Abstract
The Penicillium chrysogenum antifungal protein PAF inhibits the growth of various filamentous fungi. In this study, PAF was found to localize to the cytoplasm of sensitive aspergilli by indirect immunofluorescence staining. The internalization process required active metabolism and ATP and was prevented by latrunculin B, suggesting an endocytotic mechanism.
Antimicrobial proteins are produced by many organisms, including humans, amphibians, arthropods, plants, and fungi (6, 11, 13, 32, 37). The Penicillium antifungal protein PAF is abundantly secreted into the supernatant of the β-lactam-producing mold Penicillium chrysogenum (22). This small, basic, and cysteine-rich protein specifically inhibits the growth of numerous filamentous fungi (16). Although its primary amino acid structure resembles that of antifungal proteins isolated from other molds, e.g., Aspergillus giganteus (AFP), A. niger (ANAFP), and P. nalgiovense (NAF), significant differences exist in their species specificity (12, 20, 38). Knowledge of the bases of this selectivity is essential for determining strategies by which to overcome the resistance of important pathogens and to design new antifungal agents. Most data on the possible mechanism of action derive from studies on plant antifungal proteins and AFP which support the hypothesis of an interaction with the plasma membrane that would result in its permeabilization (18, 34, 35). So far, experiments have addressed such an interaction only indirectly. Therefore, we were interested in elucidating the site of action of the antifungal protein PAF from P. chrysogenum in order to gain better insight into putative target structures that might play a role in the activity of the protein. Here we report the localization of PAF in the sensitive molds A. nidulans, A. fumigatus, and A. niger and give evidence for an active transport of the antifungal protein into affected hyphae.
Purification of PAF and generation of polyclonal antiserum.
PAF was purified as described previously (16), and polyclonal antibodies against PAF were raised in rabbits as reported elsewhere (26). To determine antibody specificity and the optimal antibody concentration, 25 to 200 ng of PAF was immobilized on Biotrace-NT 0.45-μm nitrocellulose membrane strips (PALL Corp., Ann Arbor, Mich.) and detected with various dilutions of rabbit anti-PAF serum and alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G (IgG; 1:10,000; Sigma, Vienna, Austria) in accordance with the protocol previously described (21). The polyclonal antiserum reacted specifically with PAF, and no signals were obtained with the control serum that was collected before the first injection (data not shown).
Localization of PAF in aspergilli.
Fungi were grown overnight on glass coverslips at 30°C in complete medium CM (16) inoculated with 106 conidia/ml. The samples were stained in accordance with the protocol of Fischer et al. (9). In brief, prior to fixation, hyphae were treated with 10 to 50 μg of PAF/ml in CM for 90 min at room temperature. The samples were incubated for 60 min with anti-PAF serum diluted 1:600 in blocking buffer TBS/B (20 mM Tris-HCl [pH 8.0], 137 mM NaCl, 0.1% Tween 20, 3% bovine serum albumin), and immunocomplexes were detected by incubation for 60 min with fluorescein isothiocyanate-conjugated swine anti-rabbit IgG (Dako, Copenhagen, Denmark) diluted 1:40 in TBS/B. All specimens were embedded in Vectashield mounting medium (Vector Laboratories, Burlingame, Calif.) before visualization with a Zeiss Axioplan fluorescence microscope (Zeiss, Jena, Germany) or a Zeiss 510 confocal laser scanning microscope as previously described (16).
In A. nidulans, fluorescence signals appeared after a minimum incubation time of 15 to 30 min in hyphal tip segments, as well as in intrahyphal segments (Fig. 1A), and the fluorescence increased in intensity with longer exposures or raised protein concentrations (results not shown). The distribution of the fluorescence signal excludes compartmentalization of the protein but supports its cytoplasmic localization. The immunostaining was specific: no fluorescence was visible in the controls where either PAF or anti-PAF serum was omitted (data not shown) or after saturation of anti-PAF serum with a fourfold molar excess of PAF before use (Fig. 1D). Identical results were obtained when PAF was localized in A. fumigatus and A. niger, whereas no specific immunofluorescence signals were detected in the insensitive species A. terreus (results not shown). Heating to 95°C for 60 min before use abolished the antifungal activity of PAF as determined in microtiter plate assays (data not shown). Moreover, no uptake of heat-inactivated PAF with sensitive aspergilli was found by immunofluorescence staining (Fig. 1C), although the denatured protein was detectable by the anti-PAF serum on immunoblots (results not shown).
FIG. 1.
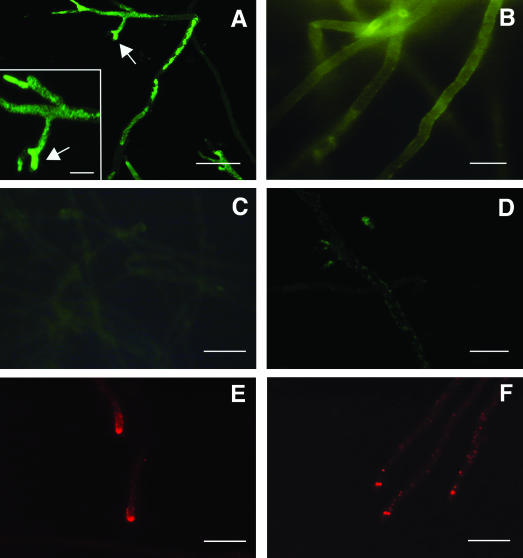
Indirect immunofluorescence staining of A. nidulans with rabbit anti-PAF serum (A to D) or mouse anti-actin monoclonal antibody (E and F). Fungi were incubated with 20 μg of PAF/ml in the absence (A) or in the presence (B) of 50 μg of latB/ml or with heat-inactivated PAF (C). Staining specificity was determined by using preadsorbed rabbit anti-PAF serum (D). Actin polymerization in untreated fungi (E) was compared to that in specimens treated with 50 μg of latB/ml (F). Arrows indicate PAF-induced tip branching. Scale bars, 30 μm (A), 10 μm (A, inset), 15 μm (B and D), and 20 μm (C, E, and F).
From these results, it can be assumed that the activity of PAF depends on its transport into hyphae and that the intact protein structure plays a significant role in the interaction of PAF with its target. These results and the species specificity of PAF strongly substantiate the existence of specific receptors or binding molecules for uptake of the protein. Regarding PAF insensitivity, it can be hypothesized that the absence or inaccessibility of a putative binding molecule prevents detrimental effects of the protein. For the latter case, a similar mechanism was shown in yeast mutants where Pirp-related proteins in the cell wall were found to determine resistance to antifungal proteins by masking or altering the structures of cell wall receptors (4, 28, 40).
Characterization of the internalization of PAF.
It has been shown that active transport of macromolecules, peptides, or proteins into hyphae of A. nidulans and various other filamentous fungi can be stopped by inhibitors of oxidative phosphorylation (7, 29, 31). In accordance with these reports, no PAF-specific immunofluorescence signals were detectable in the presence of 2.5 mM NaN3 (Sigma) or KCN (Merck, Darmstadt, Germany). Similar effects were induced by a 100 μM concentration of the uncoupler carbonyl cyanide m-chlorophenylhydrazone (Sigma), which was shown to decrease ATP levels in A. niger (14). Moreover, PAF uptake was prevented by 4°C (results not shown). These data strongly suggest that internalization of PAF depends on active cellular metabolism, as well as on the ability of the cells to provide ATP. Both are essential factors required for endocytosis (5, 17). Therefore, we exposed A. nidulans to 0.05 to 50 μg of latrunculin B (latB; Sigma)/ml in the presence of PAF. Morton et al. (24) reported that latB affects the kinetics of actin polymerization by specifically binding to actin monomers and shifting the equilibrium to the disassembled state. In the filamentous fungus Pisolithus tinctorius, a perturbation of microfilament organization by latrunculin was shown (15), and in yeast and mammalian cells, receptor-mediated endocytosis was inhibited (3, 19). We found that PAF transport into hyphal cells decreased with increasing concentrations of latB, and 50 μg of latB/ml abolished internalization (Fig. 1B). We confirmed latB-dependent perturbation of actin microfilament formation by using monoclonal mouse anti-actin clone C4 (1:500 in TBS/B; ICN, Aurora, Ohio) and tetramethyl rhodamine isothiocyanate-conjugated rabbit anti-mouse IgG (1:40 in TBS/B; Dako) (Fig. 1E and F). In contrast to conclusive evidence obtained with yeast, reports of endocytosis in filamentous fungi are controversial (2, 10, 36, 39). However, our data strengthen the assumptions that PAF uptake is dependent on intact microfilaments and that its internalization may resemble an endocytotic process. For various protein toxins, endocytosis is an essential step for delivery to the site of action (8). The plant toxin ricin (27) and various bacterial toxins, e.g., Shiga (27), anthrax (1), cholera (23), and diphtheria (30) toxins, have most diverse mechanisms of action, but they are first endocytosed before entering the cytosol from different compartments, e.g., from endosomes or from the endoplasmic reticulum, without proteolysis. Moreover, Olmo et al. (25) confirmed endocytosis of the ribotoxin α-sarcin in vivo, which excludes its direct translocation through the plasma membrane as its main entry mechanism. In our recent study, we detected the induction of multifactorial effects in sensitive fungi by PAF (16). It is conceivable that these detrimental effects are evoked by the internalized protein, which stands in contrast to reports on A. giganteus AFP, which was found to bind to the extracellular layers of sensitive fungi (33).
In conclusion, our data demonstrate that differences exist in the localization of antifungal proteins, suggesting different sites of action. Therefore, further studies are needed to identify the primary targets of PAF and those of other antifungal proteins to improve our knowledge on their antifungal effects.
Acknowledgments
We thank B. Redl for helpful discussions and R. Weiler-Goerz for technical assistance.
This work was supported by the grant P15261 from the Austrian Science Foundation and grant 9861 from the Austrian National Bank.
REFERENCES
- 1.Abrami, L., S. Liu, P. Cosson, S. H. Leppla, and F. G. van der Goot. 2003. Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. J. Cell Biol. 160:321-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkinson, H. A., A. Daniels, and N. D. Read. 2002. Live-cell imaging of endocytosis during conidial germination in the rice blast fungus, Magnaporthe grisea. Fungal Genet. Biol. 37:233-244. [DOI] [PubMed] [Google Scholar]
- 3.Ayscough, K. R. 2000. Endocytosis and the development of cell polarity in yeast require a dynamic F-actin cytoskeleton. Curr. Biol. 10:1587-1590. [DOI] [PubMed] [Google Scholar]
- 4.Bussey, H. 1991. K1 killer toxin, a pore-forming protein from yeast. Mol. Microbiol. 5:2339-2343. [DOI] [PubMed] [Google Scholar]
- 5.Connor, S. D., and S. L. Schmid. 2003. Regulated portals of entry into the cell. Nature 422:37-44. [DOI] [PubMed] [Google Scholar]
- 6.Dimarcq, J. L., P. Bulet, C. Hetru, and J. Hoffmann. 1998. Cysteine-rich antimicrobial peptides in invertebrates. Biopolymers 47:465-477. [DOI] [PubMed] [Google Scholar]
- 7.Eisendle, M., H. Oberegger, I. Zadra, and H. Haas. 2003. The siderophore system is essential for viability of Aspergillus nidulans: functional analysis of two genes encoding l-ornithine N5-monooxygenase (sidA) and a non-ribosomal peptide synthetase (sidC). Mol. Microbiol. 49:359-375. [DOI] [PubMed] [Google Scholar]
- 8.Falnes, P. O., and K. Sandvig. 2000. Penetration of toxins into cells. Curr. Opin. Cell Biol. 12:407-413. [DOI] [PubMed] [Google Scholar]
- 9.Fischer, R., and W. E. Timberlake. 1995. Aspergillus nidulans apsA (anucleate primary sterigmata) encodes a coiled-coil protein required for nuclear positioning and completion of asexual development. J. Cell Biol. 128:485-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer-Parton, S., R. M. Parton, P. C. Hickey, J. Dijksterhuis, H. A. Atkinson, and N. D. Read. 2000. Confocal microscopy of FM4-64 as a tool for analysing endocytosis and vesicle trafficking in living fungal hyphae. J. Microsc. 198:246-259. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Olmedo, F., A. Molina, J. M. Alamillo, and P. Rodriguez-Palenzuela. 1998. Plant defense peptides. Biopolymers 47:479-491. [DOI] [PubMed] [Google Scholar]
- 12.Geisen, R. 2000. P. nalgiovense carries a gene which is homologous to the paf gene of P. chrysogenum which codes for an antifungal peptide. Int. J. Food Microbiol. 62:95-101. [DOI] [PubMed] [Google Scholar]
- 13.Hancock, R. E., and G. Diamond. 2000. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 8:402-410. [DOI] [PubMed] [Google Scholar]
- 14.Hesse, S. J., G. J. Ruijter, C. Dijkema, and J. Visser. 2002. Intracellular pH homeostasis in the filamentous fungus Aspergillus niger. Eur. J. Biochem. 269:3485-3494. [DOI] [PubMed] [Google Scholar]
- 15.Hyde, G. J., D. Davies, L. Perasso, L. Cole, and A. E. Ashford. 1999. Microtubules, but not actin microfilaments, regulate vacuole motility and morphology in hyphae of Pisolithus tinctorius. Cell Motil. Cytoskelet. 42:114-124. [DOI] [PubMed] [Google Scholar]
- 16.Kaiserer, L., C. Oberparleiter, R. Weiler-Goerz, W. Burgstaller, E. Leiter, and F. Marx. 2003. Characterization of the Penicillium chrysogenum antifungal protein PAF. Arch. Microbiol. [Online.] http://dx.doi.org/10.1007/s00203-003-0578-8. [DOI] [PubMed]
- 17.Kuebler, E., and H. Riezman. 1993. Actin and fimbrin are required for the internalization step of endocytosis in yeast. EMBO J. 12:2855-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lacadena, J., A. Martinez del Pozo, M. Gasset, B. Patino, R. Campos-Olivas, C. Vazquez, A. Martinez-Ruiz, J. M. Mancheno, M. Onaderra, and J. G. Gavilanes. 1995. Characterization of the antifungal protein secreted by the mould Aspergillus giganteus. Arch. Biochem. Biophys. 324:273-281. [DOI] [PubMed] [Google Scholar]
- 19.Lamaze, C., L. Fujimoto, H. Yin, and S. Schmid. 1997. The actin cytoskeleton is required for receptor-mediated endocytosis in mammalian cells. J. Biol. Chem. 272:20332-20335. [DOI] [PubMed] [Google Scholar]
- 20.Lee, G. D., S. Y. Shin, C. Y. Maeng, Z. Z. Jin, K. L. Kim, and K. S. Hahm. 1999. Isolation and characterization of a novel antifungal peptide from Aspergillus niger. Biochem. Biophys. Res. Commun. 263:646-651. [DOI] [PubMed] [Google Scholar]
- 21.Marx, F., T. S. Gritsun, B. Grubeck-Loebenstein, and E. A. Gould. 2001. Diagnostic immunoassays for tick-borne encephalitis virus based on recombinant baculovirus protein expression. J. Virol. Methods 91:75-84. [DOI] [PubMed] [Google Scholar]
- 22.Marx, F., H. Haas, M. Reindl, G. Stoffler, F. Lottspeich, and B. Redl. 1995. Cloning, structural organization and regulation of expression of the Penicillium chrysogenum paf gene encoding an abundantly secreted protein with antifungal activity. Gene 167:167-171. [DOI] [PubMed] [Google Scholar]
- 23.Montesano, R., J. Roth, A. Robert, and L. Orci. 1982. Non-coated membrane invaginations are involved in binding and internalization of cholera and tetanus toxins. Nature 296:651-653. [DOI] [PubMed] [Google Scholar]
- 24.Morton, W. M., K. R. Ayscough, and P. J. McLaughlin. 2000. Latrunculin alters the actin-monomer subunit interface to prevent polymerization. Nat. Cell Biol. 2:376-378. [DOI] [PubMed] [Google Scholar]
- 25.Olmo, N., J. Trunay, G. Gonzales de Buitrago, I. Lopez de Silanes, J. G. Gavilanes, and M. A. Lizarbe. 2001. Cytotoxic mechanism of the ribotoxin α-sarcin. Eur. J. Biochem. 268:2113-2123. [DOI] [PubMed] [Google Scholar]
- 26.Redl, B., P. Holzfeind, and F. Lottspeich. 1992. cDNA cloning and sequencing reveals human tear prealbumin to be a member of the lipophilic-ligand carrier protein superfamily. J. Biol. Chem. 267:20282-20287. [PubMed] [Google Scholar]
- 27.Sandvig, K., S. Grimmer, S. U. Lauvrak, M. L. Torgersen, G. Skretting, B. van Deurs, and T. G. Iversen. 2002. Pathways followed by ricin and Shiga toxin into cells. Histochem. Cell Biol. 117:131-141. [DOI] [PubMed] [Google Scholar]
- 28.Schmitt, M. J., and F. Radler. 1990. Blockage of cell wall receptors for yeast killer toxin KT28 with antimannoprotein antibodies. Antimicrob. Agents Chemother. 34:1615-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simkovic, M., M. Kalinak, W. Burgstaller, and L. Varecka. 2002. Characterization of an inducible citrate uptake system in Penicillium simplicissimum. FEMS Microbiol. Lett. 213:21-26. [DOI] [PubMed] [Google Scholar]
- 30.Skretting, G., M. L. Torgersen, B. van Deurs, and K. Sandvig. 1999. Endocytic mechanisms responsible for uptake of GPI-linked diphtheria toxin receptor. J. Cell Sci. 112:3899-3909. [DOI] [PubMed] [Google Scholar]
- 31.Spathas, D. H., J. A. Pateman, and A. J. Clutterbuck. 1982. Polyamine transport in Aspergillus nidulans. J. Gen. Microbiol. 128:557-563. [DOI] [PubMed] [Google Scholar]
- 32.Tao, J., I. Ginsberg, N. Banerjee, W. Held, Y. Koltin, and J. A. Bruenn. 1990. Ustilago maydis KP6 killer toxin: structure, expression in Saccharomyces cerevisiae, and relationship to other cellular toxins. Mol. Cell. Biol. 10:1373-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Theis, T., M. Wedde, V. Meyer, and U. Stahl. 2003. The antifungal protein from Aspergillus giganteus causes membrane permeabilization. Antimicrob. Agents Chemother. 47:588-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thevissen, K., A. Ghazi, G. W. De Samblanx, C. Brownlee, R. W. Osborn, and W. F. Broekaert. 1996. Fungal membrane responses induced by plant defensins and thionins. J. Biol. Chem. 271:15018-15025. [DOI] [PubMed] [Google Scholar]
- 35.Thevissen, K., F. R. Terras, and W. F. Broekaert. 1999. Permeabilization of fungal membranes by plant defensins inhibits fungal growth. Appl. Environ. Microbiol. 65:5451-5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torralba, S., and B. I. Heath. 2002. Analysis of three separate probes suggests the absence of endocytosis in Neurospora crassa hyphae. Fungal Genet. Biol. 37:221-232. [DOI] [PubMed] [Google Scholar]
- 37.Tossi, A., L. Sandri, and A. Giangaspero. 2000. Amphipathic, alpha-helical antimicrobial peptides. Biopolymers 55:4-30. [DOI] [PubMed] [Google Scholar]
- 38.Wnendt, S., N. Ulbrich, and U. Stahl. 1994. Molecular cloning, sequence analysis and expression of the gene encoding an antifungal-protein from Aspergillus giganteus. Curr. Genet. 25:519-523. [DOI] [PubMed] [Google Scholar]
- 39.Yamashita, R. A., and G. S. May. 1998. Constitutive activation of endocytosis by mutation of myoA, the myosin I gene of Aspergillus nidulans. J. Biol. Chem. 273:14644-14648. [DOI] [PubMed] [Google Scholar]
- 40.Yun, D. J., Y. Zhao, J. M. Pardo, M. L. Narasimhan, B. Damsz, H. Lee, L. R. Abad, M. P. D'Urzo, P. M. Hasegawa, and R. A. Bressan. 1997. Stress proteins on the yeast cell surface determine resistance to osmotin, a plant antifungal protein. Proc. Natl. Acad. Sci. USA 94:7082-7087. [DOI] [PMC free article] [PubMed] [Google Scholar]


