Abstract
Combination regimens are considered a valuable tool for the fight against drug-resistant falciparum malaria. This study was conducted to evaluate the antimalarial potential of clindamycin in combination with dihydroartemisinin in continuously cultured and in freshly isolated Plasmodium falciparum parasites, measuring the inhibition of Plasmodium falciparum histidine-rich protein II synthesis. Interaction analysis revealed a synergistic or additive mode of interaction at various concentration ratios in all continuously cultured parasites at the 50% effective concentration (EC50) level. Antagonism was not found for any of the culture-adapted parasites. In fresh P. falciparum isolates, a fixed clindamycin-dihydroartemisinin combination exhibited additive activity at the EC50 and EC90 levels. The drug mixture showed no significant activity correlation to other commonly used antimalarials. The clindamycin-dihydroartemisinin combination appears to be a promising candidate for clinical investigation.
Chemotherapeutic options against Plasmodium falciparum are becoming increasingly limited in the wake of drug resistance to the most commonly used antimalarials (28, 31). In addition, the development of new compounds is wanting and use of these compounds is often not applicable in the most affected regions for economic reasons (21).Combination of common antimalarials has been advocated as a possible alternative for the treatment of resistant malaria (28). It is thought to delay the emergence of specific drug resistance, especially if partner drugs exhibit different modes of action. So far, the rationale of combination therapy is predominantly based on combining a rapidly acting short half-life drug with a longer half-life partner drug that may act more slowly (e.g., artesunate-mefloquine, artemether-lumefantrine) (28, 31). Although such combinations have shown excellent results in areas with low transmission some are not applicable to high transmission areas due to the persistence of prolonged subtherapeutic drug levels of the slowly eliminated compound, therefore increasing the risk for the development of specific drug resistance (31).
To develop suitable treatment regimens for high transmission areas, regions traditionally suffering the most from falciparum malaria, a combination of independently acting partner drugs with short or moderate half-life, exhibiting additive or synergistic activity, should be sought.
In this study we investigated mixtures of dihydroartemisinin, the active metabolite of artesunate, and clindamycin. The use of dihydroartemisinin, a sesquiterpene lactone, and its derivatives is particularly appealing because of their rapid onset of action, their favorable tolerability profile, and their inhibition of gametocyte production, potentially reducing the propagation of resistant parasite strains (25, 28). Clindamycin, a derivative of lincomycin, was first shown to possess antimalarial activity in 1967. Its activity against multidrug-resistant parasites was further investigated in vitro and, more exhaustively, in a series of in vivo studies (14, 24, 29). However, due to its slow onset of action, it is most commonly used in combination with the fast-acting antimalarial quinine.
The following in vitro study aimed at determining the antimalarial activity of clindamycin and dihydroartemisinin against P. falciparum in parasites adapted to continuous culture and in fresh parasite isolates, the interaction at different concentration ratios of the drug mixture, and the cross sensitivity patterns of the monocompounds and the drug mixture in relation to commonly used antimalarials.
MATERIALS AND METHODS
Laboratory strain and clones.
The first phase of this study was conducted with Plasmodium falciparum adapted to continuous culture, with three clones (3D7, Dd2, and 7G8) and one strain (S007). Clone 7G8 is resistant to chloroquine and pyrimethamine. Clone Dd2 shows resistance to pyrimethamine and mefloquine, and in addition, low-grade resistance against chloroquine. Clone 3D7 is drug sensitive. Strain S007 was originally isolated from a Gabonese child presenting with complicated malaria and proved to be highly chloroquine resistant but sensitive to quinine and mefloquine. Subcultures were repeatedly treated with sorbitol if synchronization was required (12).
Test compounds.
Dihydroartemisinin (Laboratory Standard of the Academy of Military Science, Beijing) was dissolved in a 1:1:7.85 mixture of Tween 80, linoleic acid, and ethanol and was further diluted in double distilled water. The maximum amount of solvent per 200 μl of blood medium mixture (BMM) was 8 × 10−7 μl. No significant difference in growth was observed between solvent and control wells. Clindamycin hydrochloride (Sigma, C5269) was dissolved and diluted in double distilled water, and stock solutions were sterilized by filtration.
Test plates were predosed with ascending concentrations of clindamycin (200 to 200,000 pmol/well, corresponding to 100 to 100,000 nmol/liter) and dihydroartemisinin (2 to 2,000 fmol/well). In addition, the compounds were tested in a checkerboard design at clindamycin concentrations of 30, 100, 300, 1,000, 3,000, 10,000, 30,000, and 100,000 nmol/liter and corresponding dihydroartemsinin concentrations of 0.01, 0.03, 0.1, 0.3, 1, 3, and 10 nmol/liter. Single drugs were tested in duplicate on each plate, control wells were predosed with diluent only, and all tests were performed on two plates in parallel.
Drug sensitivity assay.
Drug sensitivity assays were based on the quantitative detection of Plasmodium falciparum specific histidine-rich protein 2 (HRP2) by a commercially available enzyme-linked immunosorbent assay (ELISA; Malaria Ag CELISA; Cellabs Pty. Ltd., Brookvale, New South Wales, Australia) and performed according to the standard operational procedure as described recently (19). Human O+ erythrocytes were added to the viable stock cultures (2 to 5% parasitemia, 5% hematocrit) to obtain a parasitemia of 0.02%. RPMI 1640 medium was supplemented with 0.5% Albumax, 2% heat-inactivated human AB serum, 25 mM HEPES, 200 μM hypoxanthine, and 2 mM l-glutamine and used to adjust the hematocrit to 1.5%. Then 200 μl of the blood medium mixture was added to the scheduled wells of the predosed Falcon 3070 plates.
Test plates were harvested after incubation for 72 h at 37.5°C in a CO2-enriched (5%) and O2-reduced (5%) atmosphere. Test plates were freeze-thawed twice, 100 μl of the specimen was transferred into the ELISA plate, incubated for 1 h, and washed four times with the provided washing solution. Then 100 μl of the diluted antibody conjugate was added for another hour of incubation. After fourfold washing, 100 μl of diluted chromogen (tetramethylbenzidine) was put into each well and incubated for 15 min in the dark, and 50 μl of the stopping solution was added and spectrophotometric analysis was performed (Anthos Reader 2001, Anthos Labtec Instruments, Austria) at an absorbent maximum of 450 nm. Positive and negative controls were measured in duplicate, and standard serial dilutions were made on each plate. Existing levels of HRP2 were determined by freezing a sample of the BMM before incubation. An at least fourfold increase in HRP2 concentration during the incubation time was considered the threshold for further analysis. A nonlinear regression model was used to determine the amount of HRP2 of each optical density value.
All cultures and media were tested for Mycoplasma contamination by PCR amplification with genus-specific primers GPO- (5′-ACT CCT ACG GGA GGC AGC AGT A-3′) and MGSO (5′-TGC ACC ATC TGT CAC TCT GTT AAC CTC-3′) of 16S rDNA as described previously (26).
Testing of fresh Plasmodium falciparum isolates.
This part of the study took place in Lambaréné, Gabon, from February to April 2002. In this region, predominantly hyperendemic Plasmodium falciparum is known to exhibit a high degree of resistance to chloroquine and a decreasing sensitivity to antifolates but a still unaffected efficacy of quinine and mefloquine (20, 23, 30). Clindamycin and dihydroartemisinin are currently not used on a large scale in antimalarial chemotherapy in Gabon.
Outpatients of the Albert Schweitzer Hospital with P. falciparum monoinfection were invited to participate in this study. Ethical clearance was obtained from the Ethics Committee of the International Foundation of the Albert Schweitzer Hospital, and informed consent was obtained from all participants or their legal representatives. None of the participants had received antimalarial treatment for a minimum of 4 weeks prior to inclusion. We took 2 ml of venous blood in heparinized tubes, and parasitemia and hematocrit were further adjusted for in vitro testing as described above. However, gentamicin (50 μg/ml) was added to the growth medium to avoid bacterial contamination.
Drug assays were performed as described above. Plates were predosed with ascending concentrations of dihydroartemisinin (0.01 to 30 nmol/liter), clindamycin (100 to 300,000 nmol/liter), and a combination of the two compounds at a molar concentration ratio of 1:10,000 (100,001 to 300,030 nmol/liter).
In parallel, parasites were also tested for their response to chloroquine, mefloquine, and quinine. For this purpose we employed a modified schizont maturation inhibition test (World Health Organization in vitro microtest) as described previously (22).
Statistical analysis.
Individual regression analysis was based on a polynomial regression model. The software used is freely available from http://malaria.farch.net. Cumulative regression parameters and effective concentrations (EC) were calculated according to the classical method of Litchfield and Wilcoxon (16, 27). For interaction analysis, the index of the fractional inhibitory concentrations (FICI) was calculated (1). For this purpose, the concentration of drug A in the mixture was divided by the corresponding EC of the monocompound and added to the quotient of the concentration of drug B and the respective single-drug EC. FICI values were considered to indicate synergism (FICI ≤ 0.5), additive activity (0.5 < FICI ≤ 4), or antagonism (>4). Isobolograms were constructed by plotting the fractions of the EC50 values of each drug normalized to 1, with a concave curve below the isobologram line indicating synergy, a straight line close to the isobologram line additive activity, and a convex curve above the isobolgram line antagonistic interaction. Activity correlations between different drugs were analyzed by Spearman's rank correlation test. All tests were performed at a two-sided significance level of α = 5% (P < 0.05).
RESULTS
For the establishment of an adequate test system, continuously cultured parasites were exposed to clindamycin and dihydroartemisinin for 24 to 72 h. Growth inhibition by clindamycin in the HRP2 ELISA and quantitative microscopic examination were insufficient unless the incubation was prolonged to 72 h. On the contrary, EC values derived for dihydroartemisinin did not vary significantly with regard to the incubation time (data not shown). Therefore, a 72-h exposure was considered the most suitable approach for the testing of clindamycin alone and in combination with dihydroartemisinin.
Activity against strain and clones adapted to continuous culture.
Three culture-adapted P. falciparum clones as well as one strain were successfully employed for drug sensitivity testing. Individual 50% effective concentrations (EC50s) for clindamycin were 106, 89, 28, and 101 μmol/liter for the clones 3D7, 7G8, and Dd2 and the strain S007, respectively. Dihydroartemisinin showed respective EC50 values of 0.57, 0.58, 0.78, and 0.63 nmol/liter. Individual regression analysis was carried out for each fixed concentration ratio of the two compounds.
For a more detailed insight into the mode of interaction, FICI values were computed for the drug mixtures and plotted in isobolograms (Fig. 1a to d). An asymmetric line was found in all tests, indicating increased interaction at proportionally higher dihydroartemisinin activity. The isobolograms for the chloroquine-sensitive P. falciparum clone 3D7 showed an S-shaped interaction curve that was convex above the line of identity (antagonistic trend) at lower concentrations of DHA, but concave below the line of identity (synergistic trend) at higher concentrations of DHA (Fig. 1a). Drug combination ratios of dihydroartemisinin and clindamycin in the range of 1:3,000 to 1:30,000 showed highest synergistic or additive interaction in all P. falciparum employed (Table 1). Most importantly, antagonism was not observed for any of the tested clones and strain at the different drug combination ratios (Table 1).
FIG. 1.
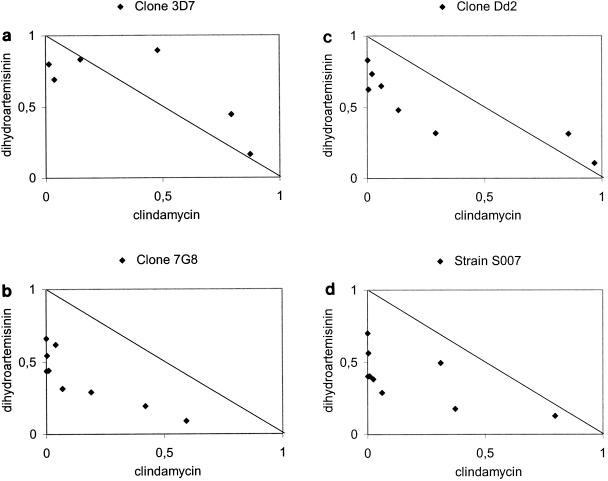
Interaction of clindamycin and dihydroartemisinin against laboratory-adapted P. falciparum. Isobolograms are drawn at the EC50 level. The straight line in each panel is representing additivity. Points located below this line indicate synergism, data points above the line indicate antagonism. EC50 values of the monocompounds are taken as 1 (x- and y-axes), and observed values of the drug mixture are plotted as proportions of this value (solid points).
TABLE 1.
Overall results of the mode of interaction for dihydroartemisinin-clindamycin drug mixtures at various molar concentration ratios against culture-adapted P. falciparum (3D7, 7G8, Dd2, and S007) at the EC50 level
| Mode of interaction | No. of isolates showing mode of interaction at dihydroartemisinin:clindamycin ratio of:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1:100 | 1:300 | 1:1,000 | 1:3,000 | 1:10,000 | 1:30,000 | 1:100,000 | 1:300,000 | 1:1,000,000 | |
| Synergistic | 0 | 2 | 0 | 2 | 1 | 2 | 1 | 0 | 0 |
| Additive | 4 | 2 | 4 | 2 | 3 | 2 | 3 | 4 | 4 |
| Antagonistic | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Testing of fresh Plasmodium falciparum isolates.
A total of 55 patients participated in this study. Patients presented with a geometric mean asexual parasitemia of 10,855/μl (range: 700 to 85,000/μl), and 29 isolates (53%) showed satisfactory growth, indicated by at least fourfold increase of HRP2 during the 72-h incubation period. When the log-concentration/probit-response regression model was calculated for clindamycin, dihydroartemisinin, and the clindamycin-dihydroartemisinin combination, the chi-square values for heterogeneity were 1.99, 8.45, and 0.68, respectively. This indicated an acceptable fit of the regression model, since these values are below the limit of 11.07.
The mean EC50 value for clindamycin was 82 nmol/liter (95% confidence interval [CI] = 25 to 270 nmol/liter). The corresponding EC90 and EC95 were 32 μmol/liter (95% CI = 1 to 1043 μmol/liter) and 174 μmol/liter (95% CI = 2 to 13103 μmol/liter), respectively. The mean EC50 for dihydroartemisinin was 0.61 nmol/liter (95% CI = 0.36 to 1.02 nmol/liter). EC values and 95% confidence intervals for both drugs and the drug mixture are shown in Table 2.
TABLE 2.
Effective concentrations of clindamycin, dihydroartemisinin, and a fixed drug mixture (1:10,000) in 29 fresh P. falciparum isolates
| Druga | EC (nmol/liter)
|
|||
|---|---|---|---|---|
| EC50 (95% CI) | EC90 (95% CI) | EC95 (95% CI) | EC99 (95% CI) | |
| CLI | 82.14 (24.97-270.27) | 32,076.82 (985.83-1,043,716.94) | 174,119.61 (2,313.81-13,102,894.60) | 4,156,790.36 (9,956.73-17,353,990.34) |
| DHA | 0.61 (0.36-1.02) | 5.61 (2.62-12.04) | 10.54 (4.36-25.44) | 34.33 (10.95-107.68) |
| CLI/DHA | 155.12 (69.18-347.86) | 8,872.78 (1,608.40-48,947.01) | 27,940.29 (3,513.07-222,215.98) | 24,0211.35 (14,070.35-4,100,928.04) |
CLI, clindamycin; DHA, dihydroartemisinin; CLI/DHA, fixed combination of clindamycin and dihydroartemisinin
Correlation analysis.
In parallel to dihydroartemisinin and clindamycin, the antimalarial activity of chloroquine, mefloquine, and quinine was tested in a modified schizont maturation inhibition test. Individual EC values were calculated for all drugs and isolates employing a nonlinear regression model (data not shown). No significant activity correlations at the EC50, EC90, EC95, and EC99 levels were observed between either clindamycin or dihydroartemisinin and standard antimalarial drugs (i.e., chloroquine, quinine, and mefloquine).
Interaction analysis.
With regard to the in vitro activity of the monocompounds and based on the results of interaction analysis, a 1:10,000 drug mixture was further investigated in parallel to the single compounds. In 29 successfully tested isolates, the geometric mean EC50 was found to be 155 nmol/liter (95% CI = 69 to 348 nmol/liter). Corresponding EC90, EC95, and EC99 values were 9, 28, and 240 μmol/liter, respectively. EC values and 95% confidence intervals are listed in Table 2.
For interaction analysis, the FICI values were calculated. The mean FICI was 1.16 (95% confidence interval: 1.10 to 1.22) at the EC50 level and 1.02 (95% confidence interval: 1.01 to 1.03) at the EC90 level, indicating an additive mode of interaction.
To identify the dominating compound in the clindamycin-dihydroartemisinin drug mixture, correlation analysis of respective EC values obtained for individual isolates (n = 29) was performed for the drug mixture and the single compounds. Dihydroartemisinin showed correlation coefficients at the EC50, EC90, EC95, and EC99 of 0.13 (P > 0.5), 0.38 (P = 0.04), 0.19 (P > 0.3), and 0.38 (P = 0.04), respectively. The corresponding correlation coefficients for clindamycin were 0.91, 0.74, 0.74, and 0.64, respectively (P < 0.001).
DISCUSSION
In the era of Plasmodium falciparum resistance to various antimalarial drugs and considering the limited number of newly developed drugs, combination therapy is an interesting approach for the development of new effective treatment regimens. For the use of such drug combinations in the most affected countries, several conditions need to be met. Clindamycin and artemisinin derivatives meet the key criteria for a reasonable combination therapy. Both monocompounds have been successfully used for many years in antimalarial therapy, demonstrating high efficacy and a favorable tolerability profile (14, 28). Although resistance to artemisinin derivatives was induced in vitro and despite reports of in vivo treatment failure of artesunate, these findings seem to be of limited clinical relevance due to high recrudescence rates when artesunate is used in monotherapy for too short a treatment duration (2, 10, 11). The development of drug resistance is further curbed by particularly short plasma half lives of both drugs (8, 9, 17, 28). Moreover the sesquiterpene lactones rapidly reduce the parasite load by a factor of approximately 10−4 per asexual life cycle (i.e., 48 h) in vivo and are known to inhibit gametocytogenesis to a certain degree, a potential asset for the prevention of propagation of resistance (25, 28).
So far there exists little information about the mode of interaction of clindamycin and artemisinin derivatives. However, clindamycin-artemisinin potentiation was observed in a screening investigation with a P. berghei mouse model and an artemisinin-resistant strain, whereas additive activity was observed in a drug-sensitive strain (5).
Concordant with previous findings, dihydroartemisinin EC50 values were found to be in a narrow range both in laboratory-adapted parasites (from 0.57 to 0.78 nmol/liter) and in fresh isolates (0.17 to 3.84 nmol/liter) (3, 13, 18). With laboratory-adapted parasites the geometric mean EC50 for clindamycin was 72 μM. These results correspond well to previous findings. In a recently published in vitro investigation, clindamycin activity was tested against various P. falciparum clones, yielding similar EC50 values (3D7: 57 μM, Dd2: 43 μM, and HB3: 66 μM) (29).So far there are no data published for the activity of clindamycin against fresh P. falciparum isolates.
However, Seaberg et al. in the 1980s described a plateau-shaped dose-response curve for clindamycin (24). This regression pattern is in contrast to that of most other antimalarials, with steep regressions in the range of EC16 to EC84 (27). In the 72-h isotopic assay, the plateau region ranged from 10−2 to 101 μg/ml (22 nM to 22 μM), indicating 45% to 50% growth inhibition. Marked differences were found if the exposure time was prolonged from 42 to 90 h, increasing growth inhibition within the plateau region from 20%, 25%, to as far as 90% to 95%.
Our investigation confirmed both the previously described plateau-shaped dose-response curve and the time dependency (Fig. 2). We observed virtually no growth inhibition by clindamycin in a 24-h schizont maturation inhibition test within the range of 100 μmol/liter (unpublished data). In unsynchronized culture adapted parasites 50% growth inhibition was observed at 72 μmol/liter in a 72-h drug exposure test. However, growth inhibition induced by clindamycin further increased in fresh isolates within 72 h. This further increase in growth inhibition was reflected by a marked reduction in the corresponding EC50 value (82 nmol/liter) and by an enhanced growth inhibition within the plateau region. Within a concentration range from 8 μmol/liter up to 4,157 μmol/liter, growth inhibition changed by 15%, from 84% to 99% (Table 2 and Fig. 2).
FIG. 2.
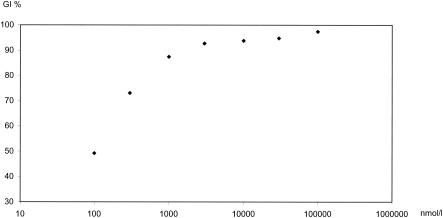
Concentration dependent growth inhibition of 29 P. falciparum isolates against clindamycin. X-axis: Clindamycin concentrations in a logarithmic scale. Y-axis: growth inhibition of P. falciparum isolates.
One might speculate that this further increase is due to the reasonably synchronized stage of the parasites in patients' peripheral blood compared to continuously cultured parasites. To test this hypothesis, highly synchronized subcultures were exposed to clindamycin in parallel to unsynchronized parasite cultures. However, no significant difference was observed in their drug response (unpublished data). Although the discrepancy of EC50 levels of laboratory-adapted strains versus fresh isolates was reproduced in another independent investigation (unpublished data), no clear explanation for the increased in vitro activity of clindamycin in freshly isolated P. falciparum can be provided until now.
However, these peculiar characteristics might, at least in part, be attributable to the unique mode of action of clindamycin. While lincosamide antibiotics act on the 50S ribosomal subunits of bacteria, their main drug target in P. falciparum is thought to be the apicoplast, an organelle that appears to have been acquired by secondary endosymbiosis of green algae (6, 7). In Toxoplasma gondii, clindamycin has been shown to inhibit plastid replication, leading to parasitic growth inhibition during the second life cycle (6). Camps et al. provided evidence that the large subunit of the apicoplast ribosome seems to be the actual target of clindamycin in T. gondii, a fact that might also hold true for P. falciparum (4).
Clindamycin's unique mode of action was also well reflected by our results. There was no association observed between clindamycin EC values and the distinct drug sensitivity pattern of the P. falciparum clones employed (3D7, 7G8, and Dd2). Furthermore, correlation coefficients of drug sensitivity tests in fresh isolates conducted in parallel for chloroquine, quinine, and mefloquine were far from any significance. These results are further indications for an independent mode of action of clindamycin compared to other antimalarial drugs. As this fact holds true for both drugs, dihydroartemisinin and clindamycin, a combination of these two drugs seems to meet a further key criterion for reasonable combination therapy.
In interaction analysis, antagonism was not found for any parasites tested in this investigation. Furthermore, the highest interaction was observed in drug mixtures in a range from 1:3,000 to 1:30,000 of dihydroartemisinin-clindamycin in culture-adapted parasites. This range of molar concentration ratios corresponds approximately to 1:3 to 1:30 ratios of clindamycin/dihydroartemisinin based on in vitro activity (clindamycin EC50: 72 μmol/liter, dihydroartemisinin EC50: 0.63 nmol/liter). Synergism was most pronounced in drug mixtures consisting of dihydroartemisinin as the proportionally more active combination partner, indicated by an asymmetric FICI curve derived from distinct concentration ratios in continuously cultured parasites. A lower degree of interaction was found in the chloroquine-sensitive P. falciparum clone 3D7 under continuous culture conditions, however this trend was not reflected in fresh isolates, as the activities of chloroquine and the clindamycin-dihydroartemisinin combination were not significantly correlated. Although the isobolar lines showed a certain degree of variability for the respective P. falciparum, the asymmetric curve shape was highly consistent, showing an increased interaction at proportionally higher dihydroartemisinin activity (Fig. 1a to d). Similarly, such a phenomenon was recently reported for in vitro activity interaction of fosmidomycin, a rapidly acting antimicrobial, and clindamycin against P. falciparum (29).
In fresh isolates, the activity equilibrium of the 1:10,000 fixed drug combination was deviated to a much higher proportion of clindamycin activity, as represented in the EC values of the monocompounds. This was further confirmed in correlation analysis of the drug mixture to the monocoumpounds. While clindamycin activity was linked very closely to the activity of the drug combination (EC50: rho = 0.91, EC90: rho = 0.74, EC95: rho = 0.74, EC99: rho = 0.64), correlation coefficients for dihydroartemisinin activity were found to be much lower (EC50: rho = 0.13, EC90: rho = 0.38, EC95: rho = 0.19, EC99: rho = 0.38). However, mean FICI values of the clindamycin, dihydroartemisinin drug mixture were found to be in the range of additivity both at the EC50 and at the EC90 levels for fresh P. falciparum isolates.
Taking into consideration that in vitro findings are often not directly applicable to the in vivo situation, EC95 and EC99 levels of the drug mixture, usually considered the MIC in semi-immune versus nonimmune patients, seem to lie within a pharmacokinetically achievable range. Peak levels of dihydroartemisinin found in Gabonese children after rectal artesunate treatment were 468 nM and therefore far beyond EC values found in the course of this study (9). Peak plasma concentrations for clindamycin are reported to be 17 μM in adults after single administration of a 600-mg clindamycin hydrochloride capsule (8). Despite the fact that EC95 and EC99 levels of clindamycin as a monocompound were above reported peak plasma levels, clindamycin is known to be curative in monotherapy against P. falciparum (14). However, peak plasma concentrations of both drugs are well beyond EC95 and EC99 values obtained for the investigated drug mixture.
In conclusion we were able to obtain strong evidence that a combination of clindamycin and dihydroartemisinin provides substantial antiparasitic efficacy in vitro, shows no cross-resistance to commonly used antimalarials, and exerts additive to synergistic antiplasmodial activity. Therefore clinical exploration of such a drug combination is warranted.
Acknowledgments
We thankfully acknowledge the participation of our patients in the Albert Schweitzer Hospital, Lambaréné. We are grateful to David Walliker, University of Edinburgh, for kindly supplying P. falciparum clones.
REFERENCES
- 1.Berenbaum, M. C. 1978. A method for testing for synergy with any number of agents. J. Infect. Dis. 137:122-130. [DOI] [PubMed] [Google Scholar]
- 2.Borrmann, S., A. A. Adegnika, M. A. Missinou, R. K. Binder, et al. 2003. Short-course artesunate treatment of uncomplicated Plasmodium falciparum malaria in Gabon. Antimicrob. Agents Chemother. 47:901-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brockman, A., R. N. Price, M. van Vugt, D. G. Heppner, D. Walsh, P. Sookto, T. Wimonwattrawatee, S. Looareesuwan, N. J. White, and F. Nosten. 2000. Plasmodium falciparum antimalarial drug susceptibility on the north- western border of Thailand during five years of extensive use of artesunate-mefloquine. Trans. R. Soc. Trop. Med. Hyg. 94:537-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camps, M., G. Arrizabalaga, and J. Boothroyd. 2002. An rRNA mutation identifies the apicoplast as the target for clindamycin in Toxoplasma gondii. Mol. Microbiol. 43:1309-1318. [DOI] [PubMed] [Google Scholar]
- 5.Chawira, A. N., D. C. Warhurst, B. L. Robinson, W. and Peters. 1987. The effect of combinations of qinghaosu (artemisinin) with standard antimalarial drugs in the suppressive treatment of malaria in mice. Trans. R. Soc. Trop. Med. Hyg. 81:554-558. [DOI] [PubMed] [Google Scholar]
- 6.Fichera, M. E., and D. S. Roos. 1997. A plastid organelle as a drug target in apicomplexan parasites. Nature. 390:407-409. [DOI] [PubMed] [Google Scholar]
- 7.Gardner, M. J., N. Hall, E. Fung, et al. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 419:498-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.J. Gatti, J. Flaherty, J. Bubp, M. White, and B. Gambertoglio. 1993. Comparative study of bioavailabilities and pharmacokinetics of clindamycin in healthy volunteers and patients with AIDS. J. Antimicrob. Agents Chemother. 37:1137-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halpaap, B., M. Ndjave, M. Paris, A. Benakis, and P. G. Kremsner. 1998. Plasma levels of artesunate and dihydroartemisinin in children with Plasmodium falciparum malaria in Gabon after administration of 50-milligram artesunate suppositories. Am. J. Trop. Med. Hyg. 58:365-368. [DOI] [PubMed] [Google Scholar]
- 10.Huong, N. M., S. Hewitt, T. M. Davis, L. D. Dao, T. Q. Toan, T. B. Kim, N. T. Hanh, V. N. Phuong, D. H. Nhan, and L. D. Cong. 2001. Resistance of Plasmodium falciparum to antimalarial drugs in a highly endemic area of southern Viet Nam: a study in vivo and in vitro. Trans. R. Soc Trop. Med. Hyg. 95:325-329. [DOI] [PubMed] [Google Scholar]
- 11.Jiang, G. F. 1992. In vitro development of sodium artesunate resistance in Plasmodium falciparum. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 10:37-39. [PubMed] [Google Scholar]
- 12.Lambros, C., and J. P. Vanderberg. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65:418-420. [PubMed] [Google Scholar]
- 13.Le Bras, F. 1998. In vitro susceptibility of African Plasmodium falciparum isolates to dihydroartemisinin and the risk factors for resistance to qinghaosu. J. Med. Trop. 58:18-21. [PubMed] [Google Scholar]
- 14.Lell, B., and P. G. Kremsner. 2002. Clindamycin as an antimalarial drug: review of clinical trials. Antimicrob. Agents Chemother. 46:2315-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis, C. 1967. Antiplasmodial activity of halogenated lincomycin analogues in Plasmodium berghei-infected mice. Antimicrobial Agents Chemother. 7:537-542. [PubMed] [Google Scholar]
- 16.Litchfield, J. T., and F. Wilcoxon. 1949. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 96:99-113. [PubMed] [Google Scholar]
- 17.Nealon, C., A. Dzeing, Muller-Romer, U., T. Planche, V. Sinou, M. Kombila, P. G. Kremsner, D. Parzy, and S. Krishna. 2002. Intramuscular bioavailability and clinical efficacy of artesunate in gabonese children with severe malaria. Antimicob. Agents Chemother. 46:3933-3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noedl, H., W. H. Wernsdorfer, S. Krudsood, P. Wilairatana, H. Kollaritsch, G. Wiedermann, and S. Looareesuwan. 2001. Antimalarial activity of azithromycin, artemisinin and dihydroartemisinin in fresh isolates of Plasmodium falciparum in Thailand. Acta Trop. 80:39-44. [DOI] [PubMed] [Google Scholar]
- 19.Noedl, H., W. H. Wernsdorfer, R. S. Miller, and C. Wongsrichanalai. 2002. Histidine-rich protein II: a novel approach to malaria drug sensitivity testing. Antimicrob. Agents Chemother. 46:1658-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Philipps, J., P. D. Radloff, W. Wernsdorfer, and P. G. Kremsner. 1998. Follow-up of the susceptibility of Plasmodium falciparum to antimalarials in Gabon. Am. J. Trop. Med. Hyg. 58:612-618. [DOI] [PubMed] [Google Scholar]
- 21.Radloff, P. D., J. Philipps, M. Nkeyi, D. Hutchinson, and P. G. Kremsner. 1996. Atovaquone and proguanil for Plasmodium falciparum malaria. Lancet. 347:1511-1514. [DOI] [PubMed] [Google Scholar]
- 22.Ramharter, M., H. Noedl, K. Thimasarn, G. Wiedermann, G. Wernsdorfer, and W. H. Wernsdorfer. 2002. In vitro activity of Tafenoquine alone and in combination with Artemisinin against Plasmodium falciparum. Am. J. Trop. Med. Hyg. 67:39-43. [DOI] [PubMed] [Google Scholar]
- 23.Schwenke, A., C. Brandts, J. Philipps, S. Winkler, W. H. Wernsdorfer, and P. G. Kremsner. 2000. Declining chloroquine resistance of Plasmodium falciparum in Lambarene, Gabon from 1992 to 1998. Wien. Klin. Wochenschr. 113:63-64, 2001. [PubMed] [Google Scholar]
- 24.Seaberg, L. S., A. R. Parquette, I. Y. Gluzman, Phillips GW Jr., T. F. Brodaskyd, and J. Krogstad. 1984. Clindamycin activity against chloroquine-resistant Plasmodium falciparum. J. Infect. Dis. 150:904-911. [DOI] [PubMed] [Google Scholar]
- 25.Targett, G., C. Drakeley, M. Jawara, L. von Seidlein, R. Coleman, J. Deen, M. Pinder, T. Doherty, C. Sutherland, G. Walraven, and P. Milligan. 2001. Artesunate reduces but does not prevent posttreatment transmission of Plasmodium falciparum to Anopheles gambiae. J. Infect. Dis. 183:1254-1259. [DOI] [PubMed] [Google Scholar]
- 26.Van Kuppeveld, F. J., J. T. van der Logt, A. F. Angulo, M. J. van Zoest, W. G. Quint, H. G. Niesters, J. M. Galama, and W. J. Melchers. 1992. Genus- and species-specific identification of mycoplasmas by 16S rRNA amplification. Appl. Environ. Microbiol. 58:2606-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wernsdorfer, W. H., and M. G. Wernsdorfer. 1995. The evaluation of in vitro tests for the assessment of drug response in Plasmodium falciparum. Mitt. Oesterr. Ges. Trop. Parasitol. 17:221-228. [Google Scholar]
- 28.White, N. J., F. Nosten, S. Looareesuwan, et al. 1999. Averting a malaria disaster. Lancet. 353:1965-1967. [DOI] [PubMed] [Google Scholar]
- 29.Wiesner, J., D. Henschker, D. B. Hutchinson, E. Beck, and H. Jomaa. 2002. In vitro and in vivo synergy of fosmidomycin, a novel antimalarial drug, with clindamycin. Antimicrob. Agents Chemother. 46:2889-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wildling, E., S. Winkler, P. G. Kremsner, C. Brandts, L. Jenne, and W. H. Wernsdorfer. 1995. Malaria epidemiology in the province of Moyen Ogooué, Gabon. Trop. Med. Parasitol. 46:77-82. [PubMed] [Google Scholar]
- 31.Wongsrichanalai, C., A. L. Pickard, W. H. Wernsdorfer, and S. R. Meshnick. 2002. Epidemiology of drug-resistant malaria. Lancet Infect. Dis. 2:209-218. [DOI] [PubMed] [Google Scholar]


