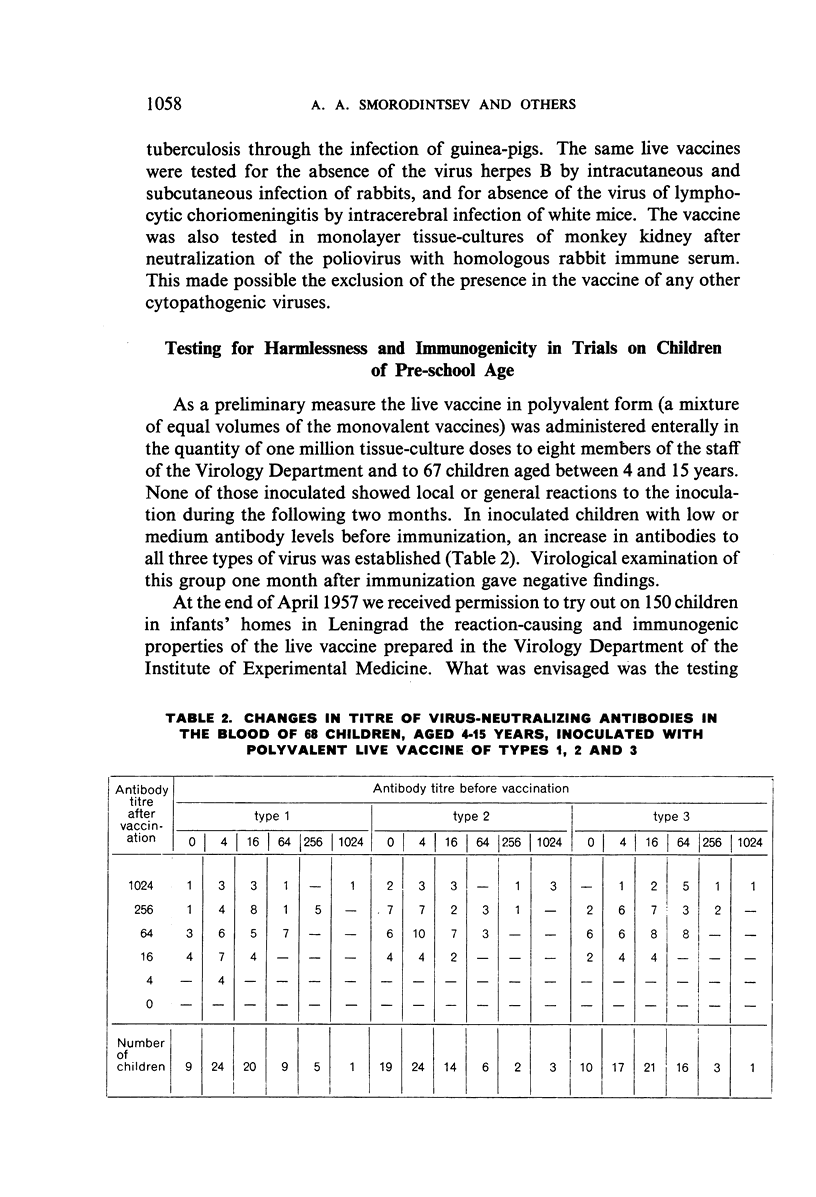
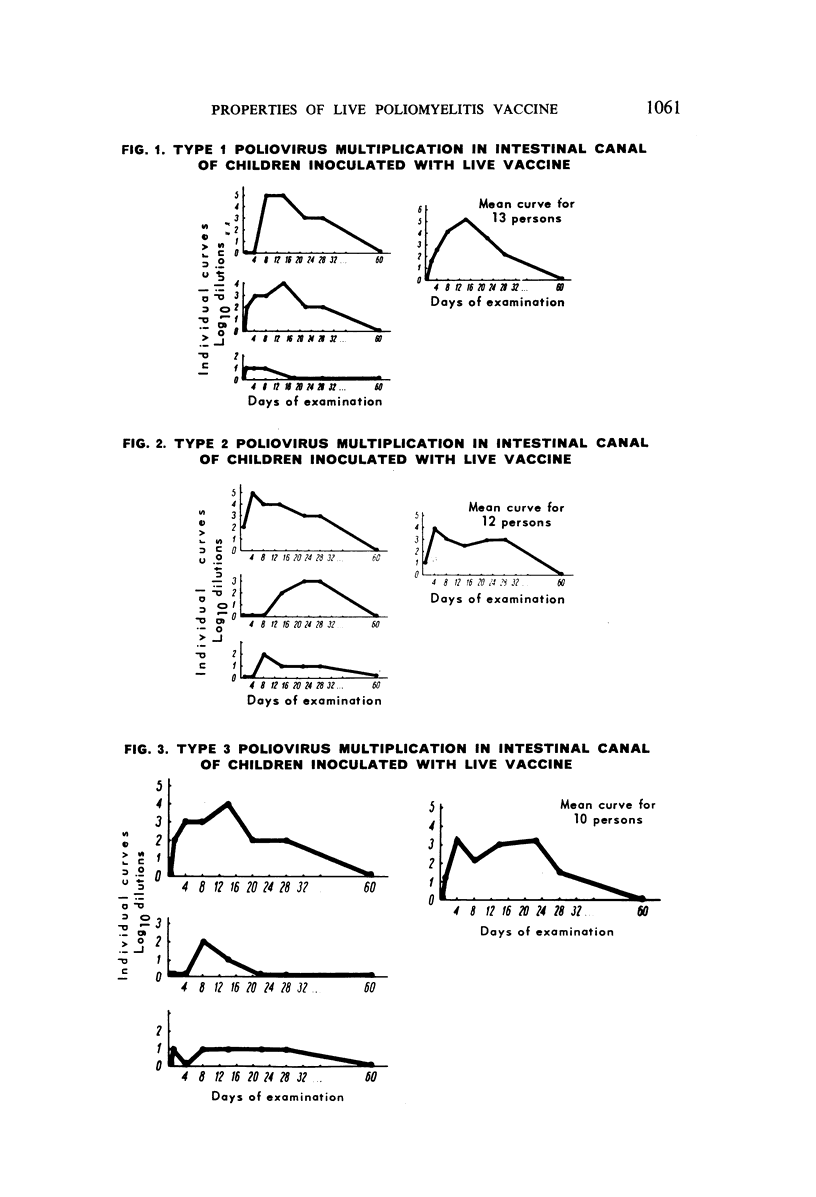
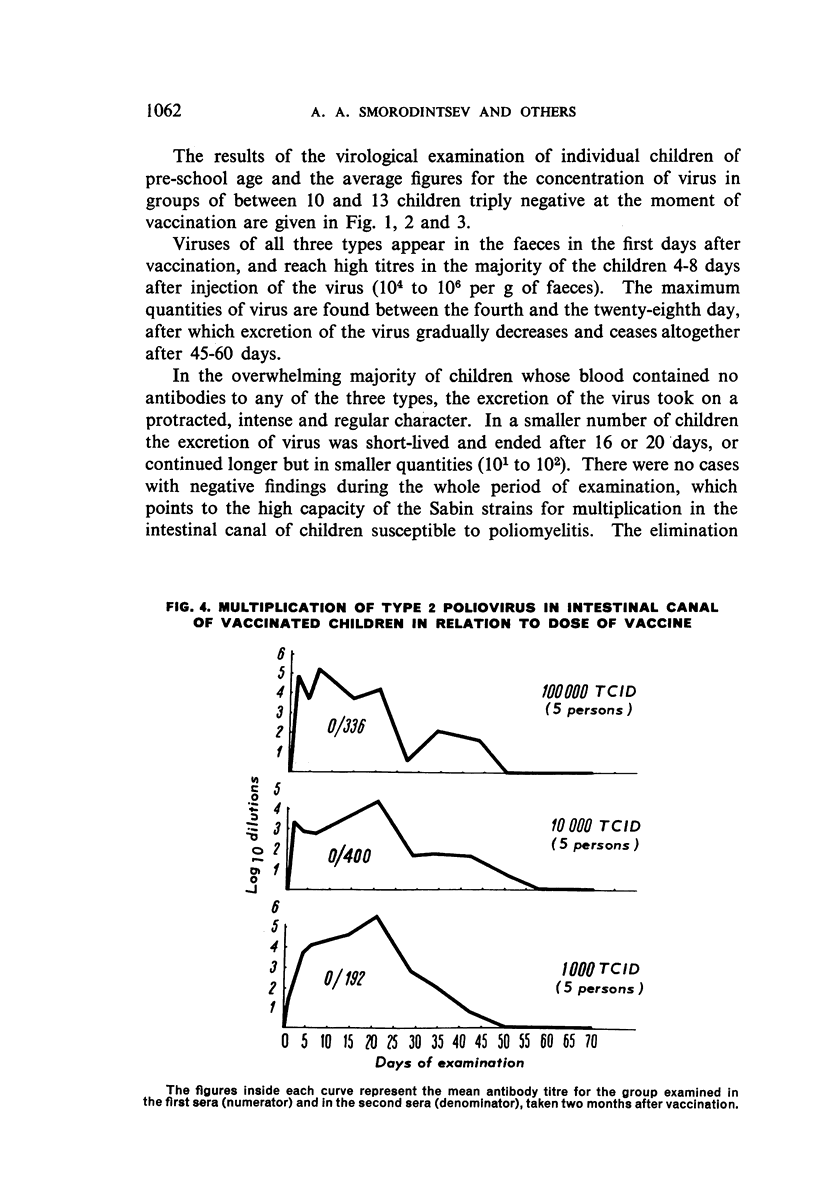
The authors have studied the harmlessness and immunogenic properties of live poliomyelitis vaccine made in Leningrad from Sabin strains of low pathogenicity for monkeys. More than 20 000 children of pre-school (6 months to 3 years) and school age (7-14 years) were each given 100 000 tissue-culture infective doses of virus of types 1, 2 and 3, injected either in three stages at monthly intervals in the form of monovaccines, or in two stages, a monovaccine of type 1 being followed after a month's interval by injection of a divalent vaccine of types 2 and 3. The vaccination caused no symptoms of lesions of the central nervous system or other organs. In the blood of the inoculated children there was a regular build-up of virus-neutralizing antibodies to the serotypes mentioned, the intensity of which depended on the antibody level before vaccination and was in a constant relationship to the multiplication of the virus in the intestinal canal. The antibody titre was maintained at high levels for 6-9 months after immunization and fell a little after 12-18 months. The vaccinal virus is easily transferred from vaccinated children to contact groups, which are gradually vaccinated by this natural means.
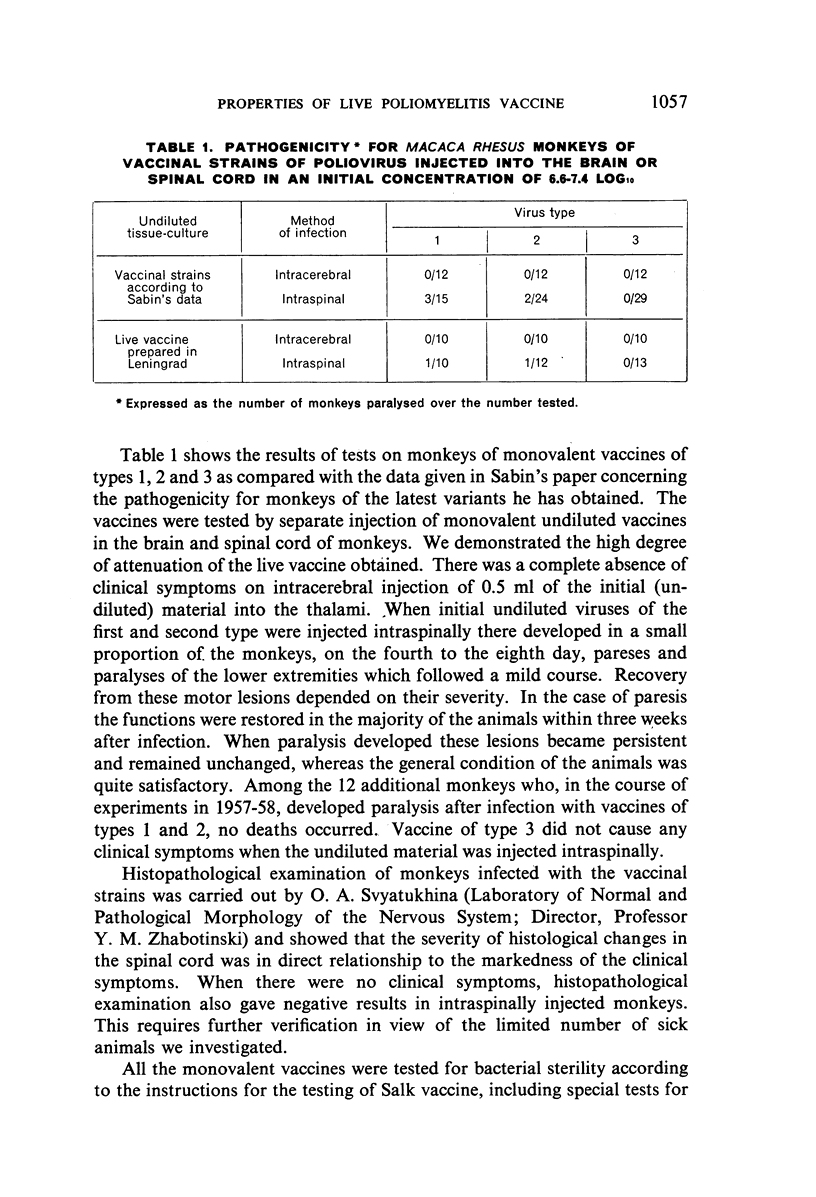
Lengthy and numerous passages of vaccinal strains through the intestinal canal of normal, susceptible children showed that strains may periodically appear which have a higher neurotropic activity for monkeys. This activity, however, did not increase in subsequent passage and returned to the initial level.
Full text
PDF





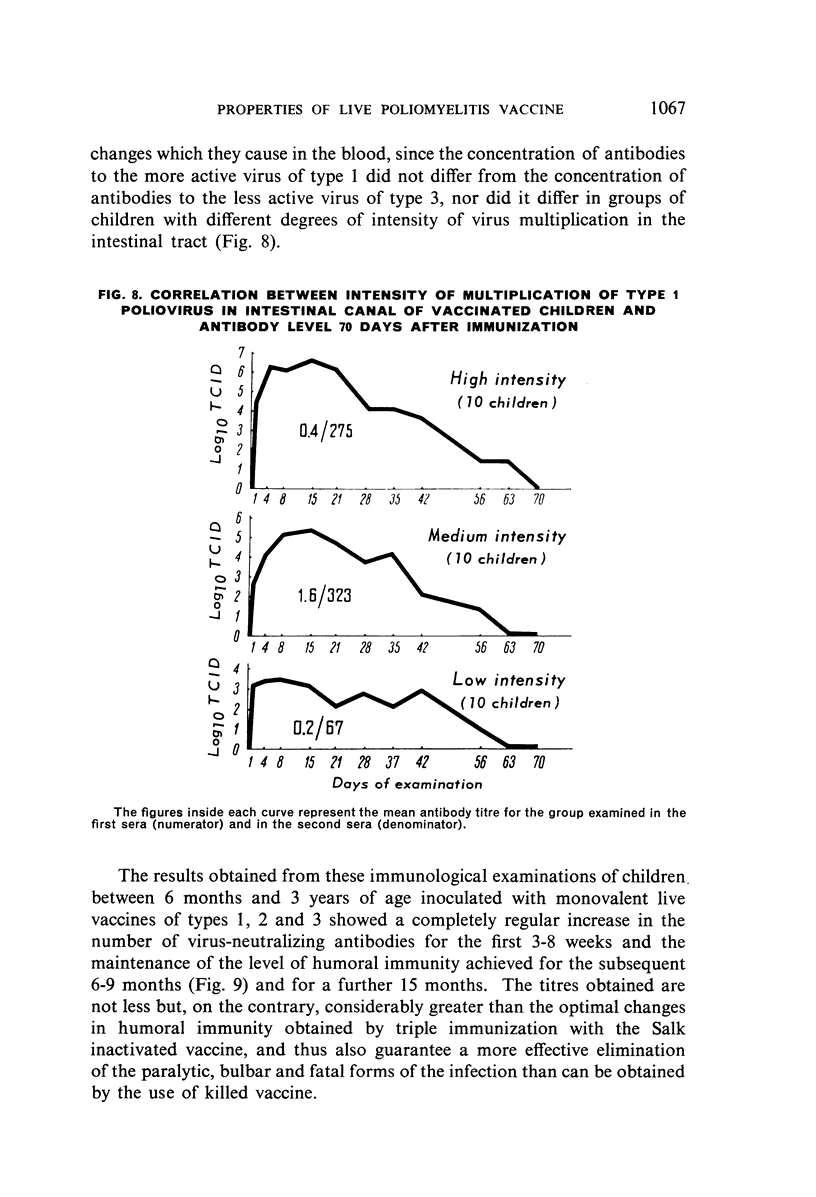
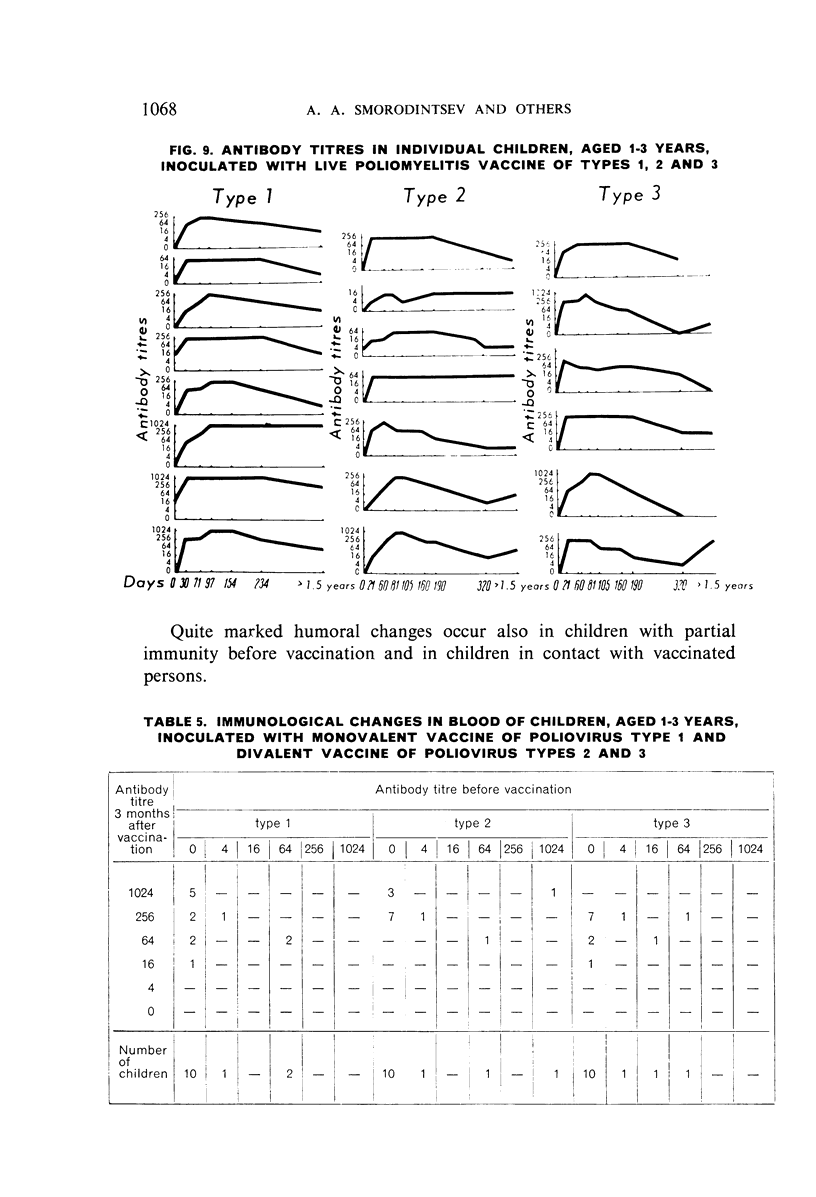


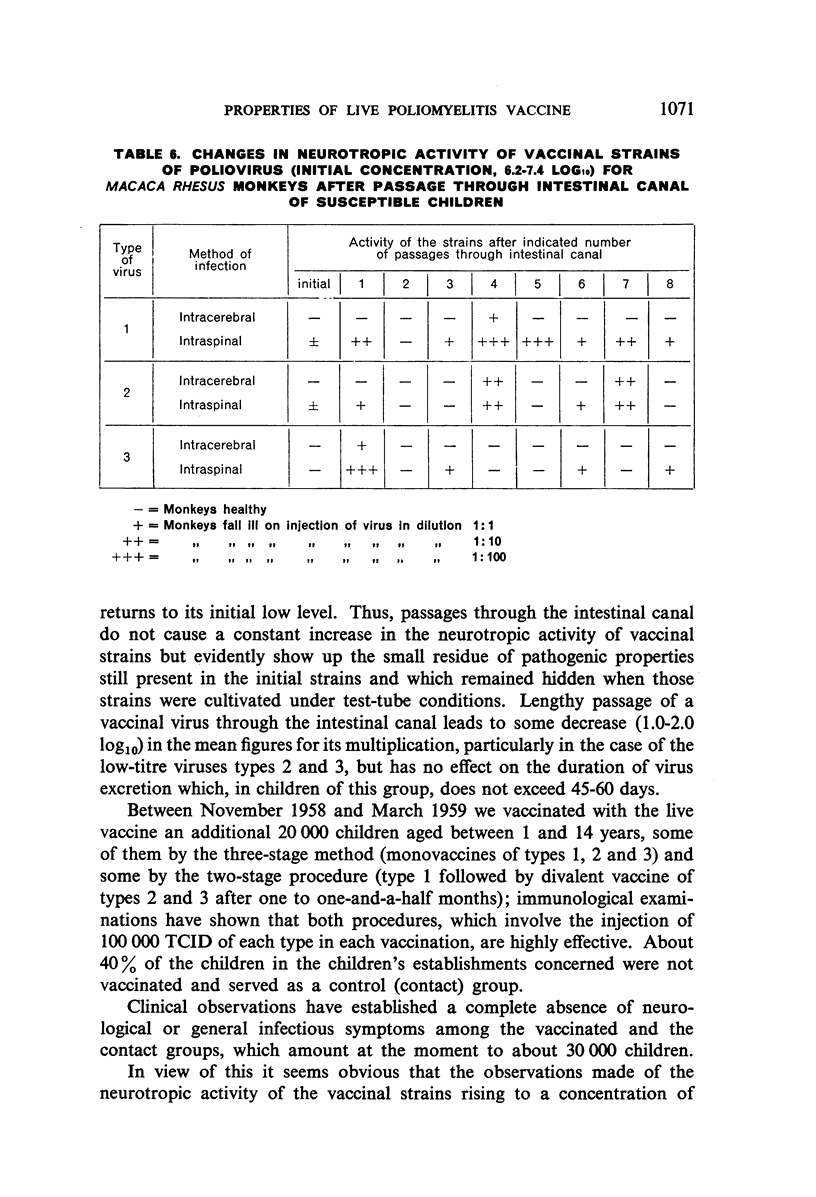



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FLACK A., HUMMELER K., HUNT A. D., Jr, JERVIS G. A., KOPROWSKI H., NORTON T. W., STOKES J., Jr Immunization of infants with living attenuated poliomyelitis virus; laboratory investigations of alimentary infection and antibody response in infants under six months of age with congenitally acquired antibodies. J Am Med Assoc. 1956 Dec 1;162(14):1281–1288. [PubMed] [Google Scholar]
- SABIN A. B. Characteristics and genetic potentialities of experimentally produced and naturally occurring variants of poliomyelitis virus. Ann N Y Acad Sci. 1955 Sep 27;61(4):924-38; discussion, 938-9. doi: 10.1111/j.1749-6632.1955.tb42551.x. [DOI] [PubMed] [Google Scholar]
- SABIN A. B. Present status of attenuated live-virus poliomyelitis vaccine. J Am Med Assoc. 1956 Dec 29;162(18):1589–1596. doi: 10.1001/jama.1956.02970350005002. [DOI] [PubMed] [Google Scholar]
- SABIN A. B. Properties and behavior of orally administered attenuated poliovirus vaccine. J Am Med Assoc. 1957 Jul 13;164(11):1216–1223. doi: 10.1001/jama.1957.62980110008008. [DOI] [PubMed] [Google Scholar]


