Abstract
Fluconazole (FLC), a triazole with limited activity against Aspergillus species, is frequently used as prophylaxis in leukemia patients and bone marrow transplant recipients. Prior FLC use has been associated with an increasing incidence of invasive aspergillosis in these patients. We hypothesized that prior exposure of Aspergillus fumigatus to FLC could result in altered in vitro susceptibility of this fungus to other, more active triazoles. Thus, we performed serial passages of conidia of 10 clinical isolates of A. fumigatus (all itraconazole [ITC] susceptible) on FLC-containing yeast agar glucose plates. The MICs and minimal fungicidal concentrations (MFCs) of amphotericin B, FLC, ITC, and voriconazole (VRC) for A. fumigatus conidia were measured following four passages on FLC-containing medium according to the National Committee for Clinical Laboratory Standards microdilution method. Serial passages on FLC-containing plates resulted in a fourfold increase in the MFCs (but not the MICs) of ITC for nine isolates. The attenuated ITC fungicidal activity against A. fumigatus following FLC preexposure was medium independent and was also observed against FLC-preexposed A. fumigatus hyphae with the viability staining FUN-1 dye. Moreover, FLC preexposure of A. fumigatus conidia resulted in an analogous increase in the MFCs (but not the MICs) of VRC. Our findings suggest that preexposure of A. fumigatus to FLC attenuates the in vitro fungicidal activity of subsequent ITC use against it. This phenotypic adaptation is not captured by a routine MIC determination but requires MFC measurement. The in vivo significance of this in vitro phenomenon requires further investigation.
Fluconazole (FLC) is a triazole with good activity against a broad spectrum of pathogenic fungi, including Candida species and Cryptococcus neoformans, but with poor activity against molds such as Aspergillus species (11). Since the early 1990s, several studies have shown that FLC prophylaxis results in a substantial decrease in the incidence of superficial and invasive candidiasis in patients at high risk for invasive fungal infections, such as patients with leukemia as well as those who have received bone marrow and solid organ transplants (1). Because of the limited toxicity and the availability of a reliable oral formulation of FLC, prolonged FLC courses have become common practice for such high-risk patients. Nevertheless, the increasing FLC use over the past decade has been associated with a higher incidence of invasive aspergillosis (IA) for these patients (12). Of the Aspergillus spp., Aspergillus fumigatus is the most common cause of IA (6). We previously hypothesized that phenotypic alteration of some Aspergillus isolates already colonizing high-risk patients with preexposure to FLC may account, at least in part, for this increasing incidence (3). We show here that FLC, despite its lack of activity against A. fumigatus, alters the in vitro susceptibility of this fungus to itraconazole (ITC) and voriconazole (VRC), two triazoles that are used increasingly for the prophylaxis and treatment of IA (4).
(Part of this work was presented at the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 27 to 30 September 2002.)
MATERIALS AND METHODS
Materials.
Yeast agar glucose (YAG) plates (0.5% yeast extract, 1.0% dextrose, 0.2% vitamin mix, 0.1% trace elements, 1.5% agar, 1% MgSO4) and RPMI 1640 (Sigma Chemical Co., St. Louis, Mo.) buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid and 2% glucose were prepared as 100-mm agar plates as described previously (2).
A. fumigatus isolates.
Ten ITC-susceptible clinical isolates of A. fumigatus obtained from the Mycology Laboratory at The University of Texas M. D. Anderson Cancer Center were used. All of the isolates were cultured on potato dextrose agar plates and then subcultured on YAG plates with or without FLC. The isolates were preserved in 20% glycerol at −80°C.
In vitro susceptibility testing.
The MICs of antifungal agents were determined on RPMI 1640 liquid medium by using NCCLS standard methods (9). Candida tropicalis strain ATCC 2697 served as a quality control isolate for all experiments. The drugs tested were amphotericin B (AMB), FLC, ITC, and VRC. FLC and VRC were obtained from Pfizer, Inc. (New York, N.Y.), ITC was obtained from Janssen Pharmaceutica (Titusville, N.J.), and AMB was obtained from Pharma-Tek (Huntington, N.Y.) All of the antifungal agents were obtained in assay powder form. Drug dilutions were prepared in 100% dimethyl sulfoxide (for AMB, ITC, and VRC) or distilled water (for FLC).
The MIC was defined as the lowest drug concentration at which there was a complete absence (for AMB) or prominent reduction of growth (corresponding to approximately 50% growth reduction for the azoles). The in vitro fungicidal activity (minimal fungicidal concentration [MFC]) of each agent was determined according to a recently proposed standardized methodology (2). Briefly, 20-μl aliquots of broth were subcultured from each well that showed complete inhibition of growth (100%, or an optically clear well), from the growth control well (drug-free medium), and from the last positive well (with growth similar to that in the growth control well) onto Sabouraud dextrose agar plates. The plates were incubated at 35°C until growth was seen in the growth control subculture (usually 48 h later). The MFC was defined as the lowest drug concentration that resulted in either no growth or growth of fewer than three colonies, which corresponds to a killing activity of 99.0 to 99.5%. The MICs and MFCs were determined in triplicate in three independent experiments.
Preexposure of Aspergillus isolates to FLC.
All 10 isolates of A. fumigatus were plated on YAG plates at 35°C for 3 days, and conidia were collected and counted by hemocytometry. Aliquots (100 μl) of a standardized conidial suspension (106 conidia/ml) were then transferred onto YAG plates containing no FLC or 8 or 64 μg of FLC/ml and incubated at 35°C. Three days later, conidia were harvested, and a similar inoculum (approximately 105 conidia) was replated on fresh YAG plates containing the same FLC concentrations and incubated as described above. The MICs and MFCs of AMB, FLC, ITC, and VRC (on RPMI 1640 liquid medium) for each A. fumigatus isolate were determined at baseline and after the first and fourth passages on FLC-containing YAG plates. To determine whether attenuation of the in vitro fungicidal activity of ITC following preexposure of A. fumigatus to FLC was medium independent, we measured the MICs and MFCs of ITC (on RPMI 1640 liquid medium) against a subset of representative Aspergillus isolates (n = 4) after growth (for four passages) on RPMI 1640 plates (instead of YAG plates) containing no FLC or 8 or 64 μg of FLC/ml.
2,3-Bis{2-methoxy-4-n-5-[(sulfenylamino)carbonyl]-2H-tetrazolium-hydroxide} (XTT) colorimetric assay.
NCCLS microtiter plates were prepared with RPMI 1640 liquid medium and incubated for 48 h at 35°C. XTT solutions were prepared as previously described (7, 8). Incubation was continued at 35°C for 2 h in the dark to allow for conversion of XTT to its formazan derivatives. The optical density (OD) was measured with a microplate spectrophotometer (Powerwave X; Bio-Tek Instruments, Winooski, Vt.) at 492 and 690 nm after shaking. The color was then assessed spectrophotometrically based on the relative OD at 492 and 690 nm, with the relative OD at 690 nm being a reference wavelength subtracted from the OD at 492 nm.
NCCLS microdilution with Aspergillus hyphae as the starting inoculum.
Conidial stock solutions from a representative isolate (A. fumigatus isolate 32) previously preexposed to different concentrations of FLC (obtained after four passages on YAG plates containing no FLC or 8 or 64 μg of FLC/ml) were diluted (1:100) in RPMI 1640 medium to obtain an inoculum of 0.4 × 104 to 5.0 × 104 cells/ml. An aliquot of each solution was added to 24-well cell culture plates containing no FLC or the aforementioned final concentrations of FLC (8 or 64 μg/ml) in liquid RPMI 1640 medium. Next, the plates were incubated at 35°C for 16 to 22 h, which allowed the transformation of more than 95% of the conidia to hyphae with lengths of 50 to 70 μm as determined with an inverted microscope (5). A 100-μl aliquot of the hyphae, washed twice with RPMI 1640 liquid medium to remove FLC, was then inoculated into a 96-well microtiter plate containing serial twofold dilutions of ITC and incubated as described above. The MIC and MFC of ITC for this isolate were then determined as described previously (2, 9).
Viability staining.
Hyphal viability was evaluated with the FUN-1 viability staining method (Molecular Probes, Inc., Eugene, Oreg.) (5). According to the FUN-1 staining patterns, the hyphae were classified as viable (green fluorescent hyphae with clearly red fluorescent vacuolar structures), impaired (green fluorescent hyphae without red fluorescent vacuolar structures), or dead (green-yellow fluorescent hyphae without any fluorescent vacuolar structures). To assess the degree of ITC antifungal activity against hyphae, conidia from A. fumigatus isolate 32 were serially plated on YAG plates containing no FLC, 8 μg of FLC/ml, and 64 μg of FLC/ml, and hypha formation was induced on 24-well cell culture plates as described above. The wells were then washed twice with RPMI 1640 liquid medium and refilled with 0.5 ml of RPMI 1640 medium containing four different concentrations of ITC (range, 0.5 to 256.0 μg/ml) and then stained with FUN-1 after the plates were incubated at 35°C for another 24 h. Isolates were then examined with a triple-band fluorescent microscope (Olympus BX-51; Olympus America, Inc., Melville, N.Y.) as described previously (5).
Stability of the attenuation of the in vitro fungicidal activity of ITC.
Conidia of the Aspergillus isolates for which the ITC MFCs were higher after serial passages on FLC-containing YAG plates were then serially subcultured on FLC-free YAG plates. Specifically, 100-μl aliquots (from a standardized conidial suspension of 106 conidia/ml) of these Aspergillus isolates (grown for four passages as described above on plates containing YAG, YAG plus 8 μg of FLC/ml, and YAG plus 64 μg of FLC/ml) were serially plated on YAG plates without FLC. After the second and fourth passages on drug-free medium, the ITC MICs and MFCs were determined and compared with the baseline values and with the MICs and MFCs for the isolates after four passages on the YAG-plus-FLC plates.
Growth characteristics of Aspergillus isolates preexposed and not preexposed to FLC determined with the XTT colorimetric assay.
To assess the growth rates of Aspergillus isolates preexposed and not preexposed to FLC, 96-well round-bottom microtiter plates were prepared by inoculating 100-μl aliquots of conidial suspensions of two representative Aspergillus isolates (A. fumigatus isolate 32 and A. fumigatus reference isolate 293) either preexposed (cultured for four passages on YAG plates containing 8 μg of FLC/ml) or not preexposed to FLC (cultured for four passages on YAG plates not containing FLC) in RPMI 1640 liquid medium as described above (final concentrations, 0.4 × 104 to 5.0 × 104 cells/ml). The plates were then incubated at 35°C. At regular intervals following the addition of the conidial suspensions (0, 4, 12, 24, 36, and 48 h), 50-μl aliquots of an XTT solution that were prepared as described elsewhere (7, 8) were added to each well in single rows corresponding to each time point. The OD at 492 and 690 nm was then measured for each row to which the XTT solution was added following a 2-h incubation in the dark; the color was assessed as described above.
Statistical analysis.
The MICs and MFCs of AMB, ITC, and VRC for the 10 Aspergillus isolates following serial growth (one and four passages) on YAG plates containing no FLC or FLC in concentrations of 8 or 64 μg/ml were compared with the values at baseline. Furthermore, the MICs and MFCs for the FLC-preexposed Aspergillus isolates were compared with those for the isolates following serial growth (four passages) on FLC-free YAG plates. The Mann-Whitney nonparametric two-tailed t test or one-way analysis of variance plus the Dunn test (analysis of variance) was used where appropriate to assess statistically significant differences in the MICs and MFCs. Results with a P value of 0.05 or less were considered statistically significant.
RESULTS
Susceptibilities of FLC-preexposed A. fumigatus isolates to ITC.
The baseline susceptibilities of the 10 A. fumigatus isolates to AMB, FLC, ITC, and VRC are summarized in Table 1. Serial passages (n = 4) on FLC-containing YAG plates (8 or 64 μg of FLC/ml) resulted in increased ITC MFCs for nine isolates (P < 0.001). In contrast, the ITC MICs did not significantly change (Table 2). Strain-to-strain variations were observed in increases in the ITC MFCs, as the MFCs (>16 μg/ml) for 6 of the 10 isolates showed marked increases, including an ITC MFC of 256 μg/ml for 1 isolate. The median increase in ITC MFCs was fourfold greater than the value at baseline and after serial passages (n = 4) on YAG plates without FLC (see below). Likewise, the ITC MFCs for A. fumigatus clinical isolate 293 exhibited a fourfold increase following four passages on YAG-plus-FLC plates without a change in the ITC MICs (data not shown). No significant differences in the ITC MFCs (and MICs) were noted after four passages of Aspergillus isolates on YAG plates without FLC (Table 2). Thus, serial growth of Aspergillus isolates on YAG plates without FLC did not change the isolates' susceptibilities to ITC.
TABLE 1.
The MICs and MFCs (in μg/ml) of antifungal agents for 10 clinical isolates of A. fumigatus and reference isolate 293a
| Isolate no. | AMB
|
ITC
|
VRC
|
FLC
|
||||
|---|---|---|---|---|---|---|---|---|
| MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | |
| 31 | 1 | 1 | 0.25 | 4 | 0.25 | 1 | >64 | N/A |
| 32 | 1 | 2 | 0.5 | 2 | 0.25 | 1 | >64 | N/A |
| 34 | 1 | 1 | 0.25 | 1 | 0.125 | 1 | >64 | N/A |
| 40 | 1 | 2 | 0.25 | 4 | 0.25 | 4 | >64 | N/A |
| 49 | 1 | 2 | 0.25 | 2 | 0.25 | 16 | >64 | N/A |
| 50 | 1 | 1 | 0.25 | 1 | 0.25 | 1 | >64 | N/A |
| 51 | 1 | 2 | 0.5 | 2 | 0.25 | 1 | >64 | N/A |
| 53 | 1 | 1 | 0.5 | 2 | 0.25 | 1 | >64 | N/A |
| 54 | 1 | 2 | 0.25 | 1 | 0.25 | 1 | >64 | N/A |
| 55 | 0.5 | 2 | 0.25 | 1 | 0.25 | 1 | >64 | N/A |
| 293 | 1 | 1 | 0.5 | 2 | 0.25 | 1 | >64 | N/A |
NCCLS microdilution method M38-P was used. MIC50s were determined for AMB (1 μg/ml), ITC (0.25 μg/ml), VRC (0.25 μg/ml), and FLC (>64 μg/ml). MFC50s were determined for AMB (1 μg/ml), ITC (2 μg/ml), and VRC (1 μg/ml). N/A, not applicable.
TABLE 2.
MICs and MFCs of ITC for 10 clinical isolates of A. fumigatus following preexposure (for four passages) to different concentrations of FLC (NCCLS microdilution method M38-P)
| Aspergillus isolates grown on YAG plates | MIC
|
MFC
|
||||
|---|---|---|---|---|---|---|
| MIC50 | Range | GMa | MFC50 | Range | GM | |
| Without FLCb | 0.25c | 0.25-0.5 | 0.35 | 2d | 1-4 | 2e |
| With 8 μg of FLC/ml | 0.5c | 0.25-0.5 | 0.475 | 32d | 1-128 | 45.3f |
| With 64 μg of FLC/ml | 0.5c | 0.25-0.5 | 0.475 | 16 | 1-256 | 43.3e,f |
| Baseline value (no passages on any media)b | 0.25 | 0.25-0.5 | 0.375 | 2 | 1-4 | 2.2 |
GM, geometric mean.
P was not significant for the ITC MICs and MFCs for Aspergillus isolates serially cultured (for four passages) on YAG plates without FLC versus Aspergillus isolates not grown on any media.
P was not significant for the ITC MICs for Aspergillus isolates not preexposed to FLC versus Aspergillus isolates preexposed to 8 μg of FLC/ml versus Aspergillus isolates preexposed to 64 μg of FLC/ml.
P = 0.001 for the ITC MFCs for Aspergillus isolates not preexposed to FLC versus Aspergillus isolates preexposed to 8 μg of FLC/ml.
P < 0.001 for the ITC MFCs for Aspergillus isolates not preexposed to FLC versus Aspergillus isolates preexposed to 64 μg of FLC/ml.
P was not significant for the ITC MFCs for Aspergillus isolates preexposed to 8 μg of FLC/ml versus Aspergillus isolates preexposed to 64 μg of FLC/ml.
The magnitude of the increases in ITC MFCs was not influenced by the FLC concentrations to which the isolates were previously exposed. There was not a statistically significant difference in the increases in the ITC MFCs following preexposure of the isolates to 8 versus 64 μg of FLC/ml (Table 2). On the other hand, the rapidity of the increases in ITC MFCs appeared to depend on the FLC concentrations to which the Aspergillus isolates were preexposed. More specifically, preexposure of isolates to 64 μg of FLC/ml resulted in marked increases in the ITC MFCs following only one passage compared to increases in the ITC MFCs following preexposure to 8 μg of FLC/ml after one passage (P = 0.02) (data not shown).
Comparison of the visual and spectrophotometric readings from the NCCLS method for testing FLC-preexposed Aspergillus isolates and the XTT colorimetric assay.
The ITC MICs for the FLC-preexposed Aspergillus isolates measured spectrophotometrically with the XTT colorimetric assay were comparable to the respective MICs estimated visually by the NCCLS method. Specifically, the ITC MICs for the FLC-preexposed isolates did not significantly differ from those for isolates not preexposed to FLC (data not shown).
Medium-independent attenuation of the in vitro fungicidal activity of ITC against A. fumigatus isolates preexposed to FLC.
Following FLC preexposure (for four passages) of four representative A. fumigatus isolates on the alternative RPMI 1640 medium (containing either 8 or 64 μg of FLC/ml), increases in the ITC MFCs comparable to those described above were seen. Again, the ITC MICs were not affected (data not shown). The attenuation of ITC's fungicidal activity after serial growth of the isolates on plates containing RPMI plus 64 μg of FLC/ml was similar to that after growth on plates containing RPMI plus 8 μg of FLC/ml. Additionally, preexposure to higher concentrations of FLC (64 μg/ml) was associated with a more rapid increase in the ITC MFCs, as we also observed on YAG plates containing FLC (data not shown).
Detecting the attenuation of the in vitro fungicidal activity of ITC against A. fumigatus hyphae preexposed to FLC with FUN-1 viability staining.
We used the representative A. fumigatus isolate (isolate 32) for which the ITC MFC showed a fourfold increase but for which the ITC MIC was unchanged after preexposure of the isolate to FLC. The same isolate unexposed to FLC was used as a control. By using hyphae as starting inocula, FLC preexposure once more resulted in a fourfold increase in the ITC MFC (2 versus 32 μg/ml) for Aspergillus hyphae. Moreover, by using the viability stain FUN-1, we found that when this isolate was not preexposed to FLC, it exhibited only a few viable hyphae following 48 h of incubation with 2 μg of ITC/ml (which was the MFC of ITC for that particular isolate) (Fig. 1A). No viable hyphae were seen after incubation with 256 μg of ITC/ml, a concentration sevenfold greater than the ITC MFC (Fig. 1D). In contrast, when the same isolate was preexposed to an FLC concentration of 8 or 64 μg/ml on YAG plates for four passages, viable hyphae could be seen after 48 h of incubation with ITC at concentrations of 2 μg/ml (Fig. 1B and E) and even 256 μg/ml (Fig. 1C and F).
FIG. 1.
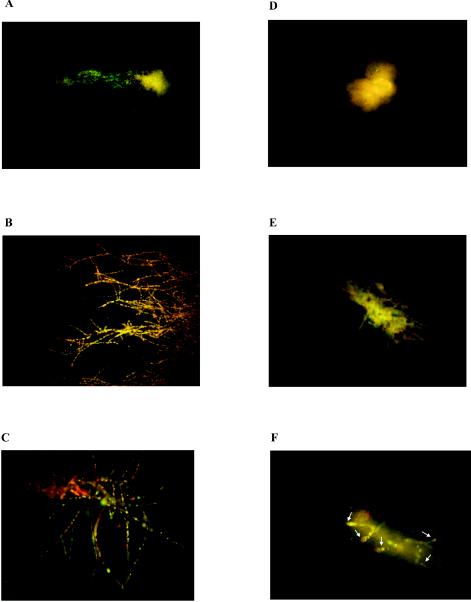
FUN-1 staining of A. fumigatus hyphae. Panels A to C show damage induced by ITC at a concentration of 2 μg/ml to hyphae in A. fumigatus isolate 32 after four passages on YAG plates containing no FLC (A), 8 μg of FLC/ml (B), or 256 μg of FLC/ml (C). Panels D to F show damage induced by ITC at a concentration of 256 μg/ml to hyphae in A. fumigatus isolate 32 after four passages on YAG plates containing no FLC (D), 8 μg of FLC/ml (E), or 256 μg of FLC/ml (F). Arrows (F) indicate viable hyphae. Compartments of Aspergillus hyphae previously exposed to FLC remained viable even after exposure to ITC at a concentration of 256 μg/ml.
Stability of the increase in the ITC MFC for A. fumigatus conidia preexposed to FLC.
The nine Aspergillus isolates for which the ITC MFCs increased after preexposure to FLC were grown on YAG plates without FLC to assess the stability of the increases in the ITC MFCs. For six of these nine isolates, the ITC MFCs decreased following four passages on FLC-free medium. Nonetheless, the ITC MFCs did not reach the baseline level and remained significantly higher than the baseline MFCs (P < 0.001) (Fig. 2).
FIG. 2.
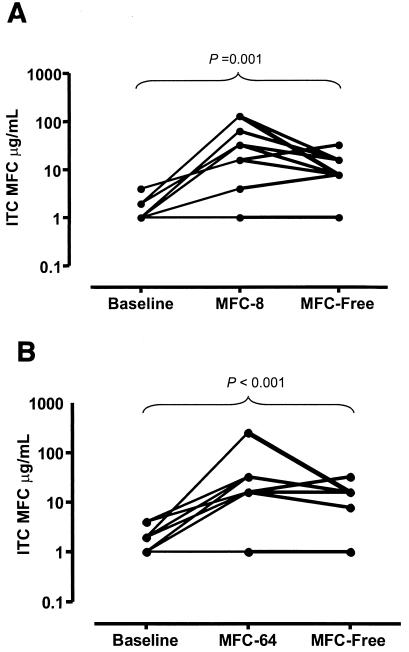
Stability of attenuation of fungicidal activity of ITC induced by prior exposure to FLC. (A) MFCs of ITC for the 10 FLC-preexposed A. fumigatus isolates at baseline, after four passages on YAG plates containing 8 μg of FLC/ml (MFC-8), and after another four passages on FLC-free medium (MFC-Free). (B) MFCs of ITC for the 10 FLC-preexposed A. fumigatus isolates at baseline, after four passages on YAG plates containing 64 μg of FLC/ml (MFC-64), and after another four passages on FLC-free medium (MFC-Free).
Susceptibilities of the FLC-preexposed A. fumigatus isolates to VRC.
Four passages of 8 of the 10 A. fumigatus isolates preexposed to 64 μg of FLC/ml resulted in a fourfold increase in the VRC MFCs compared to the MFCs at baseline and after serial passages (n = 4) on YAG plates without FLC (P < 0.001) (Table 3). Again, no statistically significant changes in the MICs of VRC were observed. The magnitude of the increases in the VRC MFCs was more marked after preexposure of the isolates to 64 μg of FLC/ml than to 8 μg of FLC/ml on YAG plates, although this difference was not statistically significant (Table 3). In agreement with our observations of the ITC MFCs, the VRC MFCs were higher after one passage on medium containing 64 μg of FLC/ml than after one passage on medium containing 8 μg of FLC/ml (data not shown).
TABLE 3.
MICs and MFCs of VRC for 10 clinical isolates of A. fumigatus following exposure to different concentrations of FLC (NCCLS microdilution method M38-P)
| Aspergillus isolates grown on YAG plates | MIC
|
MFC
|
||||
|---|---|---|---|---|---|---|
| MIC50 | Range | GMa | MFC50 | Range | GM | |
| Without FLCb | 0.25c | 0.125-0.25 | 0.24 | 1d | 1-16 | 2.8e |
| With 8 μg of FLC/ml | 0.25c | 0.25-0.5 | 0.375 | 8d | 4-12 | 13.6f |
| With 64 μg of FLC/ml | 0.5c | 0.25-0.5 | 0.425 | 16 | 4-32 | 20.4e,f |
| Baseline value (no pas- sages on any media)b | 0.25 | 0.125-0.25 | 0.26 | 1 | 1-16 | 2.9 |
GM, geometric mean.
P was not significant for the VRC MICs and MFCs for Aspergillus isolates serially cultured (for four passages) on YAG plates without FLC versus Aspergillus isolates not grown on any media.
P was not significant for the VRC MICs for Aspergillus isolates not preexposed to FLC versus Aspergillus isolates preexposed to 8 μg of FLC/ml versus Aspergillus isolates preexposed to 64 μg of FLC/ml.
P < 0.001 for the VRC MFCs for Aspergillus isolates not preexposed to FLC versus Aspergillus isolates preexposed to 8 μg of FLC/ml.
P < 0.001 for the VRC MFCs for Aspergillus isolates not preexposed to FLC versus Aspergillus isolates preexposed to 64 μg of FLC/ml.
P was not significant (0.19) for the VRC MFCs for Aspergillus isolates preexposed to 8 μg of FLC/ml versus Aspergillus isolates preexposed to 64 μg of FLC/ml.
Susceptibilities of FLC-preexposed A. fumigatus isolates to AMB.
Serial growth (for four passages) of Aspergillus isolates on FLC-containing YAG plates resulted in only onefold, statistically insignificant, increases in both the MICs and MFCs of AMB. The MICs at which 50% (MIC50s) of the FLC-unexposed and FLC-preexposed isolates were inhibited were 1 and 2 μg/ml, respectively; the MFCs at which 50% (MFC50s) of the FLC-unexposed and FLC-preexposed isolates were killed were also 1 and 2 μg/ml, respectively.
Growth characteristics of Aspergillus isolates preexposed and not preexposed to FLC as determined by the XTT colorimetric assay.
Using the XTT colorimetric assay, we evaluated whether preexposure to FLC altered the growth characteristics of two representative Aspergillus isolates. The growth rates and times to germination of these two isolates were identical in the presence and absence of FLC preexposure (Fig. 3).
FIG. 3.
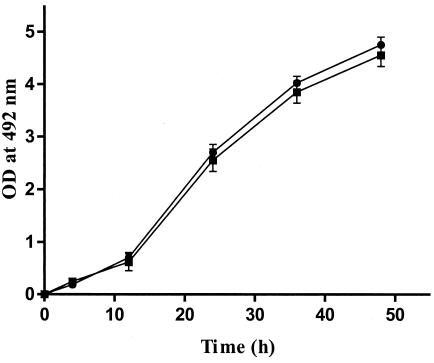
Growth rates of two A. fumigatus isolates preexposed (filled squares) and not preexposed (filled circles) to FLC as determined by the XTT colorimetric assay. T bars indicate standard deviations.
DISCUSSION
ITC, a triazole with good in vitro activity against Aspergillus spp., is used increasingly as the primary or secondary form of prophylaxis for leukemia patients and bone marrow transplant recipients (4, 11). The availability of newer broad-spectrum triazoles with enhanced activity against Aspergillus spp. means that different triazoles could be sequentially administered to patients at risk for invasive mycoses during the course of their immunosuppressive therapy. If some of these patients are already colonized with Aspergillus spp., such aspergilli would have been preexposed during the course of therapy to triazoles with inherently different activity against Aspergillus spp. Nevertheless, no studies thus far have addressed the possibility of the attenuation of the activity of a more potent triazole due to prior exposure of A. fumigatus to another, less active triazole. We previously hypothesized that this could be a concern (3). Because IA is a common breakthrough infection in patients undergoing FLC prophylaxis (12), we investigated whether preexposure of Aspergillus spp. to FLC could result in in vitro alterations in the susceptibility of this fungus to subsequent ITC (and VRC) exposure.
We found that serial passages of A. fumigatus on an FLC-containing medium resulted in significant increases in the ITC MFC. The attenuated in vitro fungicidal activity of ITC was not medium dependent, as it was seen in both of the media tested (YAG and RPMI 1640) in our study. Furthermore, comparable increases in the MFCs of ITC for A. fumigatus isolates following preexposure to FLC were seen when hyphae were used as the starting inocula, implying that this phenomenon is not affected by different experimental conditions and may be applicable to both developmental stages of A. fumigatus growth (conidia and hyphae). Conversely, the ITC MICs were not significantly affected by FLC preexposure of the isolates, according to growth rates estimated both visually with the NCCLS microdilution method and spectrophotometrically with the XTT colorimetric assay.
The attenuation of ITC's fungicidal efficacy following preexposure of the isolates to FLC was further confirmed by viability staining. FUN-1 is a halogenated dye that is converted to a red fluorescent compound in actively metabolizing fungal cells. Recently, FUN-1 was shown to be a useful indicator of A. fumigatus viability following exposure to antifungal agents (5). To assess whether preexposure to FLC influences the viability of A. fumigatus hyphae that are subsequently treated with ITC, we stained hyphae of a representative A. fumigatus isolate with FUN-1. We found that when the isolate was preexposed to FLC, it retained its viability even after exposure to ITC at a concentration sevenfold higher than its ITC MFC. Furthermore, the destructive morphological changes seen after exposure to fungicidal concentrations of ITC (Fig. 1A) were not observed when the same isolate was preexposed to FLC (Fig. 1B and C).
This phenomenon is likely to be specific for azoles, because similar to the observed increases in the ITC MFCs for A. fumigatus isolates, FLC preexposure resulted in analogous increases in the MFCs of VRC, another triazole with good activity against A. fumigatus (11). On the other hand, FLC preexposure did not significantly alter the in vitro susceptibility of A. fumigatus to AMB, suggesting that this phenomenon is likely to be specific to triazoles. Additional studies are needed to investigate whether this phenomenon applies to non-fumigatus Aspergillus spp. and non-Aspergillus molds.
An increase in the MFC of an antifungal agent differs from resistance to it. In bacteria, tolerant organisms fail to grow in the presence of an antibiotic but resume growth upon discontinuation of antibiotic exposure (10). In contrast, resistant organisms are able to grow in the presence of an antibiotic unless a very high drug concentration is present. The biology of tolerance in fungi has not been well studied to date. In an attempt to account for the attenuated in vitro fungicidal activity of ITC against A. fumigatus isolates following preexposure to FLC, we used the XTT colorimetric assay to evaluate the growth rates of two representative Aspergillus isolates either preexposed or not to FLC. We found that FLC preexposure did not affect the growth of the two isolates, as their rates of growth and conversion of conidia to hyphae were identical with and without preexposure to FLC (Fig. 3). It has been suggested that antimicrobial agents with killing ability are more efficacious against rapidly replicating microorganisms, whereas their activity is reduced when tested against slowly growing microbes. In our study, the fact that FLC preexposure did not alter the growth characteristics of A. fumigatus suggests that the observed attenuation of ITC's activity against A. fumigatus could be the result of subtle physiological adaptations (e.g., induction of detoxification mechanisms, alteration of drug influx or efflux, minor changes in ergosterol composition, or activity of target enzyme P-450-dependent 14α-demethylase), ultimately leading to attenuation of the fungicidal activity of a more potent triazole.
Furthermore, the attenuated in vitro fungicidal activity of ITC against A. fumigatus following preexposure of the isolates to FLC remained relatively stable after four passages on FLC-free plates. It is unclear, however, whether four passages on FLC-free medium are sufficient to determine whether the attenuation of ITC fungicidal activity following preexposure of the isolates to FLC reflects a selection of a genetically stable subpopulation of tolerant A. fumigatus or, alternatively, a slowly resolving phenotypic adaptation (e.g., through delays in the decay of specific mRNAs). More studies are needed to clarify the exact mechanism of these changes.
Finally, the clinical relevance of our in vitro findings is unclear and must await further confirmation by appropriate in vivo models and human studies. These observations, if further supported by future in vivo studies, may provide an alternate explanation for the suboptimal efficacy of the newer broad-spectrum triazoles, such as ITC (and perhaps VRC), in preventing and/or treating some episodes of breakthrough IA following FLC prophylaxis. In that case, since our study shows that such phenotypic changes are not captured by the conventional microbiology techniques of measuring the MIC, future clinical studies examining breakthrough Aspergillus infections during azole prophylaxis should measure the MFC to determine whether this phenomenon is relevant to clinical practice.
Acknowledgments
W. Liu and M. S. Lionakis contributed equally to this work.
REFERENCES
- 1.Bow, E. J., M. Laverdiere, N. Lussier, C. Rotstein, M. S. Cheang, and S. Ioannou. 2002. Antifungal prophylaxis for severely neutropenic chemotherapy recipients: a meta analysis of randomized-controlled clinical trials. Cancer 94:3230-3246. [DOI] [PubMed] [Google Scholar]
- 2.Espinel-Ingroff, A., A. Fothergill, J. Peter, M. G. Rinaldi, and T. J. Walsh. 2002. Testing conditions for determination of minimum fungicidal concentrations of new and established antifungal agents for Aspergillus spp.: NCCLS collaborative study. J. Clin. Microbiol. 40:3204-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kontoyiannis, D. P. 2002. Why prior fluconazole use is associated with an increased risk of invasive mold infections in immunosuppressed hosts: an alternative hypothesis. Clin. Infect. Dis. 34:1281-1283. [DOI] [PubMed] [Google Scholar]
- 4.Kontoyiannis, D. P., and G. P. Bodey. 2002. Invasive aspergillosis in 2002: an update. Eur. J. Clin. Microbiol. Infect. Dis. 21:161-172. [DOI] [PubMed] [Google Scholar]
- 5.Lass-Flörl, C., M. Nagl, C. Speth, H. Ulmer, M. P. Dierich, and R. Wurzner. 2001. Studies of in vitro activities of voriconazole and itraconazole against Aspergillus hyphae using viability staining. Antimicrob. Agents Chemother. 45:124-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Latge, J. P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meletiadis, J., J. F. G. M. Meis, J. W. Mouton, and P. E. Verweij. 2001. Analysis of growth characteristics of filamentous fungi in different nutrient media. J. Clin. Microbiol. 39:478-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meletiadis, J., J. W. Mouton, J. F. G. M. Meis, B. A. Bouman, J. P. Donnelly, P. E. Verweij, and EUROFUNG Network. 2001. Colorimetric assay for antifungal susceptibility testing of Aspergillus species. J. Clin. Microbiol. 39:3402-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. 1998. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. Proposed standard M38-P. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 10.Pootoolal, J., J. Neu, and G. D. Wright. 2002. Glycopeptide antibiotic resistance. Annu. Rev. Pharmacol. Toxicol. 42:381-408. [DOI] [PubMed] [Google Scholar]
- 11.Sheehan, D. J., C. A. Hitchcock, and C. M. Sibley. 1999. Current and emerging azole antifungal agents. Clin. Microbiol. Rev. 12:40-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh, N. 2001. Trends in the epidemiology of opportunistic fungal infections: predisposing factors and the impact of antimicrobial use practices. Clin. Infect. Dis. 33:1692-1696. [DOI] [PubMed] [Google Scholar]


