Abstract
Most patients with alveolar echinococcosis are diagnosed at a late stage when the disease has advanced to unresectable hepatic lesions. These patients require lifelong therapy with benzimidazoles, the only medical treatment currently available. To date, no treatment option remains for patients with benzimidazole intolerance or treatment failure. Amphotericin B was recently shown to exert antiparasitic activity in vitro. Here, we report the efficacy of amphotericin B in human alveolar echinococcosis. In three patients with extensive disease and without further treatment options, disease progression had been documented over several months. They were treated with amphotericin B intravenously at a dose of 0.5 mg/kg of body weight three times per week. Follow-up parameters were physical examination, laboratory parameters, and imaging techniques. Amphotericin B treatment effectively halted parasite growth in all three patients. The antiparasitic effect was most evident by spontaneous closure of cutaneous fistulae in two patients and by constant size of parasitic lesions during treatment, as assessed radiologically. Metabolic activity in parasitic areas was visualized by positron emission tomography and significantly decreased during treatment. However, progressive affection of the heart in one patient could not be stopped. All patients currently continue on amphotericin B and have been treated for 25, 17, and 14 months, respectively. We introduce amphotericin B as salvage treatment for alveolar echinococcosis patients with intolerance or resistance to benzimidazoles, as it effectively suppresses parasite growth. Amphotericin B is not parasitocidal; therefore long-term treatment has to be anticipated.
The larval stage of Echinococcus multilocularis causes alveolar echinococcosis (AE). Surgical resection of hepatic lesions is often incomplete due to the diffuse infiltration into nonresectable structures or insufficient safety margins (7).
Benzimidazoles (i.e., mebendazole and albendazole) are the only drugs licensed for the treatment of unresectable AE (6). These drugs have a parasitostatic effect on E. multilocularis metacestodes. Adverse reactions lead to treatment interruptions in up to 10% of cases (3, 25), and treatment failure has been reported in 16% of patients (4). The overall success rate ranges somewhere between 55 and 97% (19, 25). In cases with treatment failure the disease will ultimately progress to death.
New chemotherapeutic strategies against AE are needed, because alternative treatments have been tested with very limited success (11, 14, 21, 30). Recently, suppression of parasite growth with amphotericin B (AMB) was demonstrated in an in vitro tissue culture model (26). We now report on the first three AE patients treated long-term with AMB.
MATERIALS AND METHODS
AMB deoxycholate treatment.
After a loading dose of 0.5 mg/kg of body weight daily for the first 2 weeks, patients were treated as outpatients and were given AMB three times per week intravenously. For better tolerance of the drug 1 liter of isotonic sodium chloride was given concomitantly. In addition, patients were instructed to maintain a daily fluid intake of more than 4 liters. They were regularly monitored at our center at intervals of 6 to 12 weeks. In addition, patients visited their general practitioner three times weekly for the administration of AMB, as well as for clinical evaluation and follow-up of laboratory parameters. The guidelines of the University of Ulm for human experimentation were followed in the conduct of the clinical research, and written informed consent was obtained from all patients for this exploratory trial.
Diagnostic criteria and follow-up parameters.
Diagnosis of AE was either based on histology, if applicable, or on a typical morphological appearance of lesions by standardized ultrasound imaging (US) and computerized tomography (CT) or magnetic resonance imaging (MRI) in conjunction with serum antibodies (serology) (16). In addition, an FDG-PET (14F-deoxyglucose positron emission tomography) scan was used to assess parasite activity (28).
Imaging techniques.
(i) For US examinations an HDI 3000 diagnostic US unit (Advanced Technology Laboratories, Bothell, Wash.) with a 2- to 4-MHz convex transducer head was used. (ii) A standardized spiral CT instrument (Twin; Elscint, Haifa, Israel) was acquired for examination of the liver with an effective slice thickness of 5.5 mm. For contrast enhancement, the nonionic Ultravist-300 (Schering, Berlin, Germany) was used. (iii) Abdominal MRI was performed in a closed cylindrical high-field system of 1.5 T (MagnetomVision; Siemens, Munich, Germany) with a slice thickness of 8 mm, with gadolinium-diethylenetriamine-pentaacetic acid (Gd-DTPA) for postcontrast images. (iv) For cardiac MRI a cardiovascular whole-body scanner (1.5-T Intera CV; Philips, Best, The Netherlands) with a phased-array surface coil was used. First-pass perfusion and late enhancement were assessed using gradient echo sequences with Gd-DTPA. (v) FDG-PET is a noninvasive technique in nuclear medicine that has proven valuable for the detection of tissue viability and for the discrimination between active and inactive lesions of E. multilocularis in humans (28). FDG-PET was performed using a high-resolution full ring scanner (ECAT HR+; Siemens/CTI, Knoxville, Tenn.).
Laboratory parameters.
Routine laboratory tests (blood count and differential, alkaline phosphatase, aspartate transaminase, alanine transaminase, gamma-glutamyltransferase, C-reactive protein, erythrocyte sedimentation rate, and creatinine) and total and specific immunoglobulin E (Pharmacia Diagnostics, Uppsala, Sweden), as well as the Echinococcus indirect hemagglutination assay (bioMérieux, Nürtingen, Germany) and the specific E. multilocularis enzyme-linked immunosorbent assay (Bordier Affinity Products, Crissier, Switzerland), were used.
Viability testing.
In vivo viability testing was performed by injection of larval tissue into Mongolian gerbils, as described previously (15). Briefly, minced larval tissue was injected into the peritoneal cavity of two gerbils. After 6 weeks intraperitoneal larval growth was assessed, and the proliferation of vesicles confirmed the viability of the larval tissue.
RESULTS
Case 1.
Incurable AE in this 67-year-old woman was first diagnosed in 1976 with multiorgan involvement, including liver, skin, ribs, and heart. She first presented to our clinic in 1995. Repeated attempts at benzimidazole treatment failed due to marked elevation of transaminase levels, >20 times the upper normal limit. She had undergone several palliative resections of parasitic lesions and suffered from chronic cutaneous fistulae with massive suppuration from the sternal area, requiring dressing changes twice daily (Fig. 1a). Viability testing from the fistular aspirate revealed E. multilocularis larvae. The patient had been without drug treatment for several years when in 2001 an accelerated enlargement of the hepatic lesion of 4 cm had been documented by CT over 9 months. Progressive cardiac involvement consisted of a parasitic lesion at the septum and the apex of the left ventricle (volume, 110 ml). Cardiac MRI showed impairment of the left ventricular function (ejection fraction, 52%) with anteroseptal akinesia. FDG-PET revealed parasitic metabolic activity of the thoracic wall, and AMB treatment was started in May 2001.
FIG. 1.
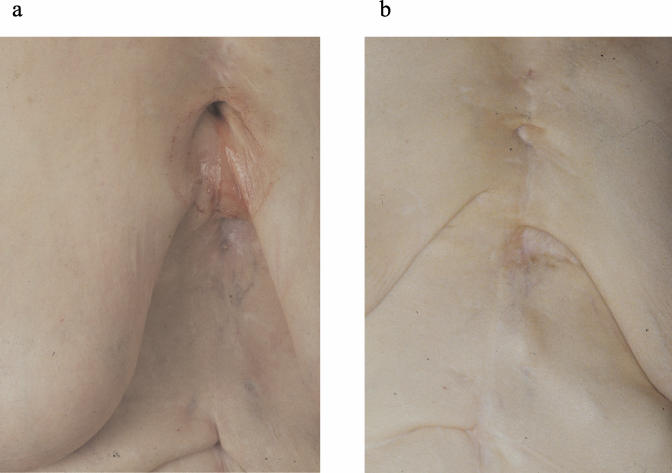
AE of the sternum with involvement of the subcutaneous tissue and cutaneous fistulation. The fistula was draining purulent liquid before AMB treatment (a) and was completely closed 4 months later (b). Viable E. multilocularis larvae were found in the aspirate from the fistulae when it was injected into Mongolian gerbils.
Course under AMB treatment.
The cutaneous fistulae spontaneously closed after 4 months of treatment with AMB (Fig. 1b). A marked regression of subcutaneous lesions of the abdominal wall was documented by CT over 25 months, the liver lesion slightly regressed, and metabolic activity in the area of parasitic lesions (including liver, subcutaneous tissue, and ribs) decreased. However, the volume of the septal cardiac infiltration increased from 110 to 160 ml. Metabolic activity in the affected heart area remained high.
Case 2.
Hepatic AE in this 17-year-old patient was first diagnosed at the age of seven. Treatment with benzimidazoles had failed to stop his extensive liver disease, because in addition to AE the patient was chronically immunocompromised due to hyper-immunoglobulin E syndrome. The latter is a rare congenital immunodeficiency disorder characterized by hyperimmunoglobulinemia and recurrent infections (12, 29). The aggressive course of disease had led to repeated surgical resections of hepatic lesions over the years, and new progression was documented by MRI over 6 months despite benzimidazole treatment (Fig. 2a and b). At least seven new lesions were detected. FDG-PET showed increased parasitic metabolic activity (Fig. 2b, right image). AMB treatment was initiated in January 2002.
FIG. 2.
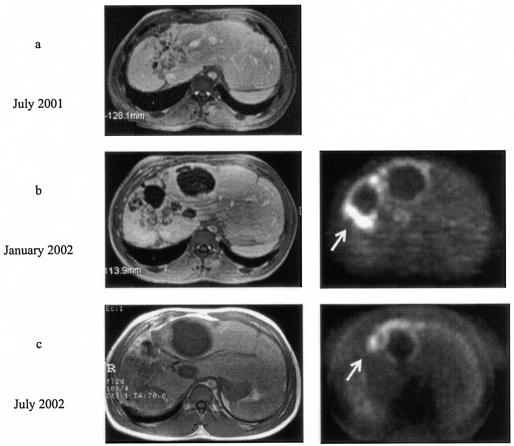
Progressive disease was noted on MRI in patient 2 during benzimidazole treatment between July 2001 and January 2002 (a and b, left images). During progression, intense metabolic activity in the parasitic area was shown on FDG-PET (b, right image). At this point AMB treatment was initiated. Progression could be halted (c, left image), and metabolic activity in the parasitic area decreased (c, right image).
Course under AMB treatment.
After a slight progression over the initial 6 weeks, the size of the hepatic lesions remained constant for the next 12 months. FDG-PET revealed decreasing metabolic activity during the course of AMB treatment, and after 7 months of AMB treatment the number of metabolically active foci had decreased (Fig. 2c, right image).
The course of treatment was complicated by nephrotoxicity with elevated creatinine levels of up to 280 μmol/liter (normal level, <96 μmol/liter). Despite vigorous hydration and sodium loading, AMB had to be reduced to a twice-weekly application, and creatinine levels remained moderately elevated hereafter.
Twelve months after initiation of AMB treatment cholangitis due to Pseudomonas aeruginosa caused obstruction of the left bile duct. Stable AE was demonstrated at this point. The left bile duct was drained by percutaneous transhepatic choledochostomy. Several weeks later, papillosphincterotomy and stenting of the bile duct were performed. Recurrent bleedings occurred from the sphincter of Oddi due to thrombocytopenia. AMB was stopped for almost 3 months during this phase of critical illness. Hepatic parameters normalized after adequate antibiotic treatment. Two months later, new disseminated infection of the left liver lobe was detected by MRI, and histological examination after fine-needle puncture revealed E. multilocularis larvae. AMB treatment is being continued.
Case 3.
In this 60-year-old man hepatic AE was first diagnosed in 1992, and hemihepatectomy was performed. Subsequently, no hepatic or extrahepatic affection was detected until 1998 when he developed a suppurating cutaneous fistula below the sternum. Viability testing from the suppurating material showed growth of E. multilocularis larvae. In September 2000, progressive disease was detected in the ventral thoracic wall, including subcutaneous nodules. Benzimidazole treatment had repeatedly failed due to hepatotoxicity with transaminase levels >10 times the upper normal limit. Until March 2002, the cutaneous fistula was increasingly suppurating and the substernal parasitic mass had progressed from 3 by 6 cm to 7 by 8 cm. FDG-PET showed hot spots in the subcutaneous tissue of the ventral abdominal wall (Fig. 3), and AMB treatment was started in April 2002.
FIG. 3.
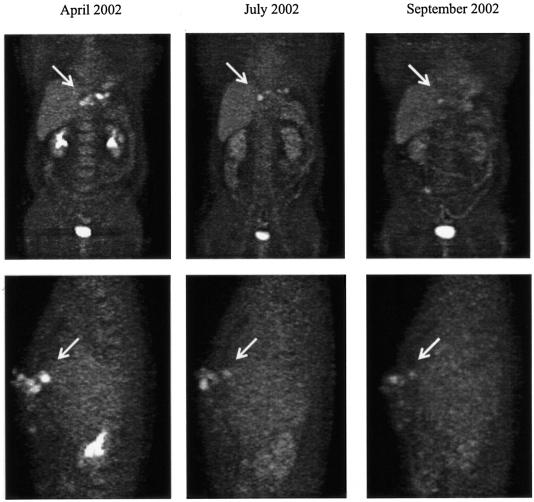
Sequence of FDG-PET images during AMB therapy in patient 3. The antiparasitic activity of AMB is shown by a significant reduction of metabolic activity in the parasitic area over time. The upper row shows coronal views with a metabolically active focus in the subcutaneous tissue of the ventral thoracic wall (arrows pointing right). In the lower row the same focus is depicted in a lateral view (arrows pointing left).
Course under AMB treatment.
The subcutaneous lesions were evaluated by CT and MRI and did not progress during AMB treatment. The substernal fistula closed after 3 months, and FDG-PET depicted a marked regression of signal intensity in the area of the subcutaneous nodules (Fig. 3). The patient has now been successfully treated for 14 months.
DISCUSSION
The treatment of patients with progressive AE after failure of conventional therapy with benzimidazoles has been limited to supportive care. Once therapeutic options such as surgical measures and benzimidazole treatment have been exhausted, the disease usually progresses to death over months or a few years (3). Therefore, alternatives to benzimidazoles are urgently needed. A novel potential chemotherapeutic option was recently discovered by demonstrating that AMB suppresses larval growth of E. multilocularis in vitro (26).
In AE, enlargement of lesions is slow and therefore demonstration of progressive disease requires a period of several months. In our patients, progression was observed over 9, 6, and 6 months, respectively. Equally, sufficient time needs to elapse before therapeutic efficacy can be reliably evaluated. In our patients, this period clearly exceeded the period of prior disease progression (25, 17, and 14 months, respectively) (Table 1).
TABLE 1.
Summary of characteristic parameters during treatment with AMBa
| Patient | Prior to AMB treatment
|
Follow-up during AMB treatment
|
|||||
|---|---|---|---|---|---|---|---|
| Patient characteristics | Treatment failure prior to AMB | Observed interval of progression | Observed interval of AMB treatment | PET | Imaging | Clinical changes during AMB | |
| 1 | 68 yr old, diagnosed 1976; affection: liver, heart, skin, ribs | Elevation of transaminases under benzimidazoles | 9 mo | 25 mo | Decreased signal intensity of all lesions except for increased activity of the affected heart | Constant size of hepatic lesions, regression of cutaneous lesions, progression of cardiac affection | Sternal fistulae closed |
| 2 | 17 yr old, diagnosed 1992; affection: liver | Progression despite treatment with benzimidazoles | 6 mo | 17 mo | Decreased signal intensity of all lesions | Constant size of right-sided hepatic lesions, new affection of the left liver lobe after interrupt ion of AMB treatment | Elevated creatinine and thrombocytopenia |
| 3 | 60 yr old, diagnosed 1992; affection: liver, sternum, skin | Elevation of transaminases under benzimidazoles | 6 mo | 14 mo | Decreased signal intensity of all lesions | Constant size of cutaneous nodules | Substernal fistula closed |
Treatment continues for all three patients.
Successful treatment comprises regression of lesions and stable disease. Stable disease is considered therapeutic success, because parasitic material is often not degraded by the host (5). In this study, AMB treatment was able to halt parasitic growth, as assessed radiologically (27). Success was also observed clinically in two cases when chronic cutaneous fistulae spontaneously closed after a few months of AMB treatment. Furthermore, FDG-PET showed a decrease of metabolic activity in parasitic areas. Interestingly, in patient 1 the lesion in the heart septum progressed during treatment, despite therapeutic success in the cutaneous and hepatic areas. We assume that these discrepant treatment responses may be due to differences in drug concentrations in tissue.
AMB not only is a broad-spectrum antifungal drug but also has diverse antiprotozoal activity against Leishmania and Trypanosoma species (13, 24, 33). Its destructive mechanism against E. multilocularis could be explained by formation of complexes with membrane phospholipids (10), which are major components of E. multilocularis larval membranes (22, 23). Furthermore, AMB (20, 32) has an effect on membrane-bound enzymes (2) and binds to sterols, thereby forming transmembrane channels (1, 8, 9, 31).
It was clear from the beginning that AMB would have to be given permanently due to its parasitostatic effect on E. multilocularis larvae in vitro (26). In those experiments AMB was effective at concentrations between 0.625 and 2.5 μg/ml, and benzimidazoles were used at 1 μg/ml. After discontinuation of AMB resurgence of vesicles was noted and vesicle growth could again be suppressed by retreatment with AMB. In the present study, the progressive infection in patient 2 after temporary discontinuation of AMB demonstrates that this parasitostatic effect holds true in vivo as well.
A potential limitation to permanent AMB treatment could be nephrotoxicity due to glomerular and tubular injury. Thus, usage of the minimal effective dose was a primary goal, and we treated our patients with 0.5 mg/kg three times per week. Recent in vitro experiments had suggested that cyclic dosing with intervals of several weeks of drug hiatus still resulted in sustained suppression of vesicle growth (26). Initially, we observed elevation of serum creatinine levels in all patients. In patient 2 only, the creatinine level rose to almost triple the upper normal limit but completely normalized after AMB administration was stopped for 2 weeks. Subsequently, the frequency of AMB administration was reduced to twice weekly in this patient, with moderate increases in creatinine levels thereafter. It should be a task for future clinical studies to investigate the role of less nephrotoxic derivatives, such as heat-treated AMB (17) or lipid-associated formulations of AMB. Besides thrombocytopenia (patient 2), no further significant alteration of laboratory parameters was noted in any patient.
Sodium loading and maintaining a high urine output of more than 4,000 ml/day during AMB treatment were shown to reduce the risk of tubular necrosis and renal failure (18). By pursuing this strategy in our patients serious organ damage was prevented.
Our patients had intravenous ports implanted to facilitate drug applications. Despite the obstacles and potential risks that salvage treatment with AMB implies, all three patients have shown excellent compliance to date. They are aware of the fact that additional therapeutic options are currently lacking and that the containment of their progressive disease is at stake.
In conclusion, AMB effectively inhibits larval growth of E. multilocularis in vivo. For salvage treatment AMB is a promising treatment modality for otherwise untreatable AE. Prolonged application of AMB over months to years appears feasible, as side effects were mild and serious organ damage was preventable.
Acknowledgments
Indispensable contributions to diagnostic procedures were provided by Sven-Norbert Reske (nuclear medicine), Wolfgang Kratzer (ultrasound), and Andreas Gabelmann (radiology). We thank our colleagues on the infectious diseases ward as well as the home physicians for their valuable efforts in patient care.
REFERENCES
- 1.Abu-Salah, K. M. 1996. Amphotericin B: an update. Br. J. Biomed. Sci. 53:122-133. [PubMed] [Google Scholar]
- 2.Abu-Salah, K. M., S. H. Sedrani, A. S. Tobia, and H. A. Gambo. 1988. Influence of amphotericin B on the transport of phosphate, sulphate and potassium ions across the human erythrocyte membrane. Acta Haematol. 79:77-80. [DOI] [PubMed] [Google Scholar]
- 3.Ammann, R. W., and J. Eckert. 1996. Cestodes. Echinococcus. Gastroenterol. Clin. N. Am. 25:655-689. [DOI] [PubMed] [Google Scholar]
- 4.Ammann, R. W., N. Ilitsch, B. Marincek, A. U. Freiburghaus, et al. 1994. Effect of chemotherapy on the larval mass and the long-term course of alveolar echinococcosis. Hepatology 19:735-742. [DOI] [PubMed] [Google Scholar]
- 5.Ammann, R. W., K. Tschudi, M. von Ziegler, F. Meister, J. Cotting, J. Eckert, F. Witassek, and A. Freiburghaus. 1988. The long-term course of 60 patients with alveolar echinococcosis in continuous therapy with MBZ (1976-85). Klin. Wochenschr. 66:1060-1073. (In German.) [DOI] [PubMed]
- 6.Anonymous. 1996. Guidelines for treatment of cystic and alveolar echinococcosis in humans. WHO Informal Working Group on Echinococcosis. Bull. W. H. O. 74:231-242. [PMC free article] [PubMed] [Google Scholar]
- 7.Bresson-Hadni, S., D. A. Vuitton, B. Bartholomot, B. Heyd, D. Godart, J. P. Meyer, S. Hrusovsky, M. C. Becker, G. A. Mantion, D. Lenys, and J. P. Miguet. 2000. A twenty-year history of alveolar echinococcosis: analysis of a series of 117 patients from eastern France. Eur. J. Gastroenterol. Hepatol. 12:327-336. [DOI] [PubMed] [Google Scholar]
- 8.Capuozzo, E., S. Jullien, C. Salerno, and C. Crifo. 1990. Inhibition of erythrocyte ghost ATPase by polyene antibiotics. Biochem. Int. 20:1135-1139. [PubMed] [Google Scholar]
- 9.Clejan, S., and R. Bittman. 1985. Rates of amphotericin B and filipin association with sterols. A study of changes in sterol structure and phospholipid composition of vesicles. J. Biol. Chem. 260:2884-2889. [PubMed] [Google Scholar]
- 10.Cohen, B. E. 1992. A sequential mechanism for the formation of aqueous channels by amphotericin B in liposomes. The effect of sterols and phospholipid composition. Biochim. Biophys. Acta 1108:49-58. [DOI] [PubMed] [Google Scholar]
- 11.Godot, V., S. Harraga, G. Podoprigora, M. Liance, K. Bardonnet, and D. A. Vuitton. 2003. IFN alpha-2a protects mice against a helminth infection of the liver and modulates immune responses. Gastroenterology 124:1441-1450. [DOI] [PubMed] [Google Scholar]
- 12.Grimbacher, B., S. M. Holland, J. I. Gallin, F. Greenberg, S. C. Hill, H. L. Malech, J. A. Miller, A. C. O'Connell, and J. M. Puck. 1999. Hyper-IgE syndrome with recurrent infections—an autosomal dominant multisystem disorder. N. Engl. J. Med. 340:692-702. [DOI] [PubMed] [Google Scholar]
- 13.Hartsel, S., and J. Bolard. 1996. Amphotericin B: new life for an old drug. Trends Pharmacol. Sci. 17:445-449. [DOI] [PubMed] [Google Scholar]
- 14.Jenne, L., J. Kilwinski, P. Radloff, W. Flick, and P. Kern. 1998. Clinical efficacy of and immunologic alterations caused by interferon gamma therapy for alveolar echinococcosis. Clin. Infect. Dis. 26:492-494. [DOI] [PubMed] [Google Scholar]
- 15.Jura, H., A. Bader, and M. Frosch. 1998. In vitro activities of benzimidazoles against Echinococcus multilocularis metacestodes. Antimicrob. Agents Chemother. 42:1052-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kern, P., K. Bardonnet, E. Renner, H. Auer, Z. Pawlowski, R. W. Ammann, D. A. Vuitton, and P. Kern. 2003. European echinococcosis registry: human alveolar echinococcosis, Europe, 1982-2000. Emerg. Infect. Dis. 9:343-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwong, E. H., M. Ramaswamy, E. A. Bauer, S. C. Hartsel, and K. M. Wasan. 2001. Heat treatment of amphotericin B modifies its serum pharmacokinetics, tissue distribution, and renal toxicity following administration of a single intravenous dose to rabbits. Antimicrob. Agents Chemother. 45:2060-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayer, J., M. Doubek, J. Doubek, D. Horky, P. Scheer, and M. Stepanek. 2002. Reduced nephrotoxicity of conventional amphotericin B therapy after minimal nephroprotective measures: animal experiments and clinical study. J. Infect. Dis. 186:379-388. [DOI] [PubMed] [Google Scholar]
- 19.Mesarina-Wicki, B. 1991. Long-term course of alveolar echinococcosis in 70 patients treated by benzimidazole derivatives (mebendazole and albendazole) (1976-1989). Inaugural dissertation. University of Zurich, Zurich, Switzerland.
- 20.Milhaud, J., M. A. Hartmann, and J. Bolard. 1989. Interaction of the polyene antibiotic amphotericin B with model membranes: differences between small and large unilamellar vesicles. Biochimie 71:49-56. [DOI] [PubMed] [Google Scholar]
- 21.Miyaji, S., K. Katakura, S. Matsufuji, Y. Murakami, S. Hayashi, Y. Oku, M. Okamoto, and M. Kamiya. 1993. Failure of treatment with alpha-difluoromethylornithine against secondary multilocular echinococcosis in mice. Parasitol. Res. 79:75-76. [DOI] [PubMed] [Google Scholar]
- 22.Persat, F., J. F. Bouhours, M. Mojon, and A. F. Petavy. 1990. Analysis of the monohexosylceramide fraction of Echinococcus multilocularis metacestodes. Mol. Biochem. Parasitol. 41:1-6. [DOI] [PubMed] [Google Scholar]
- 23.Persat, F., C. Vincent, M. Mojon, and A. F. Petavy. 1991. Detection of antibodies against glycolipids of Echinococcus multilocularis metacestodes in sera of patients with alveolar hydatid disease. Parasite Immunol. 13:379-389. [DOI] [PubMed] [Google Scholar]
- 24.Ramos, H., E. Valdivieso, M. Gamargo, F. Dagger, and B. E. Cohen. 1996. Amphotericin B kills unicellular leishmanias by forming aqueous pores permeable to small cations and anions. J. Membr. Biol. 152:65-75. [DOI] [PubMed] [Google Scholar]
- 25.Reuter, S., B. Jensen, K. Buttenschoen, W. Kratzer, and P. Kern. 2000. Benzimidazoles in the treatment of alveolar echinococcosis: a comparative study and review of the literature. J. Antimicrob. Chemother. 46:451-456. [DOI] [PubMed] [Google Scholar]
- 26.Reuter, S., M. Merkle, K. Brehm, P. Kern, and B. Manfras. 2003. Effect of amphotericin B on larval growth of Echinococcus multilocularis. Antimicrob. Agents Chemother. 47:620-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reuter, S., K. Nüssle, O. Kolokythas, U. Haug, A. Rieber, P. Kern, and W. Kratzer. 2001. Alveolar liver echinococcosis: a comparative study of three imaging techniques. Infection 29:119-125. [DOI] [PubMed] [Google Scholar]
- 28.Reuter, S., H. Schirrmeister, W. Kratzer, C. Dreweck, S. N. Reske, and P. Kern. 1999. Pericystic metabolic activity in alveolar echinococcosis: assessment and follow-up by positron-emission-tomography (PET). Clin. Infect. Dis. 29:1157-1163. [DOI] [PubMed] [Google Scholar]
- 29.Shemer, A., G. Weiss, Y. Confino, and H. Trau. 2001. The hyper-IgE syndrome. Two cases and review of the literature. Int. J. Dermatol. 40:622-628. [DOI] [PubMed] [Google Scholar]
- 30.Vanparijs, O. 1990. Chemotherapy of experimental Echinococcus multilocularis in jirds. Parasitol. Res. 76:238-240. [DOI] [PubMed] [Google Scholar]
- 31.Vertut-Doi, A., P. Hannaert, and J. Bolard. 1988. The polyene antibiotic amphotericin B inhibits the Na+/K+ pump of human erythrocytes. Biochem. Biophys. Res. Commun. 157:692-697. [DOI] [PubMed] [Google Scholar]
- 32.Whyte, B. S., R. P. Peterson, and S. C. Hartsel. 1989. Amphotericin B and nystatin show different activities on sterol-free vesicles. Biochem. Biophys. Res. Commun. 164:609-614. [DOI] [PubMed] [Google Scholar]
- 33.Yardley, V., and S. L. Croft. 1999. In vitro and in vivo activity of amphotericin B-lipid formulations against experimental Trypanosoma cruzi infections. Am. J. Trop. Med. Hyg. 61:193-197. [DOI] [PubMed] [Google Scholar]


