Abstract
To obviate the foreign protein reactions experienced with the use of hyperimmune serum in rabies-exposed individuals, an attempt was made to produce a rabies antiserum of human origin.
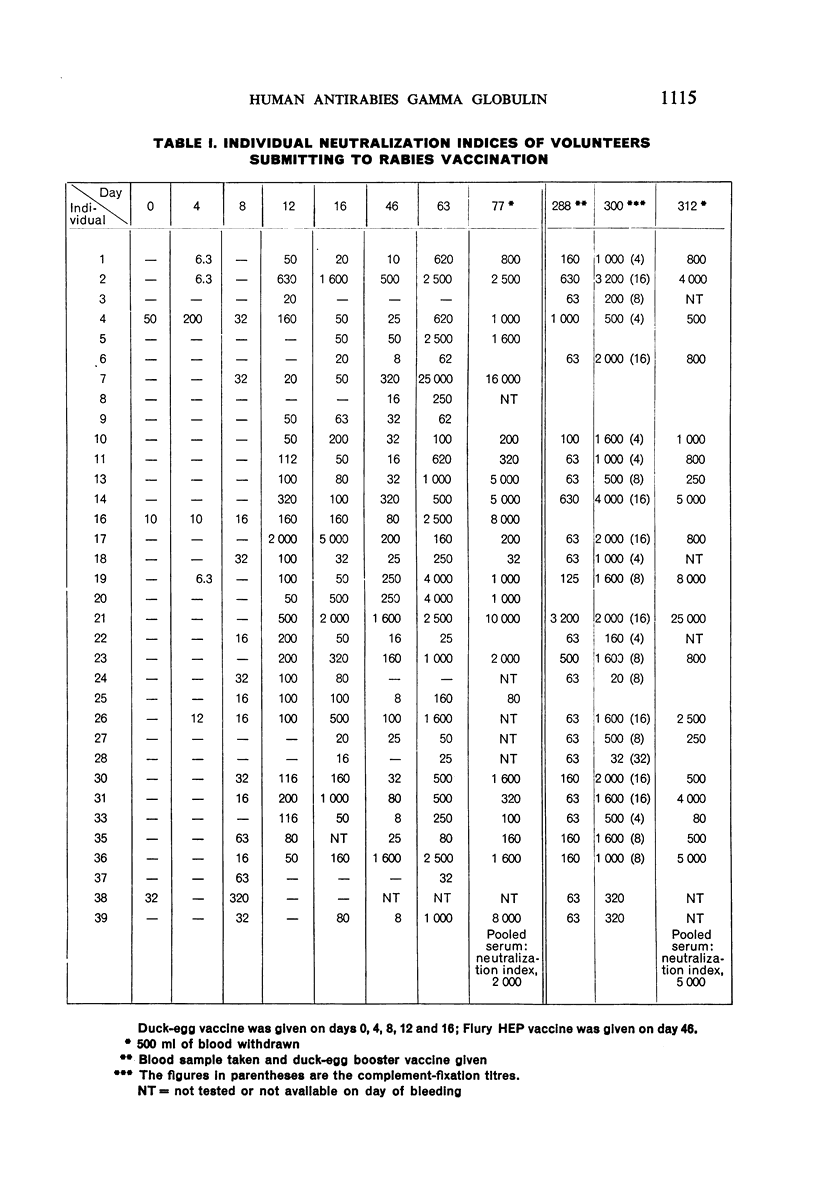
Five doses of an inactivated rabies virus duck-egg vaccine were administered to 34 volunteers at 4-day intervals (i.e., on days 0, 4, 8, 12 and 16). An additional dose of chick-embryo attenuated virus vaccine—Flury HEP (high egg passage)—was given on the 46th day, followed by a final booster dose of duck-egg vaccine on the 288th day. Twenty-four days later, i.e., on the 312th day after the first dose, the participants were bled and the serum pooled and converted to gamma globulin.
These volunteers, having no initial antibody, responded with variable titres, the pooled serum having a titre of 1: 100 against 50 LD50 of rabies virus in neutralization tests and the gamma globulin prepared from this pool a titre of 1: 300.
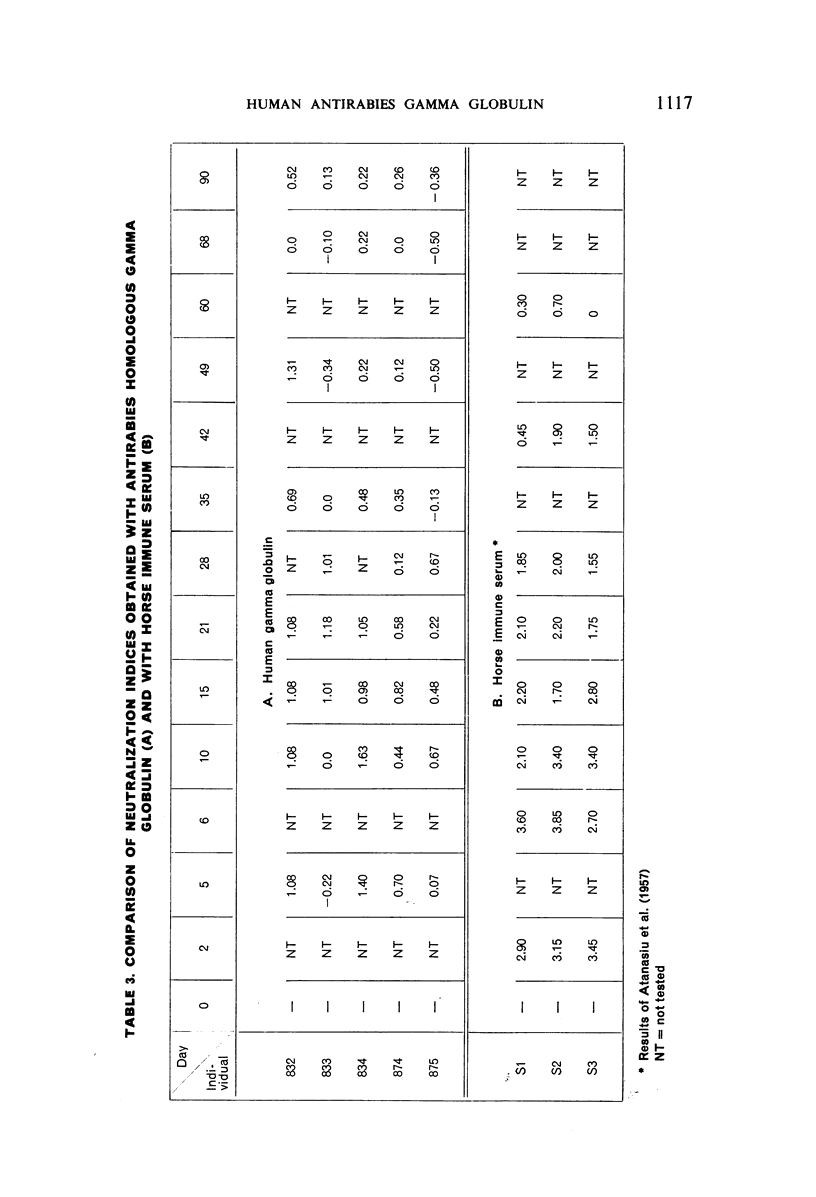
In five individuals inoculated with the antirabies gamma globulin, blood samples tested at intervals for residual antibody showed significant titres through 21 days.
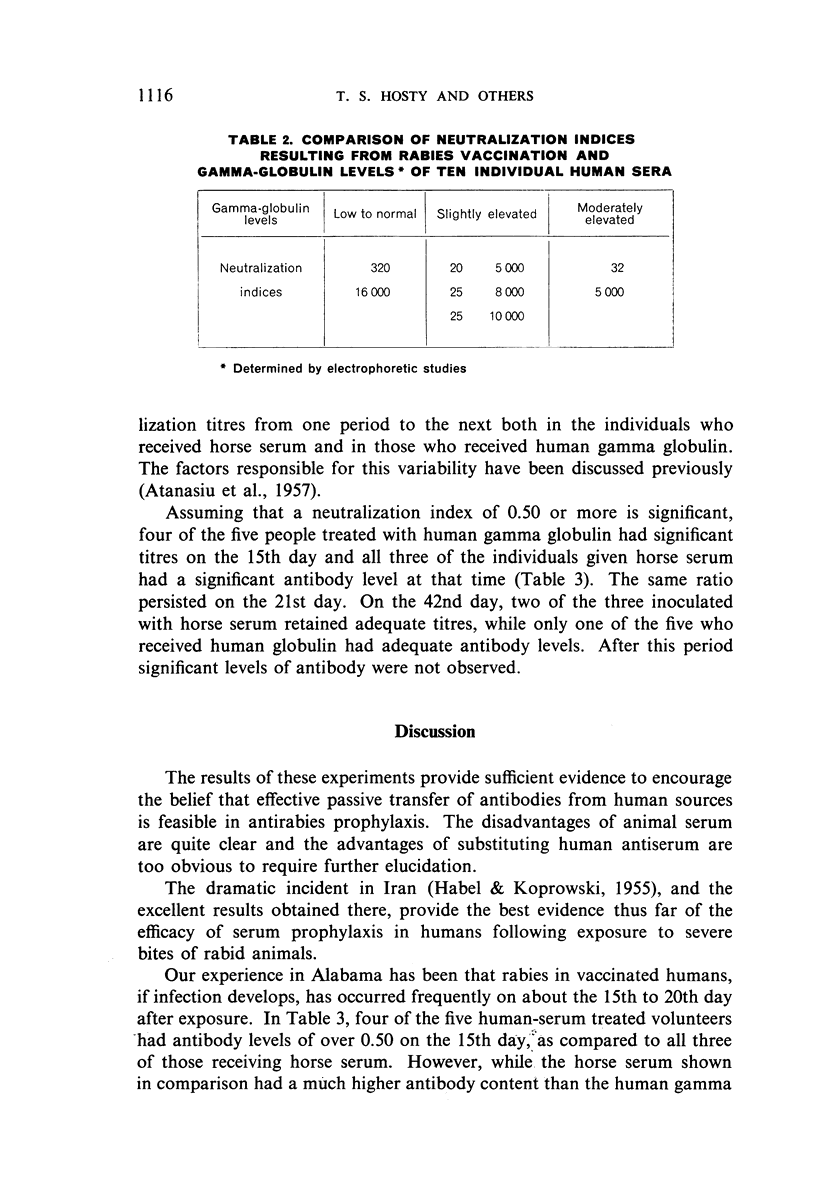
While the passive antibody levels resulting from the administration of a more potent immune horse serum were much higher than those achieved by the weaker human antirabies gamma globulin used, the decrease in titre was more gradual with the human globulin. With more booster inoculations in a larger group of human volunteers, it is believed that a human rabies immune gamma globulin could be produced which would be equal in effect to immune horse serum. The advantages of a human source of antibody in rabies prophylaxis are discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- HABEL K., KOPROWSKI H. Laboratory data supporting the clinical trial of anti-rabies serum in persons bitten by a rabid wolf. Bull World Health Organ. 1955;13(5):773–779. [PMC free article] [PubMed] [Google Scholar]
- HOSTY T. S., HUNTER F. R. Incidence of reactions to antirabies horse serum. Public Health Rep. 1953 Aug;68(8):789–791. [PMC free article] [PubMed] [Google Scholar]
- ONCLEY J. L., MELIN M. The separation of the antibodies, isoagglutinins, prothrombin, plasminogen and beta1-lipoprotein into subfractions of human plasma. J Am Chem Soc. 1949 Feb;71(2):541–550. doi: 10.1021/ja01170a048. [DOI] [PubMed] [Google Scholar]
- SMOLENS J., STOKES J., Jr, MCGEE E., HUNTER V. Feasibility and safety of frequent plasmapheresis of the same human donors. Proc Soc Exp Biol Med. 1956 Apr;91(4):611–614. doi: 10.3181/00379727-91-22347. [DOI] [PubMed] [Google Scholar]
- SMOLENS J., STOKES J., Jr, VOGT A. B. Human plasmapheresis and its effect on antibodies. J Immunol. 1957 Nov;79(5):434–439. [PubMed] [Google Scholar]


