Abstract
Listeria monocytogenes is a food-borne pathogen that can cause a variety of illnesses ranging from gastroenteritis to life-threatening septicemia. The β-lactam antibiotic ampicillin remains the drug of choice for the treatment of listeriosis. We have previously identified a response regulator of a putative two-component signal transduction system that plays a role in the virulence and ethanol tolerance of L. monocytogenes. Here we present evidence that the response regulator, CesR, and a histidine protein kinase, CesK, which is encoded by the gene downstream from cesR, are involved in the ability of L. monocytogenes to tolerate ethanol and cell wall-acting antibiotics of the β-lactam family. Furthermore, CesRK controls the expression of a putative extracellular peptide encoded by the orf2420 gene, located immediately downstream from cesRK. Inactivation of orf2420 revealed that it contributes to ethanol tolerance and pathogenesis in mice. Interestingly, we found that transcription of orf2420 was strongly induced by subinhibitory concentrations of various cell wall-acting antibiotics, ethanol, and lysozyme. The induction of orf2420 expression was abolished in the absence of CesRK. Our data suggest that CesRK is involved in regulating aspects of the cell envelope architecture and that changes in cell wall integrity provide a potent stimulus for CesRK-mediated regulation. These results further our understanding of how L. monocytogenes senses and responds to antibiotics that are used therapeutically in the treatment of infectious diseases.
Listeria monocytogenes is a gram-positive food-borne pathogen that can give rise to listeriosis, a serious disease with symptoms that include septicemia, encephalitis, meningitis, abortion, and febrile gastroenteritis. While listeriosis may occur in otherwise healthy individuals, persons primarily at risk are immunocompromised patients, pregnant women, the very young, and the elderly (37). The mortality rate is >20%, thus making listeriosis one of the most deadly bacterial infections. Individuals who develop listeriosis are usually treated with ampicillin, alone or in combination with gentamicin (33). L. monocytogenes has previously been reported to be susceptible to a wide range of antibiotics, but recently it has been found that strains of Listeria isolated from food, the environment, or patients with listeriosis have acquired resistance to a variety of antibiotics (40). It is of concern that the range of less effective antibiotics now includes ampicillin and gentamicin (30, 39). The emergence of antibiotic resistance among bacterial pathogens, including L. monocytogenes, emphasizes the need to understand how bacteria sense and respond to the presence of antimicrobial agents.
Two-component signal transduction systems allow bacteria to recognize and interpret a variety of signals from the environment (15). Two-component systems typically consist of a membrane-bound sensor histidine protein kinase and a response regulator-transcription factor. The sensor kinase allows the bacterium to sense signals from the external environment and transfer this information to the response regulator inside the cell though a phosphorylation cascade. In response to specific signals, the sensor kinase becomes phosphorylated at a conserved histidine residue though an autophosphorylation event. The phosphoryl group is subsequently transferred from the kinase to a conserved aspartic acid residue in the response regulator. Phosphorylation of the response regulator alters its DNA-binding activity, resulting in the up- or down-regulation of genes under its control.
Certain two-component signal transduction systems have been shown to contribute to the resistance or sensitivity to antibiotics. In enterococci the VanRS two-component system mediates resistance to the glycopeptide antibiotic vancomycin by controlling the expression of genes required for the synthesis of modified cell wall precursors (3). The replacement of d-alanyl-d-alanine with d-alanyl-d-lactate results in a modified peptidoglycan with a lower binding affinity for vancomycin. The presence of vancomycin or other cell wall-acting agents in the growth medium appears to generate a signal sensed by VanS; however, the reports on the specificity of induction of the VanRS two-component system are conflicting (1, 4, 12, 14, 35). In Streptococcus pneumoniae, the VncRS two-component system mediates vancomycin-induced cell death (24), thus demonstrating that signal transduction is required for the bactericidal activity of vancomycin in this pathogen. However, these conclusions were not confirmed in a more recent study (31). A second two-component system of S. pneumoniae, CiaHR, plays a role in β-lactam resistance and responds to changes in the external concentration of Ca2+ ions (8).
A previous study (20) identified three response regulators of two-component systems in L. monocytogenes LO28 that are important for virulence and growth under stress conditions found in the host environment. One of these response regulators, CesR (the cephalosporin sensitivity response regulator, formerly designated RR96), is homologous to VanR of enterococci (20). Inactivation of cesR resulted in reduced pathogenesis in mice and improved resistance to ethanol, a widely used disinfectant and food preservative. In the present study, we have investigated in more detail the molecular mechanisms underlying the reduced pathogenesis and increased ethanol resistance displayed by the mutant with the cesR insertion. We show that in-frame deletions of either cesR or the gene located downstream from cesR, cesK (cephalosporin sensitivity histidine protein kinase), which encodes a histidine protein kinase, increased the organism's sensitivity to cell wall-acting antibiotics of the β-lactam family. We have also identified a small open reading frame, orf2420, regulated by the CesRK two-component system. The transcription of orf2420 is strongly induced during the transition into stationary phase. Furthermore, various antimicrobial agents that affect the bacterial cell wall act as inducers of orf2420 expression in a CesRK-dependent manner. These results suggest a role for CesRK in sensing and responding to changes in cell wall integrity.
MATERIALS AND METHODS
Bacterial strains and growth media.
L. monocytogenes serotype 1/2c strain LO28 (36) was routinely grown in brain heart infusion (BHI) medium (Oxoid) at 37°C with shaking. BK96 is an LO28 derivative carrying an insertion in cesR (formerly rr96) (20). When required, erythromycin or kanamycin was added (final concentrations, 5 and 50 μg ml−1, respectively). Escherichia coli strain TOP10 (Invitrogen) was grown in Luria-Bertani medium. When required, 150 μg of erythromycin ml−1 was added to the medium.
DNA sequence analysis of the cesR region in L. monocytogenes LO28 and computer analyses of DNA and protein sequences.
On the basis of the genome sequence of L. monocytogenes EGD, we designed PCR primers (Table 1) for the amplification of regions in L. monocytogenes LO28 up- and downstream from cesR. For the PCRs we used LO28 chromosomal DNA as the template and Pfx DNA polymerase (Invitrogen), which possesses proofreading 3′ to 5′ exonuclease activity. DNA sequence analysis of the PCR fragments was carried out by using the CEQ dye terminator cycle sequencing kit (Beckman Coulter). Homology searches were performed with the BLAST program (2). For the identification of signal peptides, we used the Signal P program (23). For the identification of transmembrane regions, we used the TMpred program (17).
TABLE 1.
Primers used in this study
| Primer | Sequence (5′-3′) |
|---|---|
| RRA | GGGGGTCTAGAAGTGTTACAATAGGTAGAAa |
| RRB | TAATATCACTAAAGTTGGCTG |
| RRC | CAGCCAACTTTAGTGATATTAGTGATTAAGACTATTTGGG |
| RRD | GGGGGGAATTCATACTTCCCGTAAAAACAAAGb |
| RR-PRIM | TCCGCACCATCATAAGCTTG |
| HKA | GGGGTCTAGACAAGAAAAGGCCCTTGGAGa |
| HKB | CCAAAAAGATTGCATGATTCGCG |
| HKC | CGCGAATCATGCAATCTTTTTGGCGGCAATTAGTGGCGGAG |
| HKD | GGGGGAATTCGACGATAAAACTGGTTTGGCGb |
| OrfA | GGGGTCTAGATGGCAACCAAATCATGCGCa |
| OrfB | CGTAATACAAGCAACAACAATTAAAAC |
| OrfC | GTTTTAATTGTTGTTGCTTGTATTACGAAATAAATTAACATAAGTGAGAAAG |
| OrfD | CCCCGAATTCACATGATCGACTGCGAGAACb |
| Orf7 | TTTGAACTATGTACATAAATACCGC |
| KatA | GGGGGGATCCGTCAACCGGCCGATTTAAGCc |
| KatB | GGCCGGTTGATTAGAAATCCG |
| KatC | CGGATTTCTAATCAACCGGCCCATGATTTTCGCTCGAGACG |
| KatD | GGGGTCTAGATAAACGCCATACTTCCGCACa |
| Kat8 | CCTGTAAAAAAGCCGACAATC |
| ThioA | GGGGTCTAGACGAAACATTAGAAGATGAACCAGa |
| ThioB | GACCGCAAACTTTCTTTTTCCC |
| ThioC | GGGAAAAAGAAAGTTTGCGGACCACATCAGTACCGGCTCTAG |
| ThioD | CCCCGGATCCAGCAGCTGAATTGTGTCTATAGc |
| Thio1 | GCTTCTATAACCACGTCCAC |
| Thio-For | GGGGAATTCCTTGAAGAAGTAGAAGAAGCGGb |
| Thio-Rev | GGGGGATCCGCTTCTATAACCACGTCCACc |
| Kat-For | GGGGAATTCCTGTATAGTGAGTGATACb |
| Kat-Rev | GGGGGATCCGGTTCATAGAAAAAGCCTCCGc |
| RR-For | GGGGGAATTCGCTATGTGACGAAATTGAGGCb |
| RR-Rev | GGGGGGATCCCCGCACCATCATAAGCTTGc |
| Orf-For | GGGGGAATTCGTGAAGAGATTCCTAATGTCGb |
| Orf-Rev | GGGGGGATCCGTAATACAAGCAACAACAATTAAAACc |
The underlined sequence is the XbaI restriction enzyme site.
The underlined sequence is the EcoRI restriction enzyme site.
The underlined sequence is the BamHI restriction enzyme site.
Construction of strains with cesR, cesK, orf2420, lmo2423, and lmo2424 deletions.
For construction of in-frame mutants with deletions of the cesR, cesK, orf2420, lmo2423, and lmo2424 genes, L. monocytogenes LO28 chromosomal DNA was used as the template for PCR amplification of DNA fragments containing either the 5′ end of the gene and upstream sequences or the 3′ end of the gene and downstream sequences. The primers used for the PCRs are listed in Table 1. Primers RRA and RRB (210 bp) and primers RRC and RRD (232 bp) were used for the cesR gene. For the cesK gene we used primers HKA and HKB (365 bp) and primers HKC and HKD (435 bp). Primers OrfA and OrfB (455 bp) and primers OrfC and OrfD (420 bp) were used for the orf2420 gene, whereas primers KatA and KatB (456 bp) and primers KatC and KatD (423 bp) were used for the lmo2423 gene. Finally, we used primers ThioA and ThioB (241 bp) and primers ThioC and ThioD (242 bp) for the lmo2424 gene. In a second round of PCR, the cesR, cesK, orf2420, lmo2423, and lmo2424 fragments were joined by using the method of splicing by overlap extension (18), creating PCR fragments of each fragment containing in-frame deletions of 495 bp in cesR, 492 bp in cesK, 141 bp in orf2420, 498 bp in lmo2423, and 171 bp in lmo2424. The ΔcesR, ΔcesK, and Δorf2420 fragments were digested with XbaI and EcoRI, whereas the Δlmo2423 and Δlmo2424 fragments were digested with BamHI and XbaI. The fragments were then cloned into temperature-sensitive shuttle vector pAUL-A and digested with XbaI-EcoRI or BamHI-XbaI (5). The resulting plasmids were introduced into L. monocytogenes LO28 by electroporation (25), and integration of the plasmid was achieved by growing the transformed strains at 42°C in the presence of erythromycin. To allow allelic exchange between the intact genes and the deleted genes to take place, the strains containing the integrated plasmids were then grown at 30°C in the absence of erythromycin. Finally, strains carrying the desired deletions were identified by PCR, and correct deletion events were verified by DNA sequence analysis of the resulting PCR products.
Ethanol tolerance assays.
Overnight cultures of the L. monocytogenes strains in BHI medium were diluted 1:100 in fresh BHI medium containing 5% ethanol. Bacterial growth was monitored over a period of 24 h by measuring the optical density at 600 nm (OD600).
Antibiotic assays.
The sensitivities of the wild-type strain and mutant strains ΔcesR, ΔcesK, and Δorf2420 to a variety of antibiotics were assayed by agar diffusion. Overnight cultures were diluted to 107 CFU/ml and swabbed onto BHI agar. Filter disks (diameter, 6 mm; Oxoid) containing the antibiotics to be studied were placed on the surfaces of the agar plates. The plates were incubated overnight at 37°C. On the following day, the diameters of the zones of bacterial growth inhibition surrounding the disks were measured. The filter disks contained 30 μg (unless otherwise stated) of the following antibiotics: gentamicin, erythromycin, streptomycin (25 μg), rifampin, vancomycin, novobiocin, tetracycline, polymyxin B (300 μg), colistin sulfate (25 μg), minocycline, clindamycin (10 μg), kanamycin, spectinomycin (25 μg), fusidic acid (10 μg), chloramphenicol, oxytetracycline, ampicillin (10 μg), penicillin G (10 μg), cephalothin, cefaclor, cefoperazone, cephalexin, cefriaxone, cefoxitin, cefuroxime, cefotetan, and ceftazidime. For each antibiotic, at least three independent disk diffusion assays were performed per strain.
RNA techniques.
Total RNA was prepared by a hot acid phenol procedure (28). Primer extension analysis was performed as described previously (22) by using 15 μg of total RNA per reaction mixture. Primers Thio1, Kat8, RR-PRIM, and Orf7 (Table 1) labeled with 32P at the 5′ end were used for detection of the lmo2424, lmo2423, cesR, and orf2420 transcription start sites, respectively. For detection of transcription start sites, RNA was prepared from wild-type cell cultures at an OD600 of 0.6. For measurement of orf2420 induction by ethanol and cefuroxime, RNA was prepared from wild-type and ΔcesR cell cultures with and without treatment with 1% ethanol or 4 μg of cefuroxime ml. The inducers were added to the cultures at an OD600 of 0.3, and samples for RNA preparations were collected 20 min after induction. Controls without ethanol and cefuroxime treatment were included in the experiment.
Mouse virulence assay.
Overnight cultures were diluted 100-fold and grown to an OD600 of 0.6. The bacteria were washed and resuspended in phosphate-buffered saline prior to infection. Six-week-old female BALB/C mice were infected intragastrically with 2 × 109 bacteria or intraperitoneally with 1 × 10−4 bacteria. Three days after infection, the mice were killed and the spleen of each mouse was homogenized. Tenfold serial dilutions of the homogenized spleen in physiological saline buffer were plated onto BHI agar plates. Colonies were counted after overnight incubation at 37°C. The data were tested for significance by using the Student t test.
Construction of lacZ fusions to cesR, orf2420, lmo2423, and lmo2424 promoters and β-galactosidase assays.
DNA fragments containing the promoter regions of cesR, orf2420, lmo2423, and lmo2424 were amplified by PCR with primers RR-For and RR-Rev, Orf-For and Orf-Rev, Kat-For and Kat-Rev, and Thio-For and Thio-Rev, respectively (Table 1). The PCR fragments were digested with EcoRI and BamHI and cloned into EcoRI-BamHI-digested pTCV-lac, a shuttle vector for the construction of transcriptional fusions (29). Correct insertion of the fragments into pTCV-lac was verified by DNA sequencing analyses. The resulting plasmids containing fusions of the promoters to lacZ were electroporated into the wild-type and mutant strains.
For measurement of orf-lacZ expression in the presence of a variety of antimicrobial agents, wild-type, ΔcesR, and ΔcesK strains carrying the orf2420-lacZ fusion were grown in BHI medium to an OD600 of 0.2. The cultures were split, and the potential inducers were added at subinhibitory concentrations. Controls to which no inducers were added were included in the experiment. Cells were collected 1 h after the addition of the potential inducers and assayed for β-galactosidase activity. The antimicrobials tested and their final concentrations were as follows: ethanol, 1%; cephalexin, 4 μg/ml; cefuroxime, 4 μg/ml; penicillin G, 0.05 μg/ml; ampicillin, 0.05 μg/ml; vancomycin, 0.1 μg/ml; bacitracin, 4 μg/ml; and d-cycloserine, 20 μg/ml. For the lysozyme induction experiments, cells were collected 2 h after the addition of lysozyme to a final concentration of 0.2, 0.5, or 1 mg/ml.
For the β-galactosidase assay, cells were permeabilized by treatment with 0.5% toluene and 4.5% ethanol, and β-galactosidase activities were determined as described previously (21). The specific activity of β-galactosidase was calculated as [(OD420 of the reaction mixture − OD550 of the reaction mixture)/(reaction time in minutes × OD600 of the cells used in the reaction mixture)]. The specific β-galactosidase activities presented are the averages of three independent experiments, in which the observed variations did not exceed 10%.
Nucleotide sequence accession number.
The nucleotide sequence of the locus in L. monocytogenes LO28 encoding the putative proteins Lmo2424, Lmo2423, CesR, CesK, and Orf2420 was submitted to GenBank and given accession number AY281157.
RESULTS
Genetic organization of cesR region in L. monocytogenes LO28.
A gene encoding a putative response regulator, CesR (formerly designated RR96), which is involved in the virulence and ethanol tolerance of L. monocytogenes LO28, was identified previously (20). Analysis of the L. monocytogenes EGD-e genome sequence (9) revealed that cesR lies in the center of a five-gene cluster. DNA sequence analysis of the corresponding region in L. monocytogenes LO28 showed a gene organization identical to that of the cesR region in EGD-e (Fig. 1A). The product encoded by lmo2424 is a 94-amino-acid (aa) protein that shares homology with the thioredoxins of the thioredoxin-thioredoxin reductase systems implicated in the regulation of the redox homeostasis (10). lmo2423 encodes a 291-aa protein homologous to Co2+, Zn2+, and Cd2+ transporters of the cation diffusion facilitator family (26). As published previously, cesR encodes a putative response regulator (231 aa) of a two-component signal transduction system with homology to VanR of Enterococcus faecium (20). CesR contains an N-terminal receiver domain and a C-terminal DNA-binding domain characteristic of response regulator proteins of the OmpR-PhoB subfamily (15). cesK, located just downstream from cesR, encodes a putative histidine protein kinase (381 aa) of a two-component signal transduction system and shows homology to VanS of E. faecium. The CesK protein contains two potential membrane-spanning regions in the N-terminal region and a transmitter domain in the C-terminal portion of the protein, typical of sensor proteins of two-component regulatory systems (15). orf2420 encodes a 62-aa protein with no homology to any known proteins. Orf2420 possesses an N-terminal signal peptide, suggesting that it is secreted from the cell (Fig. 1B). Reverse transcription-PCR analyses suggested the presence of putative transcription terminators upstream from lmo2424 and downstream from orf2420 (Fig. 1A; data not shown). When the sequence information for the CesRK locus from L. monocytogenes LO28 and EGD-e was compared with the genome sequence of the nonpathogenic species Listeria innocua, we observed that only four of the five coding sequences were predicted in L. innocua (9). Lmo2424, Lmo2423, CesR, and CesK of L. monocytogenes LO28 correspond to Lin2518, Lin2517, Lin2516, and Lin2516 of L. innocua, respectively. However, an open reading frame corresponding to Orf2420 in L. monocytogenes LO28 and Lmo2420 in L. monocytogenes EGD-e has not been defined in L. innocua (9).
FIG. 1.
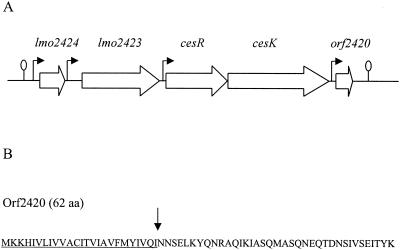
cesRK locus in L. monocytogenes LO28. (A) Genetic organization of the cesRK locus. Transcription start sites are indicated by arrows. Putative transcription terminators are indicated by lollipops. See text for a detailed description of the putative gene products. (B) The putative protein encoded by orf2420 contains a signal peptide sequence of 25 aa (underlined). The putative cleavage site is indicated by an arrow.
In order to identify the promoters at the cesRK locus, we performed primer extension analysis with RNA samples from wild-type cells in the exponential growth phase (OD600 = 0.6). We found that the transcription start sites were located upstream from lmo2424, lmo2423, cesR, and orf2420 (Fig. 2A to H). No transcription initiation site was observed in the 34-bp intergenic region between cesR and cesK (data not shown).
FIG. 2.
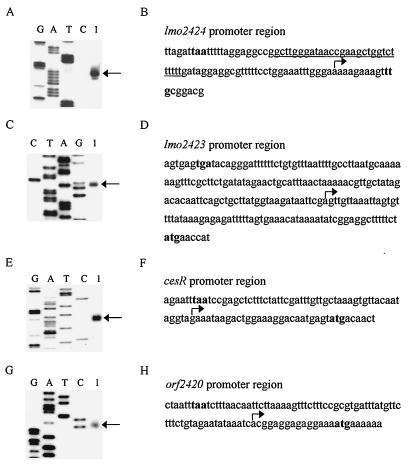
Primer extension analysis of promoters in the cesRK locus. Identification of transcription initiation sites at the promoters of lmo2424 (A), lmo2423 (C), cesR (E), and orf2420 (G). The primer extension products are indicated by arrows. Lanes G, A, T, and C are sequencing ladders. The promoter region sequences are shown for lmo2424 (B), lmo2423 (D), cesR (F), and orf2420 (H). Translation start and stop codons are indicated in boldface. The transcription start site is indicated by an arrow. A putative transcription terminator upstream from lmo2424 is underlined in panel B.
Deletion of cesR, cesK, and orf2420 results in ethanol tolerance.
We have previously shown that insertional inactivation of cesR results in ethanol resistance. In order to determine the contribution of the individual genes at the cesRK locus to this phenotype, we constructed mutants with in-frame deletions of each of the five genes (lmo2424, lmo2423, cesR, cesK, and orf2420). In the absence of ethanol we did not detect any differences in growth between the wild-type and mutant strains (data not shown). Next, we examined the growth of the wild-type and mutant strains with deletions in 5% ethanol, together with the original mutant with the cesR insertion, BK96 (20). As expected, BK96 was capable of growing in the presence of this concentration of ethanol, whereas growth of the wild-type strain was restricted (Fig. 3). Mutants carrying in-frame deletions of cesR, cesK, and, to a lesser extent, orf2420, were also able to grow in the presence of 5% ethanol, whereas mutants with in-frame deletions of lmo2424 or lmo2423 were not ethanol resistant. Our data show that cesR, cesK, and orf2420 increase the ethanol tolerance of L. monocytogenes.
FIG. 3.
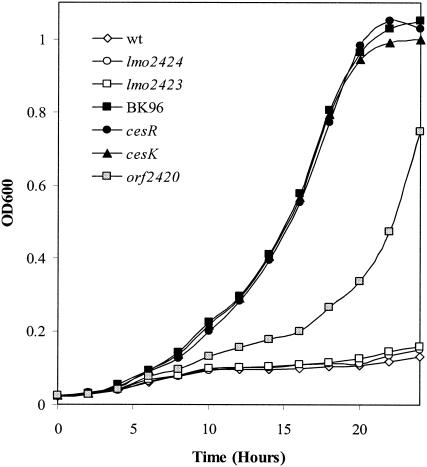
Ethanol tolerance of wild-type and mutant strains. The growth of the wild-type (wt) strain, cesR insertion mutant BK96, and strains carrying in-frame deletions of lmo2424, lmo2423, cesR, cesK, and orf2420 was monitored in BHI medium containing 5% ethanol. The data represent the means of three experiments, in which the observed variation did not exceed 10%.
Deletion of cesR, cesK, and orf2420 affects pathogenicity in mice.
Disruption of cesR leads to a significant reduction in the virulence potential in the mouse model of intragastric (i.g.) infection but not in the mouse model of intraperitoneal (i.p.) infection (20). To determine whether this effect can be attributed to the lack of a functional CesRK system, we tested the virulence of the mutants carrying in-frame deletions of cesR and cesK in i.g. infection assays. Mice were infected with the wild-type strain, the ΔcesR strain, or the ΔcesK strain. On day 3, the number of bacteria in the spleens of the mice was estimated as described in Materials and Methods. The counts of the ΔcesR and ΔcesK mutants in the spleens on day 3 corresponded to the levels previously observed for BK96, i.e., a 10-fold reduction in the number of CFU per spleen relative to the number of CFU of the wild-type strain (P < 0.03) (Fig. 4A). These results support previous observations, suggesting that the CesRK two-component system contributes to the pathogenicity of L. monocytogenes in mice.
FIG. 4.
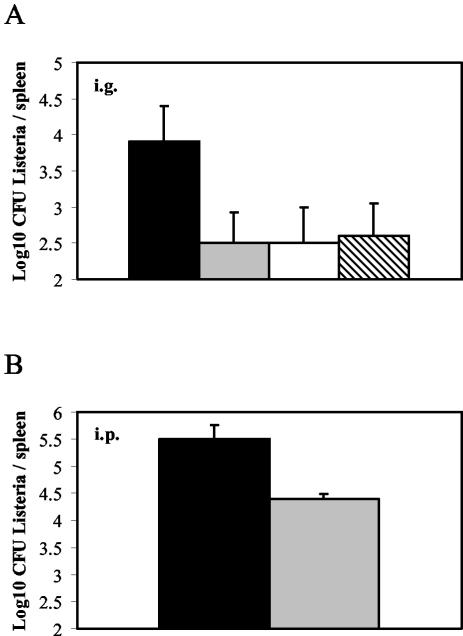
Mouse virulence assays with wild-type, Δorf2420, ΔcesR, and ΔcesK strains. The growth and survival of wild-type (black bars), Δorf2420 (gray bars), ΔcesR (white bars), and ΔcesK (hatched bars) strains in the spleens of infected mice were evaluated on day 3 after i.g. (A) or i.p. (B) injection. The log10 CFU per spleen is the mean average for five mice. The experiments were repeated twice, with similar results each time.
We also infected mice through the i.g. or i.p. route with the orf2420 deletion mutant. In both these assays, the Δorf2420 mutant was significantly impaired in its ability to colonize the spleen (Fig. 4A and B; P < 0.03). These results suggest that Orf2420 also plays a role in murine listeriosis.
Antibiotic resistance of ΔcesR, ΔcesK, and Δorf2420 strains.
The ethanol resistance phenotypes observed for the cesR, cesK, and orf2420 in-frame deletion mutants suggested that either the cell membrane or cell wall integrity might be affected by the mutations. To address this possibility we compared the levels of resistance of wild-type and mutant strains to a variety of antibiotics by using a disk diffusion assay. No significant differences in inhibition zone sizes between wild-type and mutant strains were observed for a large range of antibiotics (gentamicin, erythromycin, streptomycin, rifampin, vancomycin, novobiocin, tetracycline, polymyxin B, colistin, minocycline, clindamycin, kanamycin, spectinomycin, fusidic acid, chloramphenicol, oxytetracycline; data not shown). However, in the presence of cell wall-acting antibiotics of the β-lactam family, including ampicillin, penicillin G, and the cephalosporins, the ΔcesR and ΔcesK mutants were significantly more sensitive (Table 2). The most dramatic difference was observed for the cephalosporin cefuroxime. In this case, the size of the inhibition zone for the wild-type strain was 14.9 mm, whereas the inhibition zone sizes for the ΔcesR and ΔcesK strains were considerably larger (29.7 and 29.5 mm, respectively). No significant difference between the inhibition zone sizes of the wild-type strain and the Δorf2420 strain was observed (Table 2). These results show that the CesRK two-component system plays an important role in the resistance of L. monocytogenes to antibiotics of the β-lactam family.
TABLE 2.
β-Lactam resistance of L. monocytogenes LO28 wild-type, ΔcesR, ΔcesK, and Δorf2420 strains
| β-Lactam | Avg zone of inhibition (mm) ± SDa
|
|||
|---|---|---|---|---|
| Wild type | ΔcesR | ΔcesK | Δorf2420 | |
| Ampicillin | 35.4 ± 0.2 | 40.0 ± 0.1b | 39.6 ± 0.3b | 35.5 ± 0.2 |
| Penicillin G | 33.4 ± 0.2 | 37.7 ± 0.6b | 37.7 ± 0.3b | 33.8 ± 0.5 |
| Cephalothin (I)e | 28.8 ± 0.3 | 32.0 ± 0.1c | 32.1 ± 0.2c | NDd |
| Cefaclor (I) | 21.2 ± 0.5 | 25.3 ± 0.3c | 25.0 ± 0.1c | ND |
| Cefoperazone (III) | 20.2 ± 0.1 | 22.8 ± 0.3c | 23.2 ± 0.2c | ND |
| Cephalexin (I) | 15.9 ± 0.1 | 20.9 ± 0.1b | 20.9 ± 0.1b | ND |
| Ceftriaxone (III) | 15.7 ± 0.2 | 22.4 ± 0.2b | 22.2 ± 0.7b | ND |
| Cefoxitin (II) | 15.6 ± 0.8 | 21.8 ± 0.1b | 21.6 ± 0.1b | ND |
| Cefuroxime (II) | 14.9 ± 0.4 | 29.7 ± 0.2b | 29.5 ± 0.2b | 15.5 ± 0.5 |
| Cefotetan (III) | 12.7 ± 0.2 | 15.6 ± 0.1c | 15.4 ± 0.1c | ND |
| Ceftazidime (III) | 6.2 ± 0.1 | 7.8 ± 0.1c | 7.5 ± 0.1c | ND |
The results presented are averages of triplicate experiments.
Significant difference (P < 0.01) between the mutant strain and the wild type.
Significant difference (P < 0.05) between the mutant strain and the wild type.
ND, not determined.
The classification of the cephalosporins is indicated in parentheses (I, narrow spectrum; II, expanded spectrum; III, broad spectrum).
Stationary-phase induction of orf2420 expression depends on CesRK.
Two-component systems are often found to be autoregulated at the transcriptional level and/or located in close proximity to their target genes. To determine whether any of the promoters at the cesRK locus are regulated by the CesRK two-component system, we fused the promoter regions of lmo2424, lmo2423, cesR, and orf2420 to lacZ. Plasmids containing the promoter-lacZ fusions were introduced into wild-type, ΔcesR, and ΔcesK strains; and the specific β-galactosidase activities were determined at various time points during growth. The expression of lmo2424-lacZ, lmo2423-lacZ, and cesR-lacZ was unaffected by the growth phase (data not shown). Moreover, the expression profile was identical in wild-type, ΔcesR, and ΔcesK strains. However, expression of orf2420 varied with the growth phase and reached a maximum in stationary phase (Fig. 5). Specifically, the β-galactosidase activity in stationary phase was approximately 10-fold higher than the activity in early exponential growth phase. Furthermore, the activity of the orf2420-lacZ fusion was very low in ΔcesR and ΔcesK strains in all growth phases. The activity of orf2420-lacZ in the Δorf2420 strain was comparable to the activity measured in wild-type cells, suggesting that Orf2420 does not affect its own expression (Fig. 5). These data show that the CesRK two-component system is responsible for the induction of orf2420 expression.
FIG. 5.
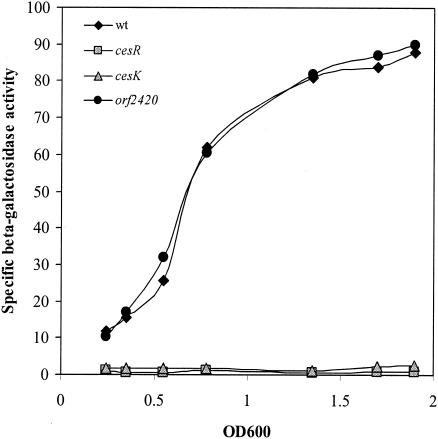
Transcription of orf2420 requires CesRK. The levels of expression of orf2420-lacZ in the wild-type (wt) strain and strains containing cesR, cesK, and orf2420 deletions were determined. Cells were grown in BHI medium. At various cell densities, the cells were harvested and subjected to β-galactosidase assays.
Antimicrobial agents affecting the bacterial cell wall induce expression of orf2420 in a CesRK-dependent manner.
The observations that in-frame deletions in cesR or cesK result in an increased sensitivity to β-lactam antibiotics and an increased resistance to ethanol suggested that CesRK might be responding to changes in cell envelope integrity. To address this hypothesis, we analyzed the transcription of orf2420, which is controlled by CesRK, in the presence of cefuroxime and ethanol. Total RNA was extracted from wild-type and ΔcesR cultures after 20 min of treatment with subinhibitory concentrations of cefuroxime or ethanol. Next, the expression of orf2420 was examined by primer extension analysis. In the wild-type strain, cefuroxime and ethanol were found to strongly induce transcription of orf2420 (Fig. 6A). Importantly, no induction of orf2420 was observed in the ΔcesR strain. This result strongly indicates that the CesRK two-component system responds to the presence of cefuroxime and ethanol in the growth medium.
FIG. 6.
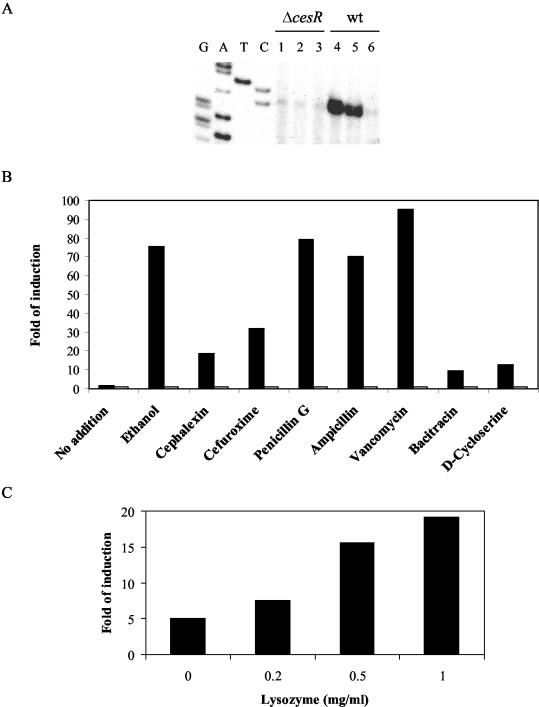
Transcription of orf2420 is induced by ethanol, cell wall-acting antibiotics, and lysozyme. (A) Expression of orf2420 in response to ethanol and cefuroxime analyzed by primer extension. The analysis was performed with RNA purified from the ΔcesR mutant strain (lanes 1 to 3) or the wild-type (wt) strain (lanes 4 to 6). Cells were grown in BHI medium to an OD600 of 0.3. The cell cultures were split and treated with 1% ethanol (lanes 1 and 4) or 4 μg of cefuroxime per ml (lanes 2 and 5) for 20 min. Controls without treatment were included (lanes 3 and 6). (B) Expression of orf2420-lacZ in response to various antimicrobial agents. The wild-type strain (black bars) and the ΔcesR mutant strain (open bars) containing the orf2420-lacZ fusion were grown in BHI medium to an OD600 of 0.2. The cell cultures were split and treated with the indicated antibiotics for 1 h. Cell pellets were harvested and subjected to β-galactosidase assays. The final concentrations of the antimicrobial agents in the medium were as follows: No addition, no antimicrobial agent; ethanol, 1%; cephalexin, 4 μg/ml; cefuroxime, 4 μg/ml; penicillin G, 0.05 μg/ml; ampicillin, 0.05 μg/ml; vancomycin, 0.1 μg/ml; bacitracin, 4 μg/ml; and d-cycloserine, 20 μg/ml. The data represent the means of three experiments, in which the observed variation did not exceed 10%. (C) Expression of orf2420-lacZ in response to lysozyme. The wild-type strain containing the orf2420-lacZ fusion was grown in BHI medium to an OD600 of 0.2. The cell culture was split and treated with the indicated concentration of lysozyme for 2 h. Cell pellets were harvested and subjected to β-galactosidase assays. The data represent the means of three experiments, in which the observed variation did not exceed 10%.
To identify more potential inducers of the CesRK two-component system, we took advantage of the orf2420-lacZ fusion. For these assays, wild-type and ΔcesR strains carrying the orf2420-lacZ fusion were grow in BHI medium to an OD600 of 0.2. The cultures were split, and the potential inducers were added at subinhibitory concentrations. Cells were collected 1 h after the addition of the potential inducers and assayed for β-galactosidase activity. In the absence of inducers, the specific activity of orf2420-lacZ in the wild-type strain was largely the same at an OD600 of 0.2 and 1 h later (OD600 = 0.4; Fig. 6B). The addition of 1% ethanol resulted in a dramatic induction of orf2420-lacZ expression (76-fold) in the wild-type strain (Fig. 6B). No induction of orf2420 was observed in the ΔcesR mutant strain (Fig. 6B). These results are consistent with the results of the primer extension analysis with orf2420 (Fig. 6A). Next, we tested the inducing capabilities of seven antibiotics targeting cell wall synthesis. Cephalexin, cefuroxime, penicillin G, ampicillin, vancomycin, bacitracin, and d-cycloserine strongly induced the expression of orf2420-lacZ, from 10-fold (bacitracin) to 95-fold (vancomycin), in a CesR-dependent fashion (Fig. 6B). Similar results were obtained for the ΔcesK strain (data not shown). Subinhibitory concentrations of the membrane-active compounds Triton X-100, Tween 20, and sodium dodecyl sulfate did not affect the expression of the orf2420-lacZ fusion, suggesting that they do not function as inducers (data not shown).
Given that ethanol and a variety of cell wall-acting antibiotics induce the orf2420 promoter, we wished to determine whether the cell wall hydrolytic enzyme lysozyme was capable of acting as an inducer. Indeed, subinhibitory concentrations of lysozyme induced orf2420-lacZ expression in a dose-dependent manner (Fig. 6C). The induction was dependent on CesR and CesK (data not shown). Collectively, these data suggest that cell wall-acting antibiotics, ethanol, and lysozyme all generate a potent inducing signal sensed by the CesRK two-component system.
DISCUSSION
We have identified a two-component system in L. monocytogenes, CesRK, which mediates sensitivity to ethanol and resistance to antibiotics of the β-lactam family and which contributes to the virulence of the organism in mice. Moreover, it is demonstrated that CesRK enables L. monocytogenes to sense the presence of a variety of cell wall-acting antimicrobial agents, including ethanol, cell wall-acting antibiotics, and the cell wall lytic enzyme, lysozyme.
We observed that the virulence potential of L. monocytogenes lacking the CesRK two-component system is reduced in comparison to that of the wild-type strain. Consequently, the target genes controlled by the CesRK two-component system may represent a collection of genes that are important for survival and persistence during an infection. In the present study, we have identified a gene regulated by CesRK. This target gene, orf2420, contributes to virulence in mice and decreases ethanol resistance. Interestingly, an open reading frame corresponding to orf2420 has not been defined in the avirulent species L. innocua, suggesting a specific role for Orf2420 in the virulence of L. monocytogenes (9). Although the sensitivity of the Δorf2420 mutant to cell wall-acting antibiotics was not significantly affected, the transcription of orf2420 was highly induced in the presence of these agents in a CesRK-dependent manner. The increased expression of orf2420 in the presence of cell wall-acting antibiotics, ethanol, and lysozyme suggests that Orf2420 may play a role in the response to cell wall damage. Studies are ongoing in our laboratory to identify the role(s) of Orf2420 in the response to alterations of cell wall integrity.
The identification of orf2420 as a target gene for CesRK provided a tool for the identification of the signal sensed by CesRK. The level of transcription of orf2420 increases about 10-fold in a CesRK-dependent manner in L. monocytogenes cells going from the early exponential phase into the stationary growth phase, suggesting that a signal sensed by CesRK is generated though this growth phase transition. In E. coli, the peptidoglycan layer is known to undergo considerable changes upon entry into stationary phase (27). Furthermore, the antimicrobial agents that induce the transcription of orf2420 in a CesRK-dependent manner affect different stages of peptidoglycan synthesis (11, 19, 32). Thus, peptidoglycan appears to play an important role in the generation of the signal sensed by CesRK. Considering the diversity and broad specificity of the inducing factors, the actual signal sensed by the two-component system most likely accumulates as a consequence of some physical changes in the cell wall induced by these factors. The signal may be an intermediate in the biosynthesis or degradation of peptidoglycan; however, its true nature remains to be determined.
The opposite responses of the cesRK deletion mutants to ethanol and β-lactam antibiotics is a paradoxical finding. In E. coli, growth in the presence of ethanol is known to result in the production of un-cross-linked peptidoglycan (19). The β-lactam antibiotics prevent cross-linking of the peptidoglycan by binding to and inhibiting the activity of the penicillin-binding protein (PBP) transpeptidases (15). Both ethanol and β-lactams induce the expression of orf2420 in a CesRK-dependent manner. However, the orf2420 gene mediates only ethanol sensitivity and not β-lactam resistance, suggesting that other genes controlled by CesRK are involved in β-lactam resistance, but not necessarily in ethanol sensitivity. Thus, different genes under the control of CesRK may promote ethanol sensitivity and β-lactam resistance.
The cephalosporins are used in the medical treatment of bacterial meningitis; however, they have no effect in cases in which L. monocytogenes is the cause of disease. The reason for this natural resistance to cephalosporins is that they have only a low affinity for the primary lethal target for active β-lactams in L. monocytogenes, PBP 3 (16, 38). The cephalosporins were, however, found to be good inhibitors of PBPs 1, 2, and 4 (38). The LisRK two-component system has recently been shown to mediate the resistance of L. monocytogenes to cephalosporins. In addition, LisRK contributes to the response to the lantibiotic nisin, ethanol, acid, and hydrogen peroxide stress; and it plays a significant role in the virulence potential of L. monocytogenes (6, 7). The similar roles played by LisRK and CesRK in mediating ethanol sensitivity and cephalosporin resistance may reflect a regulatory connection between the two signal transduction systems.
In the present study, we have identified a two-component system involved in the ability of L. monocytogenes to sense and respond to a variety of antimicrobial agents present in the environment, foods, and the infected host. Importantly, some of these agents are used therapeutically in the treatment of infections caused by L. monocytogenes and other human pathogens. It is becoming increasingly clear that a number of two-component systems of important bacterial pathogens, such as VanRS of Enterococcus faecalis (3), CiaHR and VncRS of S. pneumoniae (13, 24), and LisRK and CesRK of L. monocytogenes (7; this study), may indirectly contribute to the pathogenicity of these organisms by mediating resistance or sensitivity to antibiotics. Furthermore, CiaHR, LisRK, and CesRK play direct roles in virulence in mouse models, suggesting important roles for these systems in directly contributing to the infection process (6, 20, 34; this study). Further studies on such two-component systems will improve our understanding of how human pathogens sense and respond to commonly used antimicrobial agents and will provide a better understanding of the molecular mechanisms by which antimicrobial resistance may arise.
Acknowledgments
This work received financial support from The FREJA Program and The Plasmid Foundation.
We thank Christina Kirkegaard for excellent technical assistance.
REFERENCES
- 1.Allen, N. E., and J. N. Hobbs, Jr. 1995. Induction of vancomycin resistance in Enterococcus faecium by non-glycopeptide antibiotics. FEMS Microbiol. Lett. 132:107-114. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. Schaffer, J. Zhang, S. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arthur, M., and R. Quintiliani. 2001. Regulation of VanA- and VanB-type glycopeptide resistance in enterococci. Antimicrob. Agents Chemother. 45:375-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baptista, M. G., F. Depardieu, P. Courvalin, and M. Arthus. 1996. Specificity of induction of glycopeptide resistance genes in Enterococcus faecalis. Antimicrob. Agents Chemother. 40:2291-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakraborty, T., M. Leimeister-Wachter, E. Domann, M. Hartl, W. Goebel, T. Nichterlein, and S. Notermans. 1992. Coordinate regulation of virulence genes in Listeria monocytogenes requires the product of the prfA gene. J. Bacteriol. 174:568-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotter, P. D., N. Emerson, C. G. Gahan, and C. Hill. 1999. Identification and disruption of lisRK, a genetic locus encoding a two-component signal transduction system involved in stress tolerance and virulence in Listeria monocytogenes. J. Bacteriol. 181:6840-6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotter, P. D., C. M. Guinane, and C. Hill. 2002. The LisRK signal transduction system determines the sensitivity of Listeria monocytogenes to nisin and cephalosporins. Antimicrob. Agents Chemother. 46:2784-2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giammarinaro, P., M. Sicard, and A.-M. Gasc. 1999. Genetic and physiological studies of the CiaH-CiaR two-component signal-transduction system involved in cefotaxime resistance and competence of Streptococcus pneumoniae. Microbiology 145:1859-1869. [DOI] [PubMed] [Google Scholar]
- 9.Glaser, P., L. Frangeul, C. Buchrieser, C. Rusniok, A. Amend, F. Baquero, P. Berche, H. Bloecker, P. Brandt, T. Chakraborty, A. Charbit, F. Chetouani, E. Couve, A. de Daruvar, P. Dehoux, E. Domann, G. Dominguez-Bernal, E. Duchaud, L. Durant, O. Dussurget, K. D. Entian, H. Fsihi, F. G. Portillo, P. Garrido, L. Gautier, W. Goebel, N. Gomez-Lopez, T. Hain, J. Hauf, D. Jackson, L. M. Jones, U. Kaerst, J. Kreft, M. Kuhn, F. Kunst, G. Kurapkat, E. Madueno, A. Maitournam, J. M. Vicente, E. Ng, H. Nedjari, G. Nordsiek, S. Novella, B. de Pablos, J. C. Perez-Diaz, R. Purcell, B. Remmel, M. Rose, T. Schlueter, N. Simoes, A. Tierrez, J. A. Vazquez-Boland, H. Voss, J. Wehland, and P. Cossart. 2001. Comparative genomics of Listeria species. Science 294:849-852. [DOI] [PubMed] [Google Scholar]
- 10.Grant, C. M. 2001. Role of the glutathione/glutaredoxin and thioredoxin systems in yeast growth and response to stress conditions. Mol. Microbiol. 39:533-541. [DOI] [PubMed] [Google Scholar]
- 11.Green, D. W. 2002. The bacterial cell wall as a source of antibacterial targets. Expert Opin. Ther. Targets 6:1-19. [DOI] [PubMed] [Google Scholar]
- 12.Grissom-Arnold, J., W. E. Alborn, T. I. Nicas, and S. R. Jaskunas. 1997. Induction of VanA vancomycin resistance genes in Enterococcus faecalis: use of a promoter fusion to evaluate glycopeptide and nonglycopeptide induction signals. Microb. Drug Resist. 3:53-64. [DOI] [PubMed] [Google Scholar]
- 13.Guenzi, E., A. M. Gasc, M. A. Sicard, and R. Hakenbeck. 1994. A two-component signal-transduction system is involved in competence and penicillin susceptibility in laboratory mutants of Streptococcus pneumoniae. Mol. Microbiol. 12:505-515. [DOI] [PubMed] [Google Scholar]
- 14.Handwerger, S., and A. Kolokathis. 1990. Induction of vancomycin resistance in Enterococcus faecium by inhibition of transglycosylation. FEMS Microbiol. Lett. 70:167-170. [DOI] [PubMed] [Google Scholar]
- 15.Hoch, J. A., and T. J. Silhavy. 1995. Two-component signal transduction. American Society for Microbiology, Washington, D.C.
- 16.Hof, H., T. Nichterlein, and M. Kretschmar. 1997. Management of listeriosis. Clin. Microbiol. Rev. 10:345-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hofmann, K., and W. Stoffel. 1993. TMbase—a database of membrane spanning protein segments. Biol. Chem. Hoppe-Seyler 374:166. [Google Scholar]
- 18.Horton, R. M., Z. L. Cai, S. N. Ho, and L. R. Pease. 1990. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. BioTechniques 8:528-535. [PubMed] [Google Scholar]
- 19.Ingram, L. O., and N. S. Vreeland. 1980. Differential effects of ethanol and hexanol on the Escherichia coli cell envelope. J. Bacteriol. 144:481-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kallipolitis, B. H., and H. Ingmer. 2001. Listeria monocytogenes response regulators important for stress tolerance and pathogenesis. FEMS Microbiol. Lett. 204:111-115. [DOI] [PubMed] [Google Scholar]
- 21.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 22.Moazed, D., S. Stern, and H. F. Noller. 1986. Rapid chemical probing of conformation in 16S ribosomal RNA and 30S ribosomal subunits using primer extension. J. Mol. Biol. 187:399-416. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen, H. J., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokyotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 24.Novak, R., B. Henriques, E. Charpentier, S. Normark, and E. Tuomanen. 1999. Emergence of vancomycin tolerance in Streptococcus pneumoniae. Nature 399:590-593. [DOI] [PubMed] [Google Scholar]
- 25.Park, S. F., and G. S. A. B. Stewart. 1990. High-efficiency transformation of Listeria monocytogenes by electroporation of penicillin treated cells. Gene 94:129-132. [DOI] [PubMed] [Google Scholar]
- 26.Paulsen, I. T., and M. H. Saier, Jr. 1997. A novel family of ubiquitous heavy metal ion transport proteins. J. Membr. Biol. 156:99-103. [DOI] [PubMed] [Google Scholar]
- 27.Pisabarro, A. G., M. A. de Pedro, and D. Vazquez. 1985. Structural modifications in the peptidoglycan of Escherichia coli associated with changes in the state of growth of the culture. J. Bacteriol. 161:238-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Podbielski, A., A. Glosdorff, and J. Weber-Heynemann. 1995. The group A streptococcal virR49 gene controls expression of four structural vir regulon genes. Infect. Immun. 63:9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poyart, C., and P. Trieu-Cuot. 1997. A broad-host-range mobilizable shuttle vector for the construction of transcriptional fusions to β-galactosidase in gram-positive bacteria. FEMS Microbiol. Lett. 156:193-198. [DOI] [PubMed] [Google Scholar]
- 30.Prazak, M. A., E. A. Murano, I. Mercado, and G. R. Acuff. 2002. Antimicrobial resistance of Listeria monocytogenes isolated from various cabbage farms and packing sheds in Texas. J. Food Prot. 65:1796-1799. [DOI] [PubMed] [Google Scholar]
- 31.Robertson, G. T., J. Zhao, B. V. Desai, W. H. Coleman, T. I. Nicas, R. Gilmour, L. Grinius, D. A. Morrison, and M. E. Winkler. 2002. Vancomycin tolerance induced by erythromycin but not by loss of vncRS, vex3, or pep27 function in Streptococcus pneumoniae. J. Bacteriol. 184:6987-7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siewert, G., and J. L. Strominger. 1967. Bacitracin: an inhibitor of the dephosphorylation of lipid pyrophosphate, an intermediate in biosynthesis of the peptidoglycan of bacterial cell walls. Proc. Natl. Acad. Sci. USA 57:767-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Temple, M. E., and M. C. Nahata. 2000. Treatment of listeriosis. Ann. Pharmacother. 34:656-661. [DOI] [PubMed] [Google Scholar]
- 34.Throup, J. P., K. K. Koretke, A. P. Bryant, K. A. Ingraham, A. F. Chalker, Y. Ge, A. Marra, N. G. Wallis, J. R. Brown, D. J. Holmes, M. Rosenberg, and M. K. Burnham. 2000. A genomic ananlysis of two-component signal transduction in Streptococcus pneumoniae. Mol. Microbiol. 35:566-576. [DOI] [PubMed] [Google Scholar]
- 35.Ulijasz, A., T., A. Grenader, and B. Weisblum. 1996. A vancomycin-inducible LacZ reporter system in Bacillus subtilis: induction by antibiotics that inhibit cell wall synthesis and lysozyme. J. Bacteriol. 178:6305-6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vazquez-Boland, J. A., C. Kocks, S. Dramsi, H. Ohayon, C. Goeffroy, J. Mengaud, and P. Cossart. 1992. Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect. Immun. 60:219-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vazquez-Boland, J. A., M. Kuhn, P. Berche, T. Chakraborty, G. Dominguez-Bernal, W. Goebel, B. Gonzalez-Zorn, J. Wehland, and J. Kreft. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:1-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vicente, M. F., J. C. Perez-Daz, F. Baquero, M. Angel de Pedro, and J. Berenguer. 1990. Penicillin-binding protein 3 of Listeria monocytogenes as the primary lethal target for β-lactams. Antimicrob. Agents Chemother. 34:539-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walsh, D., G. Duffy, J. J. Sheridan, I. S. Blair, and D. A. McDowell. 2001. Antibiotic resistance among Listeria, including Listeria monocytogenes, in retail foods. J. Appl. Microbiol. 90:517-522. [DOI] [PubMed] [Google Scholar]
- 40.White, D. G., S. Zhao, S. Simjee, D. D. Wagner, and P. F. McDermott. 2002. Antimicrobial resistance of foodborne pathogens. Microbes Infect. 4:405-412. [DOI] [PubMed] [Google Scholar]


