Abstract
Rab2 immunolocalizes to pre-Golgi intermediates (vesicular-tubular clusters [VTCs]) that are the first site of segregation of anterograde- and retrograde-transported proteins and a major peripheral site for COPI recruitment. Our previous work showed that Rab2 Q65L (equivalent to Ras Q61L) inhibited endoplasmic reticulum (ER)-to-Golgi transport in vivo. In this study, the biochemical properties of Rab2 Q65L were analyzed. The mutant protein binds GDP and GTP and has a low GTP hydrolysis rate that suggests that Rab2 Q65L is predominantly in the GTP-bound–activated form. The purified protein arrests vesicular stomatitis virus glycoprotein transport from VTCs in an assay that reconstitutes ER-to-Golgi traffic. A quantitative binding assay was used to measure membrane binding of β-COP when incubated with the mutant. Unlike Rab2 that stimulates recruitment, Rab2 Q65L showed a dose-dependent decrease in membrane-associated β-COP when incubated with rapidly sedimenting membranes (ER, pre-Golgi, and Golgi). The mutant protein does not interfere with β-COP binding but stimulates the release of slowly sedimenting vesicles containing Rab2, β-COP, and p53/gp58 but lacking anterograde grade-directed cargo. To complement the biochemical results, we observed in a morphological assay that Rab2 Q65L caused vesiculation of VTCs that accumulated at 15°C. These data suggest that the Rab2 protein plays a role in the low-temperature–sensitive step that regulates membrane flow from VTCs to the Golgi complex and back to the ER.
INTRODUCTION
Protein transport through the secretory pathway is mediated by carrier vesicles and tubular elements that selectively deliver cargo between two membrane-bounded compartments. Despite this continuous movement of membrane components, intracellular organelles maintain their identity. This fact indicates that there is a mechanism that ensures temporal and spatial specificity of vesicular traffic. In recent years, a large number of polypeptides have been characterized that participate in this complex trafficking network and include members of the Rab family (Nuoffer and Balch, 1994; Pfeffer, 1994). The Rab proteins are small, monomeric GTPases that interconvert between a GDP- and GTP-bound form that catalyzes a cycle of membrane association and release to the cytosol. When these proteins are membrane associated, they show a unique subcellular location that suggests that Rab proteins regulate distinct transport events. However, the molecular role of Rab proteins in membrane traffic is not clearly understood.
Our interest in characterizing the early events of the secretory pathway has led us to study the Rab2 protein. Rab2 immunolocalizes to pre-Golgi intermediates that consist of clusters of small vesicles and tubules (termed vesicular-tubular clusters or VTCs) (Chavrier et al., 1990; Saraste and Svensson, 1991; Balch et al., 1994). Recently, it has been shown that cargo-containing vesicles that bud from the endoplasmic reticulum (ER) fuse with VTCs that then move en masse to the Golgi complex (Presley et al., 1997). During migration toward the Golgi, VTCs undergo a maturation process that involves recruitment of soluble components required for segregation of anterograde- and retrograde-transported proteins and for downstream activity (Aridor et al., 1995; Scales et al., 1997; Tisdale et al., 1997). This flow of material from pre-Golgi and Golgi compartments back to the ER is essential to preserve organelle integrity and to retrieve escaped proteins.
We have investigated previously the role of Rab2 in ER-to-Golgi transport by generating a family of mutants with altered GTP- or GDP-binding properties and then analyzing the mutants in a transient expression system (Tisdale et al., 1992). An amino acid substitution in the nucleotide-binding domain (Rab2 Q65L) resulted in a protein that was a potent inhibitor of ER-to-Golgi transport. Presumably, this mutation generates an activated Rab2 protein with impaired intrinsic and GAP-stimulated GTPase activity (equivalent to Ras Q61L) (Der et al., 1986; Hall, 1990; Maruta et al., 1991). Although these results provided the first evidence that Rab2 is required for protein traffic, the function of Rab2 in the early secretory pathway is unknown.
Recently, we reported that a peptide corresponding to the N terminus of Rab2 (residues 2–14) and the Rab2 protein stimulated COPI (coatomer and Arf) recruitment to membrane (Tisdale and Jackson, 1998). Coatomer is a soluble complex composed of seven polypeptide subunits (α, β, β′, γ, δ, ε, and ζ) (Waters et al., 1991). The binding of coatomer deforms the membranes that lead to bud formation and the eventual release of a coated vesicle that shuttles cargo to the target compartment. Vesicles containing coatomer were originally identified as intra-Golgi transport intermediates and therefore thought to participate in anterograde traffic (Balch et al., 1984; Orci et al., 1986). However, the observation that yeast mutants with a defective coatomer subunit are unable to retrieve proteins that contain a C-terminal coatomer-binding motif suggests a role for COPI in retrograde traffic (Cosson and Letourneur, 1994; Letourneur et al., 1994; Gaynor and Emr, 1997). In further support of COPI function in retrograde transport, we have shown that an anti-peptide antibody to the C-terminal retrieval motif of p53/gp58 blocks coatomer recruitment and recycling of p53/gp58 from VTCs back to the ER (Tisdale et al., 1997).
In this study, we analyzed the biochemical properties of Rab2 Q65L. The mutant protein was then introduced into several in vitro assays to determine the intracellular site of activity and the mechanism by which Rab2 Q65L inhibits transport. By analogy to other mutant Rab proteins with altered GDP- or GTP-binding properties, this mutant should inhibit a specific stage of secretion (Nuoffer et al., 1994; Pind et al., 1994; Stenmark et al., 1994). We observed that Rab2 Q65L had a profound consequence on VTC integrity. Rab2 Q65L stimulated the release of slowly sedimenting vesicles containing β-COP, Rab2, and p53/gp58 but lacking the cargo molecule vesicular stomatitis virus glycoprotein (VSV-G). These data suggest that the Rab2 protein plays a role in the low-temperature–sensitive step that regulates membrane flow from VTCs. We propose that the activated form of Rab2 initiates the recruitment of soluble components necessary for protein sorting and recycling from pre-Golgi intermediates that results in the generation of retrograde-transported vesicles.
MATERIALS AND METHODS
Materials
The monoclonal antibody to Sar 1 was a gift from Dr. Charles Barlowe (Dartmouth University, Hanover, NH). The monoclonal antibody to the Golgi complex (6F4C5) was provided by Dr. Alan Tartakoff (Case Western Reserve University, Cleveland, OH). Rab2 cDNA was acquired from Dr. Marino Zerial (EMBL, Heidelberg, Germany). CD8 cDNA and antibody were a gift from Dr. Michael Jackson (The R. W. Johnson Pharmaceutical Research Institute, La Jolla, CA). Polyclonal antiserum to VSV-G (P5D4) was purchased from Sigma (St. Louis, MO).
Purification of Recombinant Rab2 Proteins and In Vitro Prenylation
Rab2 Q65L was generated by PCR. The cDNA for Rab2 and Rab2 Q65L was cloned into pET3A (Novagen, Madison, WI) and introduced into BL21 (DE3) pLysS (Novagen). A 1-l culture was grown to an OD600 of 0.4–0.5 and induced with 0.4 mM isopropyl-β-d-thiogalactopyranoside for 3 h at 37°C. The cells were centrifuged at 5000 × g for 10 min at 4°C, and the cell pellet was resuspended in 50 mM Tris, pH 7.4, 1 mM dithiothreitol (DTT), 0.1 mM PMSF, 0.1 mM benzamidine, 1 mM EDTA, and 1% Triton X-100 and then homogenized by 20 passes with a Dounce tissue grinder. Lysozyme (400 μg/ml), DNase I (40 μg/ml), and MgCl2 (25 mM) were added to the homogenate, which was allowed to digest for 30 min at 4°C and then centrifuged at 22,000 × g for 30 min at 4°C. The supernatant was applied to a 70-ml column containing Q Sepharose Fast Flow (Pharmacia, Piscataway, NJ) equilibrated with buffer A (50 mM Tris, pH 7.4, 10 mM MgCl2, and 1.0 mM EDTA), washed with two bed volumes of buffer A, and then eluted with a linear NaCl gradient (0–400 mM) in buffer A. Three-milliliter fractions were collected, and an aliquot of each fraction was separated by SDS-PAGE and immunoblotted with a Rab2 polyclonal antibody. Rab2- or Rab2 Q65L–enriched fractions were pooled, concentrated, applied to a 200-ml column containing Sephacryl S-100 (Pharmacia), and eluted with buffer A. Fractions containing Rab2 or Rab2 Q65L protein were identified by SDS-PAGE and immunoblotting and then pooled and concentrated. To assess the purity of the pooled Rab2 and Rab2 Q65L fractions, we subjected an aliquot of each protein to SDS-PAGE and Coomassie blue staining and then scanned the gel on a densitometer (Molecular Dynamics, Sunnyvale, CA). The recombinant proteins were found to be ∼90% pure. For use in the assays, the purified proteins were first prenylated in an in vitro reaction. Briefly, the isoprenylation reaction was performed in a total volume of 50 μl that contained 0.5 μg of recombinant Rab2 or Rab2 mutant, 10 μg of geranylgeranyl pyrophosphate (Sigma), 1 mM DTT, 25 μl of rat liver cytosol, 10 mM MgCl2, 1 mM ATP, 5 mM creatine phosphate, and 0.2 U of rabbit muscle creatine kinase. The reaction was incubated for 1 h at 37°C and then desalted through a 10-ml column of Sephadex G-25 (Pharmacia) to remove incompatible reagents that may inhibit the in vitro assays. Mock prenylation reactions lacking recombinant Rab2 were performed as described above. The fraction containing prenylated Rab2 and the equivalent fraction of the mock prenylation reaction were collected and concentrated, and the protein concentration was determined by Micro BCA Protein Assay Reagent (Pierce Chemical, Rockford, IL). The mock-treated fraction (control) was added to all assays at equal amounts to the Rab2 protein. Aliquots of the prenylated protein were subjected to partitioning with precycled Triton X-114 (Sigma) as described (Bordier, 1981; Tisdale and Balch, 1996).
Guanine Nucleotide Binding and GTPase Activity
GDP binding was performed as described previously (Nuoffer et al., 1994). Briefly, recombinant Rab2 proteins (0.25 μg, ∼10 pmol) were incubated in a final volume of 100 μl containing 2.5 μM [3H]GDP (diluted to ∼500 cpm/pmol with GDP) in 50 mM HEPES-KOH, pH 8.0, 1 mM DTT, 0.1 mg/ml BSA, 5 mM EDTA, and 4.5 or 10 mM MgCl2. The samples were incubated at 32°C for 1 h, transferred to ice, diluted with ice-cold buffer containing 25 mM Tris-HCl, pH 8.0, 100 mM NaCl, 30 mM MgCl2, 1 mM DTT, and 0.1 mg/ml BSA, and immediately filtered through prewet 0.45 μM nitrocellulose filter discs. The filters were washed three times with buffer, and protein-bound [3H]GDP was quantitated by liquid scintillation counting.
The GTP-binding capacity of Rab2 proteins was analyzed by a filter binding assay (Feig et al., 1986). Rab2 proteins (10 pmol) were incubated for 60 min at 30°C with [α-32P]GTP (600 Ci/mmol) at concentrations ranging from 1 × 10−9 to 1 × 10−4 M in 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 M EDTA, 2.5 mM MgCl2, 1 mM DTT, and 40 μg of BSA. To terminate binding, 2 ml of ice-cold buffer B (25 mM Tris-HCl, pH 7.5, 20 mM MgCl2, and 100 mM NaCl) was added and immediately filtered through prewet 0.45 μM nitrocellulose filter discs. The filters were washed three times with buffer B, and protein-bound [α-32P]GTP was quantitated by liquid scintillation counting.
Measurement of Rab2 intrinsic GTPase activity was measured by a modified method (Stenmark et al., 1994). Rab2 or Rab2 Q65L (50 nM) was incubated in 20 mM Tris-HCl, pH 7.8, 100 mM NaCl, 2.5 mM MgCl2, 1 mM NaP04, 10 mM 2-mercaptoethanol, 0.1% BSA, and 1 pmol of [α-32P]GTP at 37°C. Aliquots were removed at the times indicted in RESULTS, added to an equal volume of 50 mM EDTA, pH 8.0, and then spotted onto polyethyleneimine-cellulose sheets. Chromatograms were developed in 0.6 M NaPO4, pH 3.5. The radioactive spots corresponding to [α-32P]GDP and [α-32P]GTP were quantified using a PhosphorImager (Molecular Dynamics). GTP hydrolysis was calculated as the signal in the GDP spot relative to the total signal.
Analysis of Transport In Vitro
Normal rat kidney (NRK) cells were infected for 4 h with the temperature-sensitive VSV strain ts045 and then biosynthetically labeled with 100 μCi of [35S]-protein labeling mix (New England Nuclear, Boston, MA) for 10 min at 39.5°C to accumulate the VSV-G mutant in the ER. The cells were then perforated by swelling and scraping and used in the ER-to-cis/medial-Golgi transport assay as described (Beckers et al., 1987). Briefly, transport reactions were performed in a final volume of 40 μl in a buffer containing 25 mM HEPES-KOH, pH 7.2, 75 mM KOAc, 2.5 mM MgOAc, 5 mM EGTA, 1.8 mM CaCl2, 1 mM UDP-N-acetylglucosamine, an ATP-regeneration system (1 mM ATP, 5 mM creatine phosphate, and 0.2 IU of rabbit muscle creatine phosphokinase), 5 μl of rat liver cytosol (∼20–25 μg/ml protein in 25 mM HEPES-KOH, pH 7.2, 125 mM KOAc), 125 mM KOAc, and 5 μl of semi-intact cells (∼5 x 107 cells/ml). Rab2 and Rab2 Q65L were added to obtain the final concentration as indicated in RESULTS. The reaction was preincubated on ice for 30 min and subsequently incubated for 90 min at 32°C. Transport was terminated by shifting the reactions to ice, and the membranes were pelleted, solubilized in buffer, and digested with endo H. The samples were analyzed by SDS-PAGE, and the fraction of VSV-G protein processed to the endo H–resistant forms were quantitated by a PhosphorImager (Molecular Dynamics).
Indirect Immunofluorescence
NRK cells plated on coverslips were infected with ts045 VSV-G at 39.5°C for 2–3 h and then shifted to ice and permeabilized with digitonin (20 μg/ml) as outlined previously (Tisdale and Balch, 1996). Coverslips with permeabilized cells were inverted and placed in tissue culture wells that contained the transport cocktail described above, preincubated on ice for 10 min with or without Rab2 (100 ng) or Rab2 Q65L (100 ng), and then incubated for 40 min at 32°C. Alternatively, ts045-infected NRK cells were incubated for 1 h at 39.5°C, shifted to 15°C for 2 h, and then permeabilized and incubated in transport buffer supplemented with or without Rab2 Q65L for 15 min at 15°C. To terminate transport, we transferred the cells to ice and fixed them in 3% formaldehyde and phosphate-buffered saline (PBS) for 10 min. Intracellular ts045 VSV-G was detected by repermeabilization of the fixed cells with 0.05% saponin in PBS and normal goat serum for 10 min, two washes with PBS, and then an incubation for 30 min with a monoclonal antibody to the VSV-G cytoplasmic tail (P5D4) (Sigma). Where indicated, cells were costained with antibody to β-COP (EAGE), (Pepperkok et al., 1993; Tisdale and Jackson, 1998), a polyclonal serum to p53/gp58 (Tisdale et al., 1997), or with an anti-Golgi monoclonal (6F4C5) (Chicheportiche et al., 1984). Cells were then washed with PBS, costained for 30 min with Texas Red anti-rabbit antibody or FITC anti-mouse antibody, washed, mounted, and then viewed under a Zeiss Axiovert fluorescence microscope (Thornwood, NY).
Membrane-Binding Reaction
NRK cells were washed three times with ice-cold PBS. The cells were scraped off the dish with a rubber policeman into 10 mM HEPES, pH 7.2, and 250 mM mannitol and then broken with 15 passes of a 27-gauge syringe. The broken cells were pelleted at 500 × g for 10 min at 4°C, and the supernatant was recentrifuged at 20,000 × g for 20 min at 4°C. The pellet containing ER, pre-Golgi, and Golgi membranes was washed with 1 M KCl in 10 mM HEPES, pH 7.2, for 10 min on ice and then centrifuged at 20,000 × g for 20 min at 4°C. The membranes were resuspended in 10 mM HEPES, pH 7.2, and 250 mM mannitol and used in the binding reaction as described previously (Aridor et al., 1995; Tisdale and Jackson, 1998). Membranes (30 μg of total protein) were added to a reaction mixture that contained 27.5 mM HEPES, pH 7.2, 2.75 mM MgOAc, 65 mM KOAc, 5 mM EGTA, 1.8 mM CaCl2, 1 mM ATP, 5 mM creatine phosphate, and 0.2 U of rabbit muscle creatine kinase. Rab2 Q65L was added to obtain the final concentration as indicated in RESULTS, and the reaction mix was incubated on ice for 10 min. Rat liver cytosol (50 μg) and 2.0 μM GTPγS or 2.0 μM GTP were added, and the reactions were shifted to 32°C (ts045) or 37°C and incubated for 10 min. The binding reaction was terminated by transferring the samples to ice and then centrifuging at 20,000 × g for 10 min at 4°C. The pellet (P1) was resuspended in sample buffer, and the resulting supernatant was recentrifuged at 30 psi in an airfuge to recover released vesicles (P2). In some cases, P2 was subjected to equilibrium density centrifugation as described previously (Ostermann et al., 1993). P2 was mixed with 1 ml of 70% sucrose and overlayed with 5 ml of a 30–50% sucrose. The gradient was centrifuged at 50,000 rpm for 14 h at 4°C and fractionated from the bottom into 300-μl fractions. The recovered fractions were pelleted by ultracentrifugation (100,000 rpm for 30 min at 4°C). Both pellets from the binding assay (P1 and P2) were separated by SDS-PAGE and transferred to nitrocellulose in 25 mM Tris, pH 8.3, 192 mM glycine, and 20% methanol. The membrane was blocked in Tris-buffered saline that contained 5% nonfat dry milk and 0.5% Tween-20, incubated with an affinity-purified polyclonal antibody made to the EAGE peptide of β-COP (Tisdale and Jackson, 1998), a polyclonal antibody to Rab2, a polyclonal antibody to p53/gp58 (Tisdale et al., 1997), a monoclonal antibody to VSV-G (P5D4) (Sigma), a monoclonal antibody to protein disulfide isomerase (PDI) (Stressgen, Victoria, British Columbia, Canada), a monoclonal to the c-myc epitope (9E10D) (Upstate Biotechnology, Lake Placid, NY), a polyclonal to Sar I, or a monoclonal antibody to CD8, washed, further incubated with a horseradish peroxidase–conjugated anti-rabbit or anti-mouse antibody, developed with enhanced chemiluminescence (Amersham, Arlington Heights, IL), and then quantitated by densitometry.
RESULTS
Biochemical Characterization of Purified Rab2 Proteins
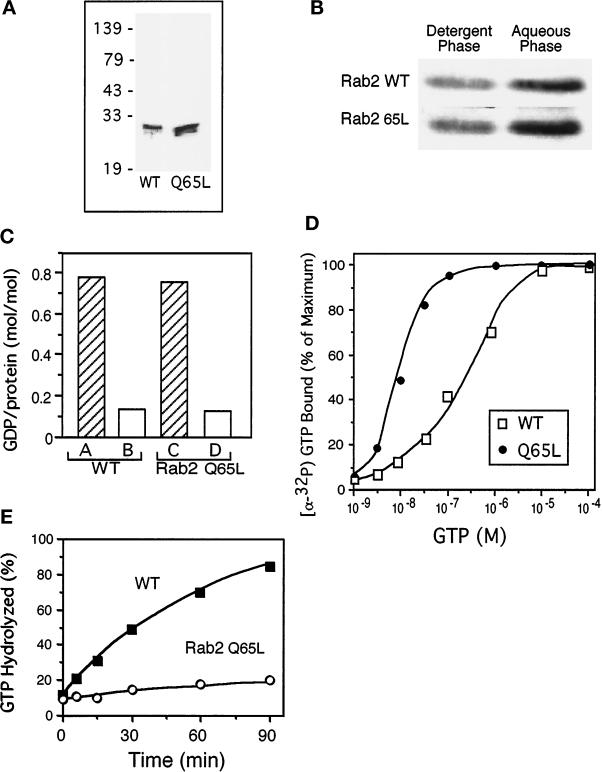
Rab2 wild type and Rab2 Q65L were purified after expression in Escherichia coli using ion exchange chromatography and gel filtration (Figure 1A). Typically, the proteins prepared by this protocol were 90% pure as assessed by SDS-PAGE and Coomassie blue staining. Pooled fractions of the purified recombinant proteins were dialyzed against a buffer containing GDP and then isoprenylated in an in vitro reaction. The proteins were subjected to phase separation in Triton X-114 to determine the extent of prenylation. In general, ∼40–45% of the total Rab2 and Rab2 Q65L proteins in the reaction distributed to the detergent phase (Figure 1B) and therefore were modified and capable of membrane association. The proteins were further characterized to establish their guanine nucleotide–binding properties. The ability to bind and exchange GDP was assayed by incubation of equal amounts of Rab2 or Rab2 Q65L in the presence of [3H]GDP and either low Mg2+ (4.5 mM MgCl2, 5 mM EDTA) that stimulates dissociation or high Mg2+ (10 mM MgCl2, 5 mM EDTA) that results in the slow exchange of exogenously added GDP or GTP. Figure 1C shows that in low Mg2+, the wild type (bar A) and mutant (bar C) efficiently exchanged protein-bound GDP for the radiolabeled nucleotide. However, in the presence of high Mg2+, the binding of [3H]GDP to Rab2 wild type (bar B) and Rab2 Q65L (bar D) was greatly reduced. It appears that the purified proteins are 70–75% active and are similar in their GDP-binding property.
Figure 1.
Guanine nucleotide–binding properties of bacterially produced Rab2 proteins. (A) Rab2 wild type and Rab2 Q65L were purified after expression in E. coli using ion exchange chromatography and gel filtration. The proteins were analyzed by separation on SDS-PAGE and Coomassie blue staining, and then the gel was scanned on a densitometer. The recombinant proteins were found to be ∼90% pure. (B) The purified proteins (0.5 μg) were prenylated in an in vitro reaction and then subjected to phase partitioning in Triton X-114, and the distribution of the Rab2 proteins was analyzed by SDS-PAGE and immunoblotting. The prenylation efficiency is ∼40–45% as indicated by partitioning with the detergent-rich phase. (C) Rab2 and Rab2 Q65L (10 pmol) were incubated for 1 h with 2.5 μM [3H]GDP (∼500 cpm/pmol) in the presence of 5 mM EDTA and 4.5 mM MgCl2 (bars A and C) or 10 mM MgCl2 (bars B and D), and the amount of protein-bound [3H]GDP was determined as described in MATERIALS AND METHODS. The recombinant proteins are ∼70–75% active based on their ability to bind and exchange GDP. (D) Equilibrium binding of [α-32P]GTP by Rab2 wild type and mutant is shown. Rab2 proteins (10 pmol) were incubated for 60 min at 30°C with increasing concentrations of [α-32P]GTP (1 × 10−9 to 1 × 10−4 M). Protein-bound [α-32P]GTP was captured on nitrocellulose membranes and then quantitated by liquid scintillation counting as described in MATERIALS AND METHODS. The results are normalized to the amount of binding at the highest concentration of [α-32P]GTP for each protein. (E) GTPase hydrolytic activity was measured by in-cubating Rab2 and Rab2 Q65L with [α-32P]GTP at 37°C. At the times indicated, the reactions were terminated with the addition of ice-cold 0.5 M EDTA, and the [32P]-labeled nucleotides bound to the proteins were analyzed by TLC. Results shown in all panels are representative of three independent protein purifications.
The proteins were then evaluated for their ability to bind GTP. Rab2 and Rab2 Q65L were incubated with increasing concentrations of [α-32P]GTP for 1 h at 30°C, and the amount of bound nucleotide was determined by a nitrocellulose filter–binding assay (Feig et al., 1986). As shown in Figure 1D, the concentration for half-maximal GTP binding by Rab2 was ∼2 × 10−7 M compared with ∼1 × 10−8 for the mutant protein. The Q65L substitution made in Rab2 resulted in ∼20-fold enhanced GTP binding without altering the mutant protein–binding capacity for GDP.
Because the mutation that was introduced into Rab2 should render the protein defective in GTPase activity, we measured the hydrolytic activity (Wagner et al., 1987; Stenmark et al., 1994). Purified proteins were incubated with [α-32P]GTP at 37°C. At the times indicated (Figure 1E), the reactions were terminated by the addition of ice-cold 0.5 M EDTA, and the [32P]-labeled nucleotides bound to the proteins were analyzed by TLC. Rab2 preloaded with [α-32P]GTP rapidly converted the bound nucleotide into [α-32P]GDP. Under the conditions used in the assay, wild-type GTPase activity increased in a linear manner for 90 min. In contrast, the GTPase activity of Rab2 65L was approximately five- to eightfold slower than was the activity of wild type that argues that the substitution at residue 65 significantly affects the ability of Rab2 to hydrolyze GTP. These combined biochemical studies demonstrate that recombinant Rab2 and Rab2 Q65L are active and exhibit similar guanine nucleotide–binding properties as has been reported for other Rab proteins and Rab mutants (Wagner et al., 1987; Touchot et al., 1989; Shapiro et al., 1993; Stenmark et al., 1994).
Rab2 Q65L Inhibits ER-to-Golgi Transport In Vitro
To study the effect of Rab2 and Rab2 Q65L on the early secretory pathway, we introduced the recombinant proteins into an in vitro transport assay. This assay uses tissue culture cells infected with ts045 VSV-G, a virus that synthesizes a protein with a thermoreversible defect that results in ER retention at 39.5°C (Gallione and Rose, 1985). The plasma membrane of these cells is perforated to release soluble content but retain functional ER and Golgi stacks. Incubation of the perforated cells at the permissive temperature of 32°C initiates export of ts045 VSV-G from the ER in the presence of cytosol and ATP. This semi-intact cell assay measures transport of ts045 VSV-G protein by following the processing of the two N-linked oligosaccharides to endo H–resistant forms (Beckers et al., 1987).
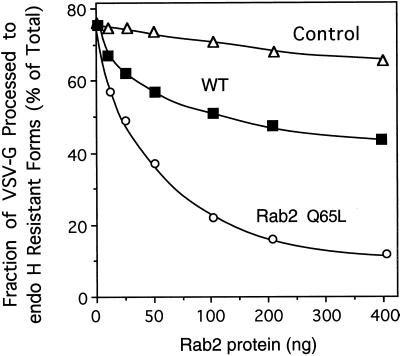
The recombinant proteins were incubated for 1 h in the presence of rat liver cytosol and geranylgeranyl pyrophosphate to promote isoprenylation and then were added to the transport assay. Rab2 Q65L inhibited processing of VSV-G to endo H–resistant forms in a dose-dependent manner (Figure 2). Transport was reduced by 50% at a mutant protein concentration of ∼50 ng (total Rab protein that includes prenylated and unprenylated forms). The mutant protein must compete with ∼5–10 ng of endogenous Rab2. Wild-type Rab2 at high concentrations (200–400 ng) resulted in a partial (30%) arrest in transport. A similar decrease in the processing of VSV-G to endo H–resistant forms has been observed after transient overexpression of Rab2 (Tisdale and Balch, 1996). It seems that the endogenous pool of Rab2 is highly controlled to ensure normal secretory activity and to maintain balance between other components of the trafficking machinery. The results of this experiment are consistent with the in vivo data and indicate that the phenotype of the wild-type and Rab2 Q65L proteins has been successfully reconstituted in vitro.
Figure 2.
Rab2 Q65L inhibits ER-to-Golgi transport in vitro. Semi-intact NRK cells were preincubated with the indicated amount of Rab2 (WT), Rab2 Q65L, or mock-prenylated control (lacking recombinant Rab2 protein) for 30 min on ice in a transport mixture as described in MATERIALS AND METHODS. The cells were then transferred to 32°C and incubated for a total of 90 min. The fraction of VSV-G processed to the endo H–resistant forms (percent of total) was determined after analysis by SDS-PAGE and fluorography. Rab2 Q65L inhibited transport in a dose-dependent manner. Results shown are representative of three independent protein purifications.
Rab2 Q65L Blocks Transport after the Calphostin C–sensitive Step
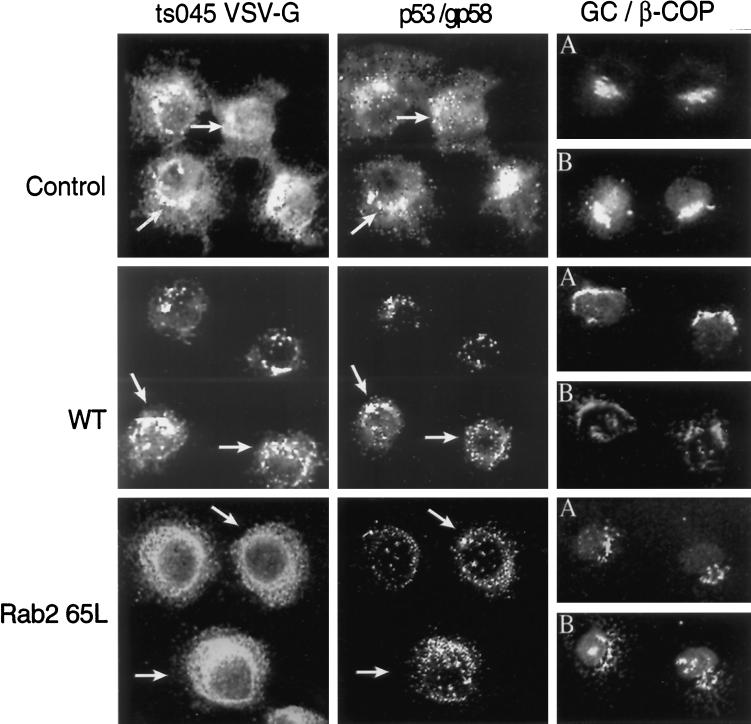
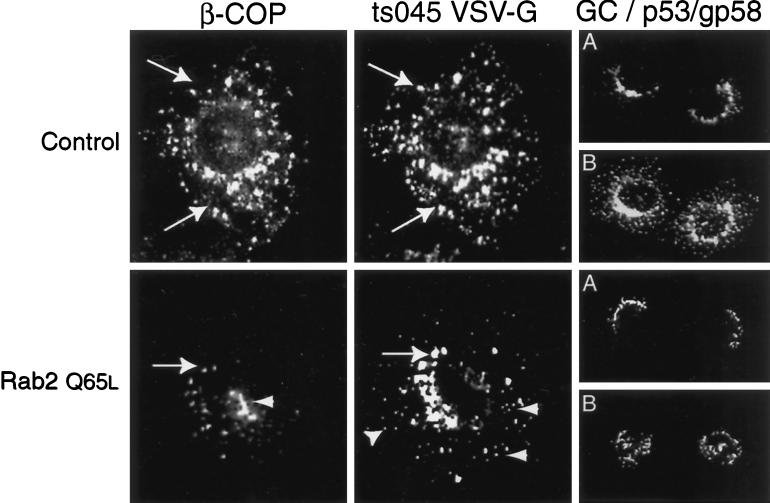
To define the morphological site of VSV-G accumulation in response to Rab2 Q65L, we infected NRK cells with ts045 VSV-G for 3 h at the nonpermissive temperature to restrict VSV-G to the ER. The cells were then permeabilized and incubated in the presence or absence of Rab2 or Rab2 Q65L for 40 min at 32°C, and the distribution of ts045 VSV-G was determined by indirect immunofluorescence. Control cells efficiently transported the reporter molecule to the Golgi complex and to perinuclear VTCs labeled with antibody to p53/gp58 and β-COP (Figure 3). In contrast, cells incubated with Rab2 Q65L failed to transport ts045 VSV-G to the Golgi complex. VSV-G was found in punctate peripheral structures and was diffusely distributed throughout the cytoplasm in an ER staining pattern. A large portion of the VSV-G accumulated in a collar-like structure composed of small vesicular elements that ringed the nucleus (Figure 3). Unlike the control, β-COP–labeled structures showed a similar distribution to numerous p53/gp58-stained intermediates that appear located on top of the nucleus. We found no change in the structure of the Golgi complex after incubation with Rab2 wild type or with the mutant protein. These morphological results suggest that Rab2 Q65L reduces ER export to pre-Golgi intermediates.
Figure 3.
Cells incubated with Rab2 Q65L fail to transport ts045 VSV-G to the Golgi complex. NRK cells grown on coverslips were infected with ts045 VSV-G for 3 h at 39.5°C to retain ts045 VSV-G in the endoplasmic reticulum. The cells were rapidly shifted to ice, permeabilized with digitonin, and then incubated in a complete transport cocktail in the absence (Control) or presence of 100 ng of Rab2 (WT) or 100 ng of Rab2 Q65L for 40 min at 32°C. The distribution of ts045 VSV-G (left), p53/gp58 (middle), β-COP (B), and Golgi (A) staining was determined as described in MATERIALS AND METHODS. Arrows denote the alignment of costained cells. GC, Golgi complex.
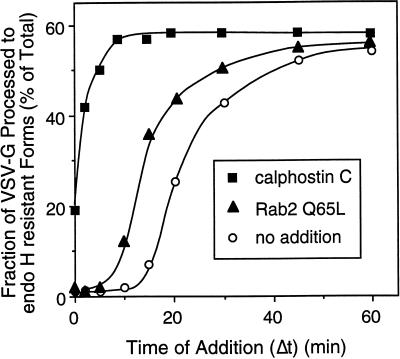
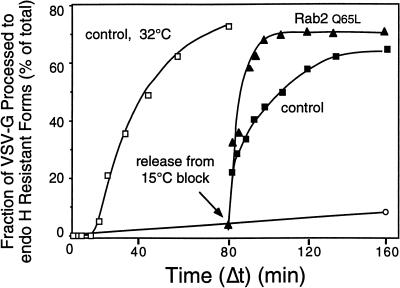
Previous in vitro studies have shown that VSV-G transport from the ER to the cis/medial-Golgi compartments requires ∼15–20 min before detection of endo H–resistant forms (Davidson and Balch, 1993). During this time, VSV-G is exported from the ER to VTCs and then subsequently transported to the Golgi complex. The time period in secretion sensitive to Rab2 Q65L was investigated by determining the effect of the mutant protein on transport relative to the site of action of calphostin C. This specific protein kinase inhibitor blocks the budding of ER-derived transport vesicles containing VSV-G (Fabbri et al., 1994). If our interpretation of the morphological data was correct, then Rab2 Q65L should arrest the transport at the stage identical to the step inhibited by calphostin C. To address this issue, semi-intact ts045-infected NRK cells were incubated at 32°C and at the indicated time (Δt) were then transferred to ice (control) or supplemented with either 100 nM calphostin C or 100 ng of Rab2 Q65L and incubated for a total of 60 min. This protocol allows any VSV-G that has migrated past the calphostin C– or Rab2 Q65L–sensitive step to continue migration to the cis-Golgi compartment. Therefore, the fraction of VSV-G processed to endo H resistance will depend on the amount of the reporter molecule that has transported beyond the calphostin C– or Rab2 Q65L–sensitive step at the time of their addition. In control cells, VSV-G endo H–resistant forms were detected after a 15-min lag. Within 20 min of incubation, 50% of the VSV-G protein was processed, indicating migration to the Golgi complex (Figure 4). Cells treated with Rab2 Q65L were blocked in transport ∼5–10 min before the processing of VSV-G to endo H–resistant forms, and by 15 min of incubation >70% of the VSV-G had transported through the Rab2 Q65L–sensitive step. This temporal site of inhibition by Rab2 Q 65L is ∼10 min later than the block by calphostin C. These results conflicted with our interpretation of the morphological data and indicated that Rab2 Q65L arrests transport after VSV-G exit from the ER but before delivery of cargo to the Golgi complex.
Figure 4.
The inhibitory activity of Rab2 Q65L is downstream from the calphostin C–sensitive step. Semi-intact NRK cells were incubated at 32°C in a transport mixture described in MATERIALS AND METHODS. At the indicated time (Δt), the control (○) was transferred to ice, or 120 nM calphostin C (▪) or 100 ng of Rab2 Q65L (▴) was added and incubated with the cells for a total of 60 min. Transport was terminated by transfer of the cells to ice, and the fraction of VSV-G processed to endo H–resistant forms was determined as described in MATERIALS AND METHODS. Results shown are representative of three independent protein purifications.
Rab2 Q65L Arrests Transport from Pre-Golgi Intermediates (VTCs)
The immunolocalization of Rab2 to VTCs (Chavrier et al., 1990) as well as our previous results that mapped Rab2 activity to VTCs (Tisdale and Balch, 1996) prompted us to study the effect of Rab2 Q65L on transport through pre-Golgi intermediates. Incubation of intact or permeabilized cells at a reduced temperature (15°C) leads to the accumulation of ts045 VSV-G protein in VTCs. To determine whether Rab2 Q65L inhibited transport from these structures, we first preincubated semi-intact cells at 15°C for 80 min. When control cells were then transferred to 32°C (Figure 5, ▪), no lag was observed in VSV-G processing to endo H–resistant forms. In contrast, export from the ER has a 15- to 20-min lag that precedes VSV-G acquisition of endo H resistance (Figure 5, □). These results indicate that VSV-G accumulated in VTCs during the preincubation period. Rab2 Q65L addition before release from the 15°C block prevented processing of ts045 VSV-G during a subsequent 80-min incubation at the permissive temperature (Figure 5, ○). However, within 7 min of incubation at 32°C, transport of VSV-G was primarily (>80%) insensitive to Rab2 Q65L (Figure 5, ▴). This movement of VSV-G to a Rab2 Q65L–insensitive step within minutes of release from the temperature block suggests that the mutant protein acts during a rate-limiting event in VTC migration to the Golgi complex.
Figure 5.
VSV-G is rapidly transported through the Rab2 Q65L–sensitive step after accumulation at 15°C. Semi-intact NRK cells were either incubated in a transport mixture for the indicated time at 32°C (□) or preincubated at a reduced temperature (15°C) for 80 min to accumulate VSV-G in pre-Golgi VTCs. Cells preincubated at 15°C were maintained at 15°C (○), shifted to 32°C and incubated for the indicated time (Δt) before transfer to ice (▪), or supplemented with 100 ng of Rab2 Q65L and incubated for a total of 80 min (▴). The fraction of VSV-G processed to endo H–resistant forms was determined as described in METHODS AND MATERIALS. Results shown are representative of three independent protein purifications
Pre-Golgi Intermediates Vesiculate in the Presence of Rab2Q 65L
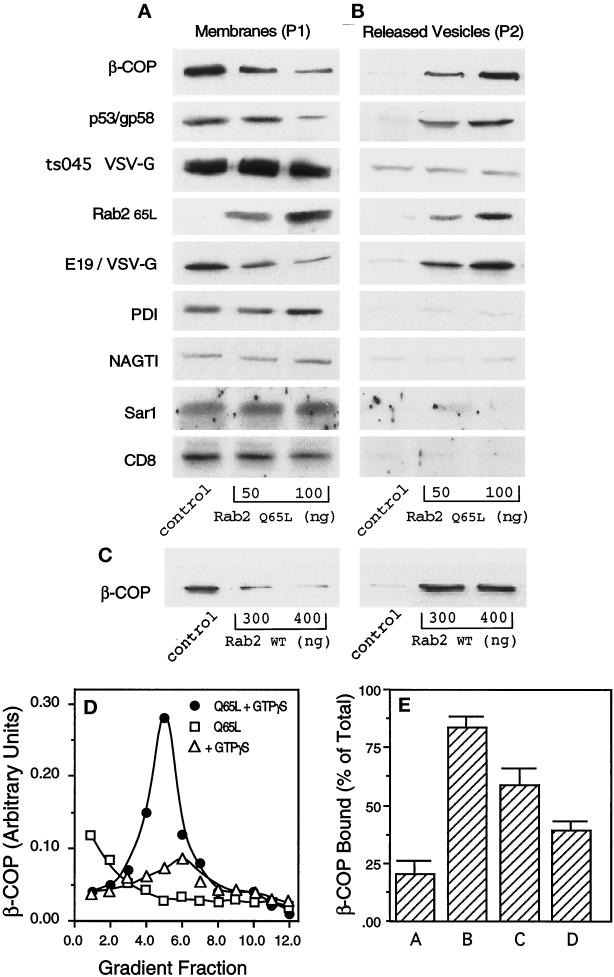
Because our previous studies showed that the Rab2 peptide and Rab2 protein promoted COPI membrane association (Tisdale and Jackson, 1998), a quantitative binding assay was used to measure β-COP recruitment in the presence of Rab2 Q65L. For this assay, microsomes were prepared from whole-cell homogenates and washed with 1 M KCl to remove prebound COPI. These membranes were preincubated in buffer for 10 min on ice with or without 50 or 100 ng of the mutant protein, then supplemented with GTPγS and rat liver cytosol, and incubated at 37°C for 10 min. To terminate the reaction, we collected membranes by centrifugation (20,000 × g). The resulting pellet (P1) containing rapidly sedimenting membranes (ER and pre-Golgi and Golgi compartments) was separated by SDS-PAGE, transferred to nitrocellulose, and then probed with an affinity-purified antibody to β-COP. Rab2 Q65L–treated membranes showed a dose-dependent decrease in β-COP recruitment (Figure 6A). Membrane-associated β-COP was reduced by 75% in the presence of 100 ng of the mutant protein. A similar decrease in β-COP binding was observed when the reaction was supplemented with GTP (our unpublished observations). On the basis of our previous observations with the Rab2 peptide and Rab2 protein, we anticipated that the activated protein would enhance COPI membrane binding. The lack of COPI recruitment by Rab2 Q65L suggested that the mutant protein either blocked binding of β-COP or generated vesicles containing β-COP. To address this question, we centrifuged the supernatant from the reaction at high speed (100,000 × g) to recover any vesicles that may have been released during the incubation. The resulting pellet (P2) was subjected to SDS-PAGE and Western blotting. Figure 6B shows that microsomes treated with increasing concentrations of the mutant protein released membranes containing β-COP into the supernatant. This result was also obtained when membranes were treated with high concentrations of Rab2 wild type (Figure 6C).
Figure 6.
Rab2 Q65L stimulates vesicle release from microsomes. (A and B) Microsomes were prepared from either NRK cell homogenates, ts045 VSV-G–infected NRK cells, or NRK cells transfected with c-myc NAGT I (Nilsson et al., 1993) or transfected with CD8 as described in MATERIALS AND METHODS and then were preincubated with 50 or 100 ng of Rab2 Q65L for 10 min on ice. Control membranes were preincubated with an equal volume of the mock-prenylated fraction (comparable with 100 ng of Rab2 Q65L), obtained as described in MATERIALS AND METHODS. Cytosol and GTPγS were added, and the incubations transferred to 37°C for 10 min to promote recruitment of soluble factors. Microsomes were collected by centrifugation (20,000 × g for 10 min) to obtain a pellet (P1). The supernatant was recentrifuged at 100,000 × g for 30 min, and the resulting pellet (P2) and P1 were separated by SDS-PAGE and immunoblotted with the respective antibodies. The blot was developed with enhanced chemiluminescence and then quantitated by densitometry. (C) Microsomes prepared from NRK cell homogenates were preincubated with 300 or 400 ng of Rab2 for 10 min on ice. Cytosol and GTPγS were added, and the incubations were then transferred to 37°C for 10 min and processed as described above. (D) P2 from control membranes incubated with GTPγS (▵), P2 from Rab2 Q65L–treated membranes incubated with GTPγS (●), or P2 from Rab2 Q65L–treated membranes incubated without GTPγS (□) was subjected to equilibrium density centrifugation as described in MATERIALS AND METHODS. The gradient was fractionated from the bottom into 300-μl fractions, and the recovered fractions were pelleted by ultracentrifugation (100,000 rpm for 30 min at 4°C) and then separated by SDS-PAGE and immunoblotted for β-COP. (E) Microsomes were preincubated in buffer with or without 200 μM BFA for 15 min on ice and then supplemented with 100 ng of Rab2 Q65L, GTPγS, and rat liver cytosol and incubated at 37°C for 10 min. The membranes were collected as described above. P1 and P2 were separated by SDS-PAGE, transferred to nitrocellulose, and then probed with an affinity-purified antibody to β-COP. Bar A, P1 from membranes incubated with Rab2 Q65L; bar B, P2 from membranes incubated with Rab2 Q65L; bar C, P1 from membranes coincubated with BFA and Rab2 Q65L; and bar D, P2 from membranes coincubated with BFA and Rab2 Q65L.
To ensure that the P2 fraction contained bona fide vesicles and not simply aggregates of β-COP that failed to bind to membrane, we subjected the slowly sedimenting pellet to equilibrium density gradient centrifugation (Ostermann et al., 1993). The gradient fractions were separated by SDS-PAGE, and the presence of β-COP protein was determined by Western blotting. In the absence of GTPγS, β-COP was found at the bottom of the gradient and is presumably soluble protein. Membrane-associated β-COP peaked at 44% (wt/wt) sucrose (Figure 6D, fraction 5). This density is slightly greater than the density of the major peak (42% wt/wt) of COPI-coated vesicles formed from Golgi membranes (Malhotra et al., 1989). It is possible that the β-COP vesicles generated after treatment with Rab2 Q65L are derived from a different subcellular compartment. As further proof that the β-COP material in the P2 fraction resided on membrane, the binding reaction was performed in the presence of brefeldin A (BFA). BFA inhibits the interaction of Arf with membrane that in turn prevents the membrane association of coatomer (Orci et al., 1991; Donaldson et al., 1992). Because previous studies showed that Arf activity was required for Rab2 to recruit COPI (Tisdale and Jackson, 1998), we predicted that the ability of Rab2 Q65L to generate coated vesicles would be lost when membranes are cotreated with BFA. To pursue this issue, microsomes were preincubated in buffer with or without 200 μM BFA for 15 min on ice and then supplemented with 100 ng of Rab2 Q65L, GTPγS, and rat liver cytosol and incubated at 37°C for 10 min. The membranes were collected as described above, separated by SDS-PAGE, transferred to nitrocellulose, and then probed with an affinity-purified antibody to β-COP. Membranes coincubated with BFA and Rab2 Q65L showed ∼50% reduction in the release of vesicles containing β-COP compared with membranes incubated with only the mutant protein (Figure 6E, compare bars B and D). These combined results are highly suggestive that the activated form of Rab2 plays a role in vesicle formation.
The protein content of P2 was further characterized by probing the blot with specific antibodies (Figure 6B). Rab2 Q65L was also found associated with these newly formed vesicles. The cytosolic contribution of wild-type Rab2 is barely detectable in control P1 and P2. Most likely the majority of the Rab2 signal is contributed by the mutant protein. The slowly sedimenting membranes (P2) are not enriched in the ER marker PDI and are devoid of the Golgi enzyme N-acetylglucosaminyltransferase I (NAGT I) (Table 1). Because ts045 VSV-G follows the secretory pathway, we prepared microsomes from cells infected with the virus to learn whether the liberated vesicles contained anterograde-directed cargo. Interestingly, Rab2 Q65L–generated vesicles contained a trivial amount of ts045 VSV-G (Figure 6B and Table 1). A second cell surface protein was then evaluated to determine whether the protein behaved in the same manner. Microsomes were prepared from NRK cells transfected with CD8 cDNA. The membranes did not release CD8-containing vesicles in the presence of Rab2 Q65L (Figure 6B). These combined results imply that the liberated vesicles may not traffic in the forward direction. We pursued this interpretation by determining whether the vesicles produced by Rab2 Q65L treatment contained recycling proteins. A reporter molecule was constructed that encodes the ecto- and transmembrane domains of VSV-G in tandem with the cytoplasmic tail of the adenoviral protein E3/19K (E19) (Herisse et al., 1980). The extreme C terminus of E19 contains the KKXX motif required for protein retrieval from post-ER compartments (Jackson et al., 1993). Membranes from NRK cells transfected with the E19/VSV-G construct were used in the binding assay. As shown in Figure 6B, slowly sedimenting vesicles that formed after incubation with Rab2 Q65L were enriched in E19/VSV-G as well as in the endogenous recycling protein p53/gp58 (Table 1). The presence of β-COP, p53/gp58, and E19/VSV-G and the exclusion of PDI, NAGT I, Sar I, and the cargo molecules ts045 VSV-G and CD8 in vesicles formed after incubation with Rab2 Q65L are highly suggestive that the released membranes are retrograde-directed vesicles.
Table 1.
Fold enrichment of Proteins in P2
| Protein | Rab2 Q65L
|
|
|---|---|---|
| 50 ng | 100 ng | |
| β-COP | 6.0 | 11.5 |
| p53/gp58 | 4.2 | 6.2 |
| ts045 VSV-G | 0 | 0 |
| Rab2 Q65L | 5.3 | 14.0 |
| E19/VSV-G | 8.8 | 13.8 |
| PDI | 0 | 0 |
| NAGTI | 0 | 0 |
| Sar1 | 0 | 0 |
| CD8 | 0 | 0 |
The high-speed pellet (P2) from the microsomal binding assay was separated by SDS-PAGE and immunoblotted with a battery of antibodies. The blot was developed with enhanced chemiluminescence and then quantitated by densitometry. Total signal for each protein in P2 (control) was normalized to a value of 1 (see Figure 6). Fold enrichment was calculated based on chemiluminescent signal of each marker in the P2 fraction.
These results were further investigated in the morphological assay. ts045 infected–NRK cells were first incubated for 80 min at 15°C to accumulate VSV-G in peripheral pre-Golgi intermediates. The cells were then permeabilized and incubated at 15°C for 15 min in the absence or presence of 100 ng of Rab2 Q65L. The distribution of ts045 VSV-G and β-COP was analyzed by indirect immunofluorescence. In control cells, anti-β-COP antibody labeled the juxtanuclear Golgi complex and vesicular structures scattered throughout the cytoplasm and in proximity to the Golgi complex. The majority of these structures codistribute with intermediates that label with antibody to VSV-G (Figure 7). The staining pattern of the VTC marker p53/gp58 was similar to that observed with antibody to β-COP and VSV-G.
Figure 7.
Peripheral VTCs containing β-COP become undetectable after incubation with Rab2 Q65L. ts045 VSV-G–infected NRK cells were incubated for 80 min at 15°C to accumulate VSV-G in VTCs. The cells were then digitonin permeabilized and incubated in transport buffer in the absence or presence of 100 ng of Rab2 Q65L for 15 min at 15°C. The distribution of β-COP (left), ts045 VSV-G (middle), p53/gp58 (B), and Golgi (A) staining was determined as described in MATERIALS AND METHODS. Arrows denote the alignment of costained cells. Arrowheads indicate peripheral VTCs.
We detected a striking change in VTC distribution after addition of Rab2 Q65L (Figure 7). Unlike control cells, the cell periphery of mutant-treated cells was sparsely decorated with punctate β-COP–labeled structures. In fact, peripheral VTCs containing β-COP that had accumulated during the 15°C incubation became nearly undetectable. Instead, the cells displayed vesicular aggregates containing β-COP over the nucleus that appeared to overlap with p53/gp58-stained structures (Figure 7). These β-COP–labeled aggregates did not codistribute with VSV-G. A less dramatic change in the distribution of VSV-G occurred after incubation with Rab2 Q65L. Mutant-treated cells contained VSV-G–stained structures that were much smaller than the VSV-G–labeled VTCs found in the control. However in stark contrast to β-COP, the VSV-G–containing elements remained in a peripheral distribution. These results suggest that a sorting event has occurred in which β-COP–containing structures have segregated from VSV-G–containing elements. Importantly, the effect of Rab2 Q65L is specific to VTCs. In no case did we observed a change in the structure of the Golgi complex.
DISCUSSION
The role of the highly conserved DTAGQE motif in Rab2 (Ras numbering 57–62) was investigated because it has been shown that an amino acid substitution made at Q61 reduces Ras GTPase activity that favors formation of the active, GTP-bound form (Der et al., 1986; Maruta et al., 1991). We found that the equivalent mutation in Rab2 (Rab2 Q65L) resulted in a protein that was a potent inhibitor of ER-to-Golgi transport when transiently overexpressed (Tisdale et al., 1992). Here, the purification of Rab2 Q65L was pursued to characterize biochemically the protein and to determine the mechanism of transport inhibition by the mutant.
The nucleotide-binding properties were first analyzed to ensure that the recombinant proteins were active and therefore could be studied in vitro. Both Rab2 and Rab2 Q65L bound and exchanged a comparable amount of GDP. Although both proteins were capable of binding GTP, the mutant protein possessed an ∼20-fold apparent increase in GTP-binding affinity. It is reasonable to assume that this finding reflects the low GTP hydrolysis rate of Rab2 Q65L compared with that of the wild-type protein. This property stabilizes the GTP-bound form and thereby promotes and prolongs events regulated by Rab proteins (Rybin et al., 1996).
The recombinant proteins are biologically active. Similar to the results obtained in the transient expression system, the Rab2 Q65L protein was an effective trans-dominant inhibitor of ER-to-Golgi traffic. Indirect immunofluorescence studies suggested that Rab2 Q65L interfered with VSV-G exit from the ER or soon after ER export. However, kinetic experiments designed to map the site of Rab2 Q65L action in the early secretory path indicated that the mutant blocked protein traffic downstream from the site of action of calphostin C. This protein kinase inhibitor prevents vesicle budding and export from the ER (Fabbri et al., 1994). Although this result suggested that Rab2 Q65L does not inhibit budding from the ER, the possibility remained because the release of single vesicles cannot be detected. These conflicting interpretations caused us to study the consequence of Rab2 Q65L on VSV-G transit from VTCs. The fact that Rab2 immunolocalizes to pre-Golgi intermediates is highly suggestive that the mutant protein could interfere with VTC function.
We evaluated the biochemical effect of Rab2 Q65L on VSV-G transport from VTCs and found that the temperature-dependent event that regulates VTC migration to the Golgi complex is sensitive to Rab2 Q65L. Additionally, a striking change in VTC distribution occurred at 15°C. Unlike the prominent β-COP– and p53/gp58-labeled cytoplasmic VTCs found in control cells, peripheral VTCs containing β-COP and p53/gp58 became undetectable after the addition of Rab2 Q65L. Most likely vesicles containing these markers exist but are too small to be observed by indirect immunofluorescence. The biochemical data from the microsomal binding assay support this interpretation. In that regard, membranes incubated with increasing concentrations of Rab2 Q65L released vesicles containing β-COP, Rab2, and the recycling proteins p53/gp58 and E19/VSV-G but lacking anterograde-directed cargo. These vesicles are slightly greater in density than are the Golgi-derived coated vesicles. Although the microsomal binding reaction used in this study has been reported to promote coated vesicle formation from Golgi membranes (Orci et al., 1993; Palmer et al., 1993), NAGT I was not detected in the high-speed pellet. The absence of this Golgi marker is caused by the specific activity of Rab2 within VTCs. We learned from previous studies that the Rab2 peptide does not effect transport through the Golgi complex or alter Golgi structure (Tisdale and Balch, 1996). Because the density of membranes involved in secretion decreases from the ER through the trans-Golgi network, it is possible that the released vesicles are derived from pre-Golgi compartments. On a similar note, the intermediate compartment marker protein p58 was shown to distribute to a slightly denser fraction than Golgi membranes when separated on a Nycodenz gradient (Hammond and Helenius, 1994). On the basis of these results, we believe that Rab2 Q65L promoted vesicle formation from peripheral VTCs.
The ability of the mutant protein to stimulate vesicle budding from peripheral VTCs is not limited to Rab2 Q65L. Indeed, we found that membranes treated with high concentrations of Rab2 wild type also released vesicles containing β-COP. These observations support our proposal that Rab2 plays a role in protein sorting and recycling from pre-Golgi intermediates. Because anterograde and retrograde transport are coupled, the block in ER-to-Golgi transport obtained in the biochemical and morphological assays is presumably caused by enhanced VTC fragmentation in response to the mutant protein. The carriers that bud from VTCs contain Rab2 Q65L and, because of the protein’s slow dissociation from membrane, potentiate COPI recruitment. The retrograde vesicles are therefore unable to uncoat and ultimately retrieve components of the trafficking machinery. This would explain the reticular staining pattern of ts045 VSV-G in the presence of Rab2 Q65L. The initial target for newly budded ER vesicles is VTCs (Presley et al., 1997). In their absence or failure to obtain a critical size, ER-derived transport vesicles are stalled in forward movement.
Our previous studies showed that the Rab2 peptide caused a dramatic increase in COPI recruitment, yet we did not detect released coated vesicles. This result suggests that the peptide might interfere with VTC vesiculation by interaction with a downstream effector required for budding. Alternatively, specific domains of the intact protein may be required to recruit a soluble factor or complex necessary for fission. We favor this interpretation based on results showing that an N-terminal deletion mutant (Rab2 Q65L Δ15) stimulates vesicle formation comparable with that of the intact mutant (our unpublished observations). This result is also consistent with studies performed with Rab5 that showed a requirement for the activated form to associate with effector proteins (Stenmark et al., 1995; Horiuchi et al., 1997). Although the Rab2 peptide can indirectly stimulate coatomer recruitment, the intact protein is required for vesicle budding. The Rab2 protein therefore plays a critical role in maintaining the steady-state distribution of VTCs.
ACKNOWLEDGMENTS
We thank Drs. Nick Davis and Bonnie Sloane for critical reading of the manuscript. This work was supported in part by a grant from the American Cancer Society (IN-162).
REFERENCES
- Aridor M, Bannykh SI, Rowe T, Balch W. Sequential coupling between COPII and COPI vesicle coats in endoplasmic reticulum to Golgi transport. J Cell Biol. 1995;131:875–893. doi: 10.1083/jcb.131.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch WE, Dunphy WG, Braell WA, Rothman JE. Reconstitution of the transport of protein between successive compartments of the Golgi measured by coupled incorporation of N-acetylglucosamine. Cell. 1984;39:405–416. doi: 10.1016/0092-8674(84)90019-9. [DOI] [PubMed] [Google Scholar]
- Balch WE, McCaffery JM, Plutner H, Farquhar MG. Vesicular stomatitis virus glycoprotein is sorted and concentrated during export from the endoplasmic reticulum. Cell. 1994;76:841–852. doi: 10.1016/0092-8674(94)90359-x. [DOI] [PubMed] [Google Scholar]
- Beckers CJM, Keller DS, Balch WE. Semi-intact cells permeable to macromolecules: use in reconstitution of protein transport from the endoplasmic reticulum to the Golgi complex. Cell. 1987;50:523–534. doi: 10.1016/0092-8674(87)90025-0. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981;256:1604–1607. [PubMed] [Google Scholar]
- Chavrier P, Parton R, Hauri HP, Simons K, Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990;62:317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- Chicheportiche Y, Vassalli P, Tartakoff AM. Characterization of cytoplasmically oriented Golgi proteins with a monoclonal antibody. J Cell Biol. 1984;99:2200–2210. doi: 10.1083/jcb.99.6.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosson P, Letourneur F. Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science. 1994;263:1629–1631. doi: 10.1126/science.8128252. [DOI] [PubMed] [Google Scholar]
- Davidson HW, Balch WE. Differential inhibition of multiple vesicular transport steps between the endoplasmic reticulum and trans Golgi network. J Biol Chem. 1993;268:4216–4226. [PubMed] [Google Scholar]
- Der CJ, Finkel T, Cooper GM. Biological and biochemical properties of human H-ras genes mutated at codon 61. Cell. 1986;44:167–176. doi: 10.1016/0092-8674(86)90495-2. [DOI] [PubMed] [Google Scholar]
- Donaldson J, Finazzi D, Klausner R. Brefeldin A inhibits Golgi membrane-catalyzed exchange of guanine nucleotide onto ARF protein. Nature. 1992;360:350–352. doi: 10.1038/360350a0. [DOI] [PubMed] [Google Scholar]
- Fabbri M, Bannykh S, Balch WE. Export of protein from the endoplasmic reticulum is regulated by a diacylglycerol/phorbol ester binding protein. J Biol Chem. 1994;269:26848–26857. [PubMed] [Google Scholar]
- Feig L, Bin-Tao P, Roberts T, Cooper G. Isolation of ras GTP-binding mutants using an in situ colony-binding assay. Proc Natl Acad Sci USA. 1986;83:4607–4611. doi: 10.1073/pnas.83.13.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallione CJ, Rose JK. A single amino acid substitution in a hydrophobic domain causes temperature-sensitive cell-surface transport of a mutant viral glycoprotein. J Virol. 1985;54:374–382. doi: 10.1128/jvi.54.2.374-382.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor EC, Emr SD. COPI-independent anterograde transport: cargo-selective ER-to-Golgi protein transport in yeast COPI mutants. J Cell Biol. 1997;136:789–802. doi: 10.1083/jcb.136.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Ras and GAP—who’s controlling whom? Cell. 1990;61:921–923. doi: 10.1016/0092-8674(90)90054-i. [DOI] [PubMed] [Google Scholar]
- Hammond C, Helenius A. Quality control in the secretory pathway: retention of a misfolded viral membrane glycoprotein involves cycling between the ER, intermediate compartment, and Golgi apparatus. J Cell Biol. 1994;126:41–52. doi: 10.1083/jcb.126.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herisse J, Courtois G, Galibert F. Nucleotide sequence of the ecoRI fragment of Adenovirus 2 genome. Nucleic Acids Res. 1980;8:2173–2192. doi: 10.1093/nar/8.10.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi H, et al. A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell. 1997;90:1149–1159. doi: 10.1016/s0092-8674(00)80380-3. [DOI] [PubMed] [Google Scholar]
- Jackson MR, Nilsson T, Peterson PA. Retrieval of transmembrane proteins to the endoplasmic reticulum. J Cell Biol. 1993;121:317–333. doi: 10.1083/jcb.121.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneur F, Gaynor E, Hennecke S, Demolliere C, Duden R, Emr S, Riezman H, Cosson P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Malhotra V, Seratini T, Orci L, Shepherd J, Rothman JE. Purification of a novel class of coated vesicles mediating biosynthetic protein transport through the Golgi stack. Cell. 1989;58:329–336. doi: 10.1016/0092-8674(89)90847-7. [DOI] [PubMed] [Google Scholar]
- Maruta H, Holden J, Sizeland A, D’Abaco G. The residues of ras and rap proteins that determine their GAP specificities. J Biol Chem. 1991;266:11661–11668. [PubMed] [Google Scholar]
- Nilsson T, Pypaert M, Hoe MH, Slusarewicz P, Berger EG, Warren G. Overlapping distribution of two glycosyltransferases in the Golgi apparatus of HeLa cells. J Cell Biol. 1993;120:5–13. doi: 10.1083/jcb.120.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuoffer C, Balch WE. GTPases: multifunctional molecular switches regulating vesicular traffic. Annu Rev Biochem. 1994;63:949–990. doi: 10.1146/annurev.bi.63.070194.004505. [DOI] [PubMed] [Google Scholar]
- Nuoffer C, Davidson HW, Matteson J, Meinkoth J, Balch WE. A GDP-bound form of Rab2 inhibits protein export from the endoplasmic reticulum and transport between Golgi compartments. J Cell Biol. 1994;125:225–237. doi: 10.1083/jcb.125.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Glick BS, Rothman JE. A new type of coated vesicular carrier that appears not to contain clathrin: its possible role in protein transport within the Golgi stack. Cell. 1986;46:171–184. doi: 10.1016/0092-8674(86)90734-8. [DOI] [PubMed] [Google Scholar]
- Orci L, Palmer D, Ravazzola M, Perrelet A, Amherdt M, Rothman JE. Budding from Golgi membranes requires the coatomer complex of nonclathrin coat proteins. Nature. 1993;362:648–651. doi: 10.1038/362648a0. [DOI] [PubMed] [Google Scholar]
- Orci L, Tagaya M, Amherdt M, Perrelet A, Donaldson J, Lippincott-Schwartz J, Klausner R, Rothman J. Brefeldin A, a drug that blocks secretion, prevents the assembly of nonclathrin-coated buds on Golgi cisternae. Cell. 1991;64:1183–1195. doi: 10.1016/0092-8674(91)90273-2. [DOI] [PubMed] [Google Scholar]
- Ostermann J, Orci L, Katsuko T, Amherdt M, Ravazzola M, Elazar Z, Rothman J. Stepwise assembly of functionally active transport vesicles. Cell. 1993;75:1015–1025. doi: 10.1016/0092-8674(93)90545-2. [DOI] [PubMed] [Google Scholar]
- Palmer D, Helms JB, Beckers CJM, Orci L, Rothman JE. Binding of coatomer to Golgi membranes requires ADP-ribosylation factor. J Biol Chem. 1993;268:12083–12089. [PubMed] [Google Scholar]
- Pepperkok R, Scheel J, Horstmann H, Hauri HP, Griffiths G, Kreis TE. β-COP is essential for biosynthetic membrane transport from the endoplasmic reticulum to the Golgi complex in vivo. Cell. 1993;74:71–82. doi: 10.1016/0092-8674(93)90295-2. [DOI] [PubMed] [Google Scholar]
- Pfeffer S. Rab GTPases: master regulators of membrane trafficking. Curr Opin Cell Biol. 1994;6:522–526. doi: 10.1016/0955-0674(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Pind S, Nuoffer C, McCaffery JM, Plutner H, Davidson H, Farquhar MG, Balch WE. Rab2 and Ca2+ are required for the fusion of carrier vesicles mediating endoplasmic reticulum to Golgi transport. J Cell Biol. 1994;125:239–252. doi: 10.1083/jcb.125.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley JF, Cole NB, Schroer TA, Hirschberg K, Zaal KJM, Lippincott-Schwartz J. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- Rybin V, Ullrich O, Rubino M, Alexandrov K, Simon I, Seabra M, Goody R, Zerial M. GTPase activity of Rab5 acts as a timer for endocytic membrane fusion. Nature. 1996;383:266–269. doi: 10.1038/383266a0. [DOI] [PubMed] [Google Scholar]
- Saraste J, Svensson K. Distribution of the intermediate elements operating in ER to Golgi transport. J Cell Sci. 1991;100:415–430. doi: 10.1242/jcs.100.3.415. [DOI] [PubMed] [Google Scholar]
- Scales SJ, Pepperkok R, Kreis TE. Visualization of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell. 1997;90:1137–1148. doi: 10.1016/s0092-8674(00)80379-7. [DOI] [PubMed] [Google Scholar]
- Shapiro AD, Riederer MA, Pfeffer SR. Biochemical analysis of Rab9, a ras-like GTPase in protein transport from late endosomes to the trans Golgi network. J Biol Chem. 1993;268:6925–6931. [PubMed] [Google Scholar]
- Stenmark H, Parton RG, Steele-Mortimer O, Lutcke A, Gruenberg J, Zerial M. Inhibition of Rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H, Vitale G, Ullrich O, Zerial M. Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell. 1995;83:423–432. doi: 10.1016/0092-8674(95)90120-5. [DOI] [PubMed] [Google Scholar]
- Tisdale EJ, Balch WE. Rab2 is essential for the maturation of pre-Golgi intermediates. J Biol Chem. 1996;271:29372–29379. doi: 10.1074/jbc.271.46.29372. [DOI] [PubMed] [Google Scholar]
- Tisdale EJ, Bourne JR, Khosravi-Far R, Der CJ, Balch WE. GTP-binding mutants of Rab1 and Rab2 are potent inhibitors of vesicular transport from the endoplasmic reticulum to the Golgi complex. J Cell Biol. 1992;119:749–761. doi: 10.1083/jcb.119.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale EJ, Jackson MR. Rab2 protein enhances coatomer recruitment to pre-Golgi intermediates. J Biol Chem. 1998;273:17269–17277. doi: 10.1074/jbc.273.27.17269. [DOI] [PubMed] [Google Scholar]
- Tisdale EJ, Plutner H, Matteson J, Balch WE. p53/58 binds COPI and is required for selective transport through the early secretory pathway. J Cell Biol. 1997;137:581–593. doi: 10.1083/jcb.137.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touchot N, Zahraoui A, Vielh E, Tavitian A. Biochemical properties of the YPT-related Rab1B protein. FEBS Lett. 1989;256:79–84. doi: 10.1016/0014-5793(89)81722-3. [DOI] [PubMed] [Google Scholar]
- Wagner P, Molenaar CMT, Rauh AJG, Brokel R, Schmitt HD, Gallwitz D. Biochemical properties of the ras-related YPT protein in yeast: a mutational analysis. EMBO J. 1987;6:2373–2379. doi: 10.1002/j.1460-2075.1987.tb02514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MG, Seratini T, Rothman J. “Coatomer”: a cytosolic protein complex containing subunits of nonclathrin-coated Golgi transport vesicles. Nature. 1991;349:248–251. doi: 10.1038/349248a0. [DOI] [PubMed] [Google Scholar]