Abstract
The in vitro activity of recombinant lysostaphin was tested against a collection of well-characterized clinical Staphylococcus aureus isolates by disk diffusion (429 isolates) and minimum bactericidal concentration (10 isolates) assays. Minimum bactericidal concentrations of 0.16 μg/ml and zones of inhibition ranging between 15 and 21 mm in diameter demonstrate that lysostaphin was highly active against all isolates tested.
Oxacillin-resistant Staphylococcus aureus (ORSA) strains have become a widespread problem in many countries (7), which has become more complicated by the presence of vancomycin-intermediate S. aureus (VISA) (15) and, more recently, vancomycin-resistant (2, 3) strains. This latest development raises fears of increased therapeutic failures. Therefore, prevention of infections due to S. aureus and new treatments for S. aureus infections are of utmost importance not only for the safety of patients but also in terms of treating an infection by cost-effective means.
Nasal carriage has been identified as a risk factor for the pathogenesis of S. aureus infections and as a source of S. aureus bacteremia (10, 14, 17, 18). For patients with S. aureus bacteremia, a strong correlation between strains colonizing the anterior nares and strains isolated from blood was described, supporting strategies to prevent systemic S. aureus infections by eliminating nasal carriage (17). Indeed, suppression or eradication of S. aureus in a patient's nose has been associated with reduced rates of endogenous S. aureus infection in multiple studies (1, 8, 13, 16).
Lysostaphin, an endopeptidase produced by Staphylococcus simulans biovar Staphylolyticus cleaves the polyglycine interpeptide bridges of staphylococcal cell walls (4). With the recent formulation of lysostaphin in a nasal cream consisting of fatty acid esters and lysostaphin mixed in as an aqueous suspension (5%), a new product might be available for the nasal eradication of S. aureus (9).
Here, we tested the in vitro activity of recombinant lysostaphin against a well-characterized S. aureus strain collection using (i) a disk diffusion method and (ii) a minimum bactericidal concentration (MBC) assay with selected strains.
All together, 429 well-characterized S. aureus isolates were tested. All isolates were collected in Germany during the course of a multicenter study including 32 university and community hospitals (17); only one isolate per patient was included. Two hundred ten S. aureus isolates were obtained from nasal swabs during routine surveillance; the other 219 isolates were collected from the blood of patients with S. aureus bacteremia. The strains were collected in a setting with a low level of ORSA (between 3.5 and 9.1% of isolated S. aureus strains during the study period), with only 23 methicillin-resistant strains being part of the strain collection. These 23 isolates were confirmed to be methicillin resistant by using disks with 5 μg of oxacillin. Confirmation was accomplished by supplementation of the Mueller-Hinton agar with 2% NaCl; zones of inhibition were read after incubation at 30°C for 48 h. Isolates were considered resistant if they had a zone of inhibition of ≤15 mm according to Deutsches Institut für Normung document 58940-3 (6) and by detection of the mecA gene as previously reported (11). Results for the reference strains ATCC 33592 and ATCC 29213 were within acceptable limits throughout testing.
To determine the activity of recombinant lysostaphin versus the S. aureus strain collection, the following disk diffusion method was used. Six-millimeter-diameter disks were cut out of filter paper and then autoclaved. Sterile disks were impregnated with 50 μg of mature recombinant lysostaphin in 7 μl of phosphate-buffered saline (PBS) (a 7.1-μg/μl stock solution of lysostaphin). Disks were dried at room temperature for 30 min and stored at 20°C in a sealed container until used. S. aureus strains (with an inoculum at a McFarland standard of 0.5, corresponding to cell counts ranging between 5 × 107 and 1 × 108) were plated on cation-adjusted Mueller-Hinton agar supplemented with 2% NaCl, and 50-μg lysostaphin disks were added.
To quantitate lysostaphin in solution, 8 mg of powder (95% lysostaphin by weight) was dissolved in 1 ml of PBS. The concentration was determined by measuring the optical density at 280 nm of two dilutions: 1:40 and 1:80. The resulting optical density was multiplied by the dilution factor and by 0.49, which is the reciprocal of the extinction coefficient. The concentration given (in milligrams per milliliter) was adjusted to the desired final concentration for the production of disks.
The MBC assay was performed with selected strains isolated from blood (n = 5) and anterior nares (n = 5) exhibiting various zones of inhibition by the disk diffusion method and was carried out as follows. Diluted S. aureus cultures (grown overnight in Trypticase soy broth) were added to twofold serial dilutions of lysostaphin (ranging from 10 to 0.16 μg/ml) in PBS—with added bovine serum albumin (0.1%) to prevent nonspecific sticking of lysostaphin to plastic (5)—to a final concentration of approximately 106 CFU/ml (maximum range, from 5 × 105 to 5 × 106). A dilution series of untreated S. aureus samples was also used to determine the starting titer of bacteria (Table 1). S. aureus was incubated with lysostaphin for 30 min at room temperature with vigorous shaking. Subsequently, proteinase K (10 mg/ml) was added to each tube to neutralize the remaining lysostaphin (9) and 100 μl of each tube was plated on Trypticase soy agar. After overnight incubation, colonies were counted and the MBC was determined as the concentration of lysostaphin required to cause a 3-log or greater drop from the initial bacterial titer.
TABLE 1.
Activity of lysostaphin against selected S. aureus strains of different origins
| Strain | Origin | ORSA | Disk diffusion method inhibition zone (mm) | MBC assay
|
||
|---|---|---|---|---|---|---|
| Starting titer | Final titer (at MBC) | MBC (μg/ml) | ||||
| 1 | Blood | No | 15 | 4.69 × 106 | 7.70 × 102 | 0.16 |
| 2 | No | 16 | 7.72 × 105 | 1.85 × 102 | 0.16 | |
| 3 | Yes | 17 | 7.32 × 105 | 0.05 × 102 | 0.16 | |
| 4 | No | 19 | 1.63 × 106 | 2.95 × 102 | 0.16 | |
| 5 | Yes | 21 | 2.58 × 106 | 0.80 × 102 | 0.16 | |
| 6 | Anterior nares | No | 15 | 1.22 × 106 | 5.70 × 102 | 0.16 |
| 7 | No | 17 | 1.50 × 106 | 1.95 × 102 | 0.16 | |
| 8 | No | 18 | 1.59 × 106 | 0.65 × 102 | 0.16 | |
| 9 | Yes | 19 | 7.07 × 105 | 2.20 × 102 | 0.16 | |
| 10 | No | 20 | 1.06 × 106 | 1.35 × 102 | 0.16 | |
All together, the in vitro activity of lysostaphin was tested against 429 well-characterized S. aureus strains collected during a multicenter study. Following incubation for 24 h, disks with 50 μg of lysostaphin had zones of inhibition ranging between 15 and 21 mm in diameter, without there being any significant differences between those of strains isolated from S. aureus nasal carriers and those of strains obtained from patients with S. aureus bacteremia (Fig. 1). For all strains tested, there was no growth of single colonies at 24 h within the zone of inhibition. Differences between methicillin-susceptible and methicillin-resistant strains were also not observed. Following further incubation (up to 48 h), the sizes of zones of inhibition increased, representing a “second zone” of inhibition (up to 31 mm when cultures were incubated for 48 h) probably due to continued diffusion of lysostaphin through the agar. In this second zone of inhibition, growth was strongly reduced. However, following incubation for 48 h, the primary zones of inhibition remained at a diameter of >14 mm, without any colonies being detectable within this zone. Additional testing of selected colonies from the second zone of inhibition showed again zones of inhibition with diameters of >14 mm following incubation for 24 h, indicating that lysostaphin had the same activity against these colonies.
FIG. 1.
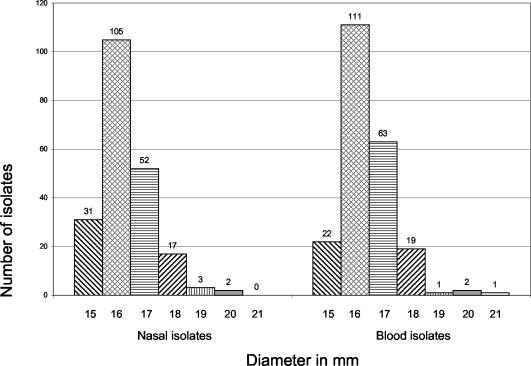
Distribution of zone diameters around lysostaphin disks following incubation for 24 h. Zones of inhibition are given for isolates recovered from nasal carriers (n = 210) and from blood (n = 219) of patients with S. aureus bacteremia.
Three in vitro-isolated lysostaphin-resistant strains served as controls and did not reveal any zone of inhibition, while four mupirocin-resistant strains—two with low-level resistance (MICs, 8 and 32 μg/ml) and two with high-level resistance (MICs, >512 μg/ml; kindly provided by W. Witte and G. L. Archer)—showed zones of inhibition similar in size to those of mupirocin-susceptible strains.
Ten selected strains exhibiting zones of inhibition of various sizes (ranging from 15 to 21 mm in diameter) were further tested in an MBC assay. For all strains tested by MBC assay, including three ORSA strains, MBCs were 0.16 μg/ml. Following incubation of S. aureus with lysostaphin for only 30 min, we observed a 3-log or a greater drop from the initial bacterial titer (99.9% reduction in the original number of CFU) for all strains, irrespective of the methicillin resistance phenotype of the isolates or of the zones of inhibition observed with the disk diffusion method (Table 1).
Previous studies revealed that lysostaphin has potent antistaphylococcal activity, including against both ORSA and VISA strains (5, 12). In a rabbit model of endocarditis caused either by ORSA or by VISA, treatment with lysostaphin reduced mean aortic valve vegetation counts by >8.0 log10 CFU/g compared to those for untreated controls (5, 12).
Most recently, S. aureus colonies isolated from the noses of nasally colonized cotton rats treated with either 0.5% (actual dose, ∼150 μg of lysostaphin) or 0.125% lysostaphin formulated in cream were tested by either the MIC assay or the disk diffusion method to determine whether lysostaphin resistance could emerge in the nares of treated cotton rats (9). In these assays, none of the isolates from cotton rat noses treated with lysostaphin formulated in cream was found to be lysostaphin resistant. The MICs of lysostaphin for the isolated colonies matched those for the parental strains, or the diameters of the zones of inhibition in the disk diffusion method were all >12 mm. In that study, S. aureus isolates were considered lysostaphin resistant if the diameter of the zone of inhibition was less than 12 mm (9).
Lysostaphin formulated in cream, allowing increased residence time and preserved enzyme activity, may prove to be a superior alternative to other antimicrobial agents for the clearance of S. aureus nasal colonization. In our study, lysostaphin revealed high in vitro activity against S. aureus strains obtained during the course of a German multicenter study of patients nasally colonized with S. aureus as well as of patients with S. aureus bacteremia, irrespective of the oxacillin resistance phenotype of the strains. Lysostaphin as formulated in the present study would be highly active at concentrations that can easily be achieved with topical application, indicating that this agent should be effective for topical decolonization of S. aureus.
Acknowledgments
We sincerely thank S. Weber and S. Deiwick for excellent technical assistance. We are grateful to J. Fischer for kind advice and helpful discussions.
This work was supported by a grant from Biosynexus Inc.
REFERENCES
- 1.Boelaert, J. R., H. W. Van Landuyt, B. Z. Gordts, Y. A. De Baere, S. A. Messer, and L. A. Herwaldt. 1996. Nasal and cutaneous carriage of Staphylococcus aureus in hemodialysis patients: the effect of nasal mupirocin. Infect. Control Hosp. Epidemiol. 17:809-811. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2002. Vancomycin-resistant Staphylococcus aureus—Pennsylvania, 2002. Morb. Mortal. Wkly. Rep. 51:902. [PubMed] [Google Scholar]
- 3.Chang, S., D. M. Sievert, J. C. Hageman, M. L. Boulton, F. C. Tenover, F. P. Downes, S. Shah, J. T. Rudrik, G. R. Pupp, W. J. Brown, D. Cardo, and S. K. Fridkin. 2003. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N. Engl. J. Med. 348:1342-1347. [DOI] [PubMed] [Google Scholar]
- 4.Climo, M. W., K. Ehlert, and G. L. Archer. 2001. Mechanism and suppression of lysostaphin resistance in oxacillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:1431-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Climo, M. W., R. L. Patron, B. P. Goldstein, and G. L. Archer. 1998. Lysostaphin treatment of experimental methicillin-resistant Staphylococcus aureus aortic valve endocarditis. Antimicrob. Agents Chemother. 42:1355-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deutsches Institut für Normung e.V. 1990. Methoden zur Empfindlichkeitsprüfung von mikrobiellen Krankheitserregern gegen Chemotherapeutika. Part 3, DIN 58940-3. Deutsches Institut für Normung-Taschenbuch 222, Medizinische Mikrobiologie, 3rd ed. Beuth Verlag, Berlin, Germany.
- 7.Fluit, A. C., C. L. Wielders, J. Verhoef, and F. J. Schmitz. 2001. Epidemiology and susceptibility of 3,051 Staphylococcus aureus isolates from 25 university hospitals participating in the European SENTRY study. J. Clin. Microbiol. 39:3727-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalmeijer, M. D., H. Coertjens, P. M. Nieuwland-Bollen, D. Bogaers-Hofman, G. A. de Baere, A. Stuurman, A. van Belkum, and J. A. Kluytmans. 2002. Surgical site infections in orthopedic surgery: the effect of mupirocin nasal ointment in a double-blind, randomized, placebo-controlled study. Clin. Infect. Dis. 35: 353-358. [DOI] [PubMed] [Google Scholar]
- 9.Kokai-Kun, J. F., S. M. Walsh, T. Chanturija, and J. J. Mond. 2003. Lysostaphin cream eradicates Staphylococcus aureus nasal colonization in a cotton rat model. Antimicrob. Agents Chemother. 47:1589-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mest, D. R., D. H. Wong, K. J. Shimoda, M. E. Mulligan, and S. E. Wilson. 1994. Nasal colonization with methicillin-resistant Staphylococcus aureus on admission to the surgical intensive care unit increases the risk of infection. Anesth. Analg. 78:644-650. [DOI] [PubMed] [Google Scholar]
- 11.Murakami, K., W. Minamide, K. Wada, E. Nakamura, H. Teraoka, and S. Watanabe. 1991. Identification of methicillin-resistant strains of staphylococci by polymerase chain reaction. J. Clin. Microbiol. 29:2240-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patron, R. L., M. W. Climo, B. P. Goldstein, and G. L. Archer. 1999. Lysostaphin treatment of experimental aortic valve endocarditis caused by a Staphylococcus aureus isolate with reduced susceptibility to vancomycin. Antimicrob. Agents Chemother. 43:1754-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perl, T. M., J. J. Cullen, R. P. Wenzel, M. B. Zimmerman, M. A. Pfaller, D. Sheppard, J. Twombley, P. P. French, and L. A. Herwaldt. 2002. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N. Engl. J. Med. 346:1871-1877. [DOI] [PubMed] [Google Scholar]
- 14.Pujol, M., C. Pena, R. Pallares, J. Ariza, J. Ayats, M. A. Dominguez, and F. Gudiol. 1996. Nosocomial Staphylococcus aureus bacteremia among nasal carriers of methicillin-resistant and methicillin-susceptible strains. Am. J. Med. 100:509-516. [DOI] [PubMed] [Google Scholar]
- 15.Tenover, F. C., M. V. Lancaster, B. C. Hill, C. D. Steward, S. A. Stocker, G. A. Hancock, C. M. O'Hara, S. K. McAllister, N. C. Clark, and K. Hiramatsu. 1998. Characterization of staphylococci with reduced susceptibilities to vancomycin and other glycopeptides. J. Clin. Microbiol. 36:1020-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thodis, E., S. Bhaskaran, P. Pasadakis, J. M. Bargman, S. I. Vas, and D. G. Oreopoulos. 1998. Decrease in Staphylococcus aureus exit-site infections and peritonitis in CAPD patients by local application of mupirocin ointment at the catheter exit site. Perit. Dial. Int. 18:261-270. [PubMed] [Google Scholar]
- 17.von Eiff, C., K. Becker, K. Machka, H. Stammer, and G. Peters. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. N. Engl. J. Med. 344:11-16. [DOI] [PubMed] [Google Scholar]
- 18.Wenzel, R. P., and T. M. Perl. 1995. The significance of nasal carriage of Staphylococcus aureus and the incidence of postoperative wound infection. J. Hosp. Infect. 31:13-24. [DOI] [PubMed] [Google Scholar]


