Abstract
Minocycline-EDTA (M-EDTA) flush solution has been shown to prevent catheter-related infection and colonization in a rabbit model and in hemodialysis patients. We undertook this study in order to determine the activities of M-EDTA against organisms embedded in fresh biofilm (in vitro) and mature biofilm (ex vivo). For the experiment with the in vitro model, a modified Robbin’s device (MRD) was used whereby 25 catheter segments were flushed for 18 h with 106 CFU of biofilm-producing Staphylococcus epidermidis, Staphyloccocus aureus, and Candida albicans per ml. Subsequently, each of the catheter segments was incubated in one of the following solutions: (i) streptokinase, (ii) heparin, (iii) broth alone, (iv) vancomycin, (v) vancomycin-heparin, (vi) EDTA, (vii) minocycline (high-dose alternating with low-dose), or (viii) M-EDTA (low-dose minocycline alternating with high-dose minocycline were used to study the additive and synergistic activities of M-EDTA). All segments were cultured quantitatively by scrape sonication. For the experiment with the ex vivo model, 54 catheter tip segments removed from patients and colonized with bacterial organisms by roll plate were longitudinally cut into two equal segments and exposed to either saline, heparin, EDTA, or M-EDTA (with high-dose minocycline). Subsequently, all segments were examined by confocal laser electron microscopy. In the in vitro MRD model, M-EDTA (with a low concentration of minocycline) was significantly more effective than any other agent in reducing colonization of S. epidermidis, S. aureus, and C. albicans (P < 0.01). M-EDTA (with a high concentration of minocycline) eradicated all staphylococcal and C. albicans organisms embedded in the biofilm. In the ex vivo model, M-EDTA (with a high concentration of minocycline) reduced bacterial colonization more frequently than EDTA or heparin (P < 0.01). We concluded that M-EDTA is highly active in eradicating microorganisms embedded in fresh and mature biofilm adhering to catheter surfaces.
Infections are the most serious complications associated with indwelling central venous catheters (CVCs) (14). It is estimated that more than 200,000 catheter-related bloodstream infections (CRBSI) occur annually in the United States (D. M. Kluger and D. G. Maki, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 514, 1999). Staphylococcus epidermidis, Staphylococcus aureus, and Candida species are the leading organisms causing CRBSI (14, 22, 27).
Because intraluminal colonization is the major source for the migration of organisms leading to bloodstream infections in long-term silicone catheters (19), recent guidelines have proposed the use of intraluminal antimicrobial lock solutions for the prevention and treatment of CRBSI (15, 17). Most long-term CVCs are flushed with heparin. An antimicrobial-anticoagulant combination consisting of vancomycin and heparin with and without ciprofloxacin was used in several studies and was demonstrated to reduce the risk of catheter-related bacteremia caused by gram-positive organisms (2, 10, 26). However, with vancomycin-resistant gram-positive bacteria increasing, concerns have been raised over the use of vancomycin flush solutions and their potential for increasing the risk of vancomycin resistance (30).
EDTA is a metal chelator with established anticoagulant activity and inhibitory activity against staphylococci and Candida spp. (9, 24, 25). Recently, we demonstrated that a flush solution consisting of minocycline and EDTA (M-EDTA) is highly efficacious in preventing catheter-related colonization, bacteremia, and endocarditis in rabbits (21). Compared to a heparin flush solution, M-EDTA was found to decrease the risk of catheter-related colonization and infection in hemodialysis and pediatric cancer patients (1, 3).
The present study was conducted to determine the activity of an M-EDTA flush solution against Candida and staphylococcal organisms embedded in biofilm by using in vitro and ex vivo models of catheter colonization.
(Part of this research was presented at the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, California, 27-30 September 2002).
MATERIALS AND METHODS
In vitro model of colonization.
The in vitro model utilized a modified Robbin’s device (MRD) to study the colonization of catheter segments by organisms embedded in biofilm. The MRD has been described previously (6, 12, 20) and is constructed from an acrylic block 42 cm long with a lumen of 2 by 10 mm. It consists of 25 evenly spaced specimen plugs, each connected to a silicone catheter segment (Allegiance Healthcare Corp., McGaw Park, Ill.) whose anterior surface (0.3 cm2) comes in contact with the flushed infusate. After the catheter segments were placed in the specimen plug of the MRD, the entire apparatus was gas-sterilized by using ethylene oxide. A 500-ml bag of 5% dextrose in water was connected to the MRD through an intravenous tubing administration set and was subsequently infected with an inoculum of 108 CFU of methicillin-resistant Staphylococcus epidermidis (MRSE) or methicillin-resistant Staphylococcus aureus (MRSA) per ml to produce an infected infusate at the concentration of 2 × 105 CFU/ml. The biofilm-producing S. epidermidis isolates and the S. aureus isolates were obtained from patients with CRBSI. In another series of experiments, a 500-ml bag of 5% dextrose in water was infected with biofilm-producing Candida albicans obtained from a patient with catheter-related candidemia by using an inoculum of 106 CFU/ml to produce an infected infusate at the concentration of 2 × 103 CFU/ml. The whole system was incubated at 37°C, and the infected infusate was flushed through the MRD by using a peristaltic pump, permitting the infusate to flow at the rate of 60 ml/h for 8 h. The MRD was left to incubate for a total of 18 h (another 10 h). Subsequently, the infected bag was removed, and a bag of 250-ml sterile saline was infused through the MRD at 125 ml/h for 2 h in order to remove all free-floating organisms. To ensure biofilm formation, at least three catheter segments were randomly removed from the 25 evenly spaced catheter segments in the MRD and studied by scanning electron microscopy. This process was repeated for every organism tested.
Exposure to anticoagulants and antimicrobials.
The remaining catheter segments were removed, and each segment was placed in a tube containing 2 ml of one of the following broth solutions: (i) streptokinase (5,000 units/ml; Hoechst Marion Roussel Deutschland Gmbh, Marburg/Lahn, Germany), (ii) heparin (100 units/ml; Elkins-Sinn, Inc., Cherry Hill, N.J.), (iii) Mueller-Hinton broth (Becton Dickinson and Co., Cockeysville, Md.), (iv) vancomycin (3 mg/ml; Eli Lilly & Co., Indianapolis, Ind.), (v) a vancomycin-heparin combination with a final concentration of 3 mg of vancomycin and 100 units of heparin per ml, (vi) EDTA (30 mg/ml; Abbott Laboratories, North Chicago, Ill.), (vii) a high concentration of minocycline (3 mg/ml; Wyeth-Ayerst Laboratories, Collegeville, Pa.), (viii) a low concentration of minocycline (0.1 mg/ml; since M-EDTA synergy by time-kill kinetics was previously demonstrated only through the use of a low concentration of minocycline, we opted to perform several [five] twofold dilutions of a high concentration of minocycline to arrive at the 0.1-mg of minocycline/ml concentration), (ix) a combination of high-concentration minocycline (3 mg/ml) and EDTA (MH-EDTA), or (x) a combination of low-concentration minocycline (0.1 mg/ml) and EDTA (ML-EDTA). For every organism, the experiments were repeated in triplicate or quadruplicate, and during each experiment, two to five catheter segments were exposed to the same solution for at least 24 h at 37°C. Subsequently, the catheter segments were removed and cultured by scrape sonication. The surface of each catheter segment that was exposed to the infected infusate was scraped with a sterile wooden applicator stick and placed, along with the stick, in a tube containing 0.5 ml of Trypticase soy broth. The tubes were sonicated for 5 min; 0.1 ml of the sonicated broth solution in the tube was pipetted and plated over a blood agar plate, which was incubated at 37°C for 24 h (27). The agar plates were checked for any contaminants, and the isolated organisms had to be of the same species and colonial morphology as the original organism used to infect the infusate. The number of colonies quantitated from the agar plate was multiplied by five to correct for the dilution factor and to determine the total number of colonies isolated from a particular catheter segment. A confluent growth of 100 or greater was calculated as ≥500 colonies, which was considered the detection limit of the in vitro model.
Ex vivo model of colonization.
All 54 of the CVC tips colonized with >100 colonies of a bacterial organism were obtained from the clinical microbiology laboratory of the M. D. Anderson Cancer Center over a 3-month period. All of the CVCs obtained were previously indwelling in patients and were subsequently removed and cultured because of a suspected infection. The 54 CVC tips were grouped into three test groups, with each group consisting of 18 tips. Each CVC tip was cut longitudinally into two equal segments with a sterile scalpel, exposing the lumen of the CVC tip. One longitudinal segment of each of the 18 CVC tips from the first group was immersed in an MH-EDTA solution consisting of minocycline (3 mg/ml) and EDTA (30 mg/ml) for 24 h at 37°C, while the mirror image longitudinal segments were immersed in saline. Similarly, one longitudinal segment of each CVC tip from the second group of 18 tips was immersed in an EDTA (30 mg/ml) solution for 24 h at 37°C while the mirror image segments were placed in saline. Finally, the longitudinal segment of each CVC tip from the third group of 18 tips was immersed in heparin (100 units/ml) for 24 h at 37°C, while the mirror image segments were placed in saline. After 24 h of incubation, all segments were removed, dried, and sent for staining and study by confocal scanning laser microscopy (CSLM). CSLM was used as previously described (4) to visualize and count live bacterial cells adhering to catheter surfaces after exposure to the solutions described above. The MH-EDTA concentration was used in this model (rather than ML-EDTA) because the same MH-EDTA concentration was previously proven in animal and clinical studies to be efficacious in preventing catheter infections (9, 24, 25).
Bacterial staining.
The tested catheter segments were stained with a Live/Dead BacLight Bacterial Viability kit as previously described (4) (part no. L-7012; Molecular Probes, Eugene, Oreg.). The viability kit consists of two nucleic acid stains: (i) SYTO 9 (excitation maximum, 508 nm; emission maximum, 527 nm), a lipophilic membrane-permeable cationic stain that labels live bacteria with green fluorescence (13), and (ii) propidium iodide (excitation maximum, 536 nm; emission maximum, 620 nm), a membrane-impermeable anionic stain that labels membrane-compromised bacteria with red fluorescence (4, 31). The stains were prepared by using autoclaved double-filtered nanopure water at a concentration of 0.1% (vol/vol). The tested material was directly stained with 0.4 ml of propidium iodide and 0.4 ml of SYTO 9 and allowed to react for 5 min. The sample was then gently washed with autoclaved nanopure water to remove excess stain and minimize background fluorescence. The specimens were then directly imaged by CSLM.
CSLM.
An Olympus BH-2 microscope with a 50× ultralong working distance objective was used for epifluorescence microscopy. Images were collected with an Optronics charge-coupled device (Optronics Engineering, Goleta, Calif.) and the imaging program Image Pro-Plus 3.0 for Windows 95 (Media Cybernetics, Silver Spring, Md.). CSLM was performed with a Leica TCS-NT confocal microscope (Leica, Heidelberg, Germany). The objectives used for CSLM imaging were 100 X1.4 N.A. Oil Plan Apo and 63X0-0.7 N.A. Plan Fluotar. The confocal microscope was optimally configured for SYTO 9-propidium iodide analysis by using the 488-nm excitation laser with a 488-, 568-, 633-nm dichroic mirror and relatively short-pass filter of 580 nm in the first beam splitter position. A band filter allowing wavelengths of 525 to 550 nm to pass to the first photo-multiplier tube was used for imaging the SYTO 9 stain. A long-pass filter of 645 nm was used for imaging the propidium iodide. The number of bacteria on each catheter segment and the relative surface (internal or external) area covered with bacteria were compared between catheter segment surfaces exposed to anticoagulant solution (M-EDTA, EDTA, or heparin) and saline as previously described (4). The detection limit is 107 bacteria per microscopic field. Catheter segments were submerged in 50 ml of buffered saline, put on ice, and sonicated at power setting 5 for 1 min to determine if adhering bacteria and the biofilm in which they reside could be removed. Consistent with standards traceable to the National Institute of Standards and Technology, 20 fields were examined. The sensitivity of the BacLight Bacterial Viability kit was consistent with results from a previous study demonstrating the positive correlation of staining with the BacLight viability stain and plate counts (31). An anticoagulant and antimicrobial solution such as M-EDTA, EDTA, or heparin was considered to have decreased colonization on a particular catheter surface (internal or external surface of a CVC tip segment) if the colony count by CSLM was 10-fold lower than the colony count calculated on a similar surface (internal or external) of the counterpart mirror image segment exposed to saline. The microscopists assessing growth on the catheter surfaces by CSLM were blinded as to which agent the catheter had been exposed to.
Statistical analysis.
The significance of differences in colony counts related to the in vitro model with continuous variables was tested by the nonparametric Mann-Whitney U test, while the significance of proportional differences pertaining to the decrease in colonization in the ex vivo model were tested by Fisher's exact test. Differences were considered significant at a P value of ≤0.05.
RESULTS
In vitro model with S. epidermidis.
By using the MRD in vitro model of colonization, it was found that S. epidermidis highly colonized catheter segments and embedded itself in biofilm, as determined by scanning electron microscopy of these randomly selected catheter segments prior to exposure to any agent. In addition, confluent growth was noted on catheter segments incubated in Mueller-Hinton broth. Similarly, incubating catheter segments in heparin and streptokinase broth solution had no impact on reducing catheter colonization and, as shown in Fig. 1A, confluent growth was shown in all of the catheter segments exposed to these two agents. Neither a concentration of 3 mg of vancomycin/ml nor a vancomycin-heparin combination had significant impact on reducing colonization of catheter segments, with colony counts of 492.5 ± 7.5 and 422 ± 58 CFU of S. epidermidis organisms per catheter segment (means ± standard errors [SE]), respectively, for each of these two agents. A low concentration of minocycline (0.1 mg/ml) alone or EDTA (30 mg/ml) alone reduced S. epidermidis catheter colonization by almost threefold to means (± SE) of 148 ± 49 and 116 ± 55 CFU/catheter segment, respectively (P < 0.01). However, the combination of 0.1 mg of minocycline/ml and EDTA was highly efficacious in reducing the colonization of S. epidermidis to a low level of 13.1 ± 5.5 CFU/catheter segment (P < 0.001). This reduction represented a significant decrease in colonization compared to that for either minocycline alone or EDTA alone (P ≤ 0.02) as well as a significant reduction compared to heparin, streptokinase, vancomycin, and vancomycin in heparin (P ≤ 0.001). A higher concentration of minocycline (3 mg/ml) alone or a combination of a high concentration of minocycline and EDTA consisting of 3 mg of minocycline/ml and 30 mg of EDTA/ml resulted in complete eradication of S. epidermidis organisms from catheter segments.
FIG. 1.
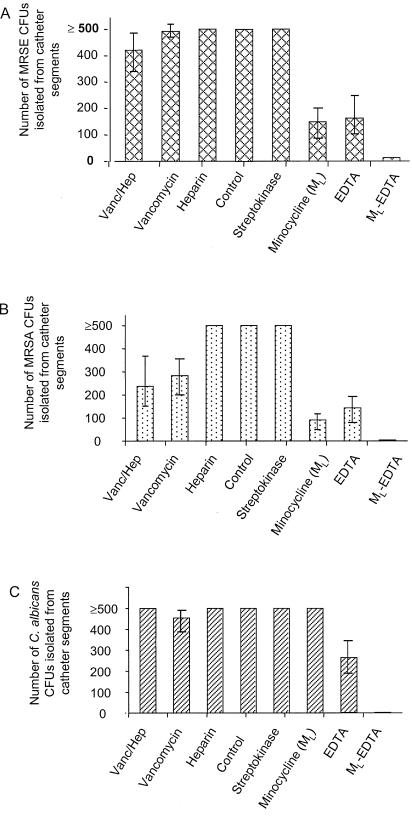
MRD in vitro model of the colonization of methicillin-resistant S. epidermidis (MRSE) (A) and MRSA (B) and of C. albicans (C) in the presence of various drugs, including 0.1 mg of minocycline/ml, 30 mg of EDTA/ml, and ML-EDTA. Vanc/Hep, vancomycin-heparin.
In vitro model with S. aureus.
Similar to results for the S. epidermidis model, exposure of catheter segments to MRSA resulted in the formation of a mature biofilm on three catheter segments. In addition, confluent colonization with this organism after exposure to heparin and streptokinase in broth was equivalent to incubation in broth alone for 24 h (Fig. 1B). A high concentration of vancomycin (3 mg/ml) and 3 mg of vancomycin/ml plus heparin in broth did have the significant impact of decreasing colonization compared to that for broth, heparin, or streptokinase, resulting in a mean colonization (± SE) of 284.5 ± 64.7 and 237.5 ± 84.3 CFU/catheter segment for vancomycin and vancomycin-heparin, respectively (P ≤ 0.01). Minocycline (0.1 mg/ml broth) alone or EDTA (30 mg/ml) alone reduced colonization to a level of 92.5 ± 16.3 and 143 ± 39.4/catheter segment, respectively (P ≤ 0. 01). The ML-EDTA combination in broth reduced MRSA colonization by more than 100-fold compared to that for heparin and streptokinase (P ≤ 0.001), to a low level of 5 ± 2.5 CFU of S. aureus organisms per catheter segment (P < 0.001). In addition, the ML-EDTA combination was significantly more effective in reducing MRSA colonization than either a low concentration of minocycline or EDTA alone (P ≤ 0.04) (Fig. 1B). Higher concentrations of minocycline (3 mg/ml), as well as MH-EDTA, resulted in complete eradication of MRSA from catheter surfaces in a similar fashion to that for the S. epidermidis in vitro model.
In vitro model with C. albicans.
Candida embedded in biofilm was also demonstrated to be present on three randomly selected catheter segments from the C. albicans in vitro model. As expected, streptokinase, heparin, vancomycin, and vancomycin in heparin had no impact in reducing catheter colonization compared to that for broth (Fig. 1C). Similarly, low-dose minocycline (0.1 mg/ml) did not reduce catheter colonization with C. albicans while EDTA alone resulted in partial reduction to a mean colony count of 263 ± 65.4. The combination of a low concentration of minocycline (0.1 mg/ml) with EDTA resulted in a significant decrease in catheter colonization, to a low level of 3.8 ± 3.8 CFU of C. albicans organisms per catheter segment, compared with that for all of the other agents, including heparin, vancomycin, a low concentration of minocycline (0.1 mg/ml) alone, and EDTA alone (P < 0.01). Higher concentrations of minocycline and MH-EDTA resulted in the complete eradication of C. albicans in a fashion similar to that for the S. epidermidis and S. aureus in vitro models.
Ex vivo model.
As determined by CSLM, all of the 54 colonization catheter segments that were exposed to saline were found to have bacterial colony counts greater than or equal to 102. MH-EDTA (with a concentration of 3 mg of minocycline and 30 mg of EDTA/ml) decreased the density of colonization by at least 10-fold or greater compared to that for saline in 67% (12/18) of the catheter segments. As shown in Table 1, a reduction of at least 105-fold was noted in 50% (9/18) of catheter segments exposed to MH-EDTA. EDTA alone or heparin alone reduced colonization by 10-fold in only 11% (2/18) of the catheter segments exposed to either one of these two anticoagulant solutions, a result which was significantly lower than that for MH-EDTA (P ≤ 0.01). The types of organisms cultured from the catheter segments that were exposed to MH-EDTA and studied by CSLM are shown in Table 1.
TABLE 1.
Results for ex vivo model of bacterial organisms colonizing 18 catheter segments exposed to MH-EDTA as determined by CSLM testing
| Reduction in no. of bacterial colonies (fold) | No. of catheters | Type of organism (no. of catheters with a specific organism) |
|---|---|---|
| <10 | 6 | Coagulase-negative staphylococci (3) |
| Staphylococcus aureus (1) | ||
| Corynebacterium spp. (1) | ||
| Alcaligenes xylosoxidans (1) | ||
| 10-104 | 3 | Staphylococcus aureus (2) |
| Coagulase-negative staphylococci (1) | ||
| 105 | 4 | Coagulase-negative staphylococci (1) |
| Staphylococcus aureus (1) | ||
| Enterococcus spp. (1) | ||
| Pseudomonas aeruginosa (1) | ||
| 106 | 5 | Coagulase-negative staphylococci (3) |
| Enterococcus spp. (1) | ||
| Pseudomonas aeruginosa (1) |
DISCUSSION
When embedded in biofilm, microorganisms become resistant to agents that are active against the free-floating form of the same organisms in suspension (5-7, 12). This finding was demonstrated in several models, including the MRD in vitro model of colonization (6, 12). By using the MRD in vitro model of colonization, in this study M-EDTA was found to be highly effective in reducing the colonization of S. epidermidis, S. aureus, and C. albicans organisms embedded in biofilm. ML-EDTA was significantly more effective than various other agents such as streptokinase, heparin, vancomycin (at a high concentration of 3 mg/ml), vancomycin and heparin, a low concentration of minocycline (0.1 mg/ml) alone, or EDTA alone in reducing the colonization of staphylococcal and Candida organisms on the surfaces of silicone catheters. In the same in vitro model, MH-EDTA (at a concentration of 3 mg of minocycline/ml) eradicated all staphylococcal and C. albicans organisms embedded in the biofilm.
As determined by CSLM, the ex vivo model for the colonized CVC tips showed that MH-EDTA reduced the colonization of bacterial cells more frequently than EDTA or heparin alone (P < 0.01). Due to the fact that the MH-EDTA concentration was used successfully in the prevention and treatment of catheter infections in previous animal and clinical studies (1, 3, 18, 21), this same concentration was used in the ex vivo model. Furthermore, although ML-EDTA resulted in a significant reduction of microbial growth, it failed to completely eradicate all growth in the in vitro model. Therefore, ML-EDTA was used to demonstrate a possible synergistic or additive effect of minocycline and EDTA in vitro but was considered suboptimal to MH-EDTA, since failure to eradicate microbial growth in vitro may select for resistant organisms. However, both the in vitro and the ex vivo models of colonization used showed that MH-EDTA is highly effective in reducing colonization of organisms embedded in biofilm on catheter surfaces.
Heparin is the standard anticoagulant used to flush the lumens of short-term and long-term CVCs in order to prevent thrombotic occlusions. However, as shown in the in vitro model in this study, heparin has no antimicrobial activity and is not different from broth alone in promoting the growth of organisms embedded in biofilm. Furthermore, in both the in vitro and ex vivo models, M-EDTA was significantly more effective than heparin. This data supports previously published animal and clinical data comparing MH-EDTA to heparin (1, 3, 21, 31). In a recent animal study, all of the rabbits whose catheters were injected intraluminally with a high inoculum of biofilm-producing S. epidermidis and subsequently maintained on heparin (100 IU/ml) developed catheter-related bacteremia, septic thrombosis, and endocarditis (21). In contrast, none of the animals that received MH-EDTA (at a concentration similar to the high-dose MH-EDTA used in the present study) flush solution through the previously challenged catheters developed catheter-related bacteremia, septic thrombosis, or endocarditis (P < 0.01). In a recent clinical study involving pediatric cancer patients, the MH-EDTA lock solution significantly decreased the risk of port infections and thrombotic occlusions compared to that for a heparin flush solution (P = 0.05) (3). In another prospective randomized study involving long-term catheters in hemodialysis patients, the MH-EDTA flush solution was significantly more effective than heparin in decreasing the risk of colonization and infection of catheters, as well as the risk of thrombotic occlusions (P < 0.05) (1).
Since staphylococci are the leading cause of catheter-related bacteremia, antimicrobial catheter flush solutions consisting of vancomycin and heparin have been proposed as an alternative to heparin. Several prospective randomized studies showed the combination of vancomycin and heparin in an antibiotic lock solution to be superior to heparin alone in preventing catheter-related bacteremia (2, 10, 26), while another prospective randomized controlled trial failed to show any benefit in a pediatric patient population (23).
Our data based on an in vitro model of colonization show that a one-time exposure to vancomycin-heparin or vancomycin alone does not significantly reduce the colonization of S. epidermidis, S. aureus, or C. albicans compared to that for heparin. As a glycopeptide antimicrobial, vancomycin was previously reported to have limited activity against microbial organisms embedded in a biofilm on a catheter surface (6, 7). In addition, the extracellular slime consisting of the exopolysaccharide produced by S. epidermidis and S. aureus has been shown to inhibit the antimicrobial activity of vancomycin (8). These findings could explain the limited activity against a high level of colonization of catheter surfaces with staphylococcal organisms embedded in biofilm, as described in the in vitro model of the present study. In a rabbit model of catheter-related S. epidermidis bacteremia, vancomycin-heparin did not significantly decrease the risk of catheter-related bacteremia and septic thrombosis compared to the decreased risk with heparin alone (21).
As shown in Fig. 1A and B, vancomycin and vancomycin-heparin had a greater effect in reducing S. aureus colonization in the in vitro model than in the S. epidermidis model. This finding is consistent with our findings in the animal study (21) and might raise the question of whether S. epidermidis embedded in biofilm becomes more resistant to vancomycin than does S. aureus. In addition to its limited activity against staphylococcal organisms embedded in biofilm, vancomycin has a few other limitations. First, vancomycin has an antimicrobial spectrum limited to gram-positive organisms but has no activity against other organisms, such as C. albicans, that could cause catheter-related bloodstream infections. The second limitation is that vancomycin is an independent risk factor for the acquisition of vancomycin-resistant enterococci, which led the Centers for Disease Control and Prevention to recommend against the use of vancomycin flush solution for the prevention of catheter-related bloodstream infections (8, 30). With the recent reports describing the emergence of S. aureus organisms with intermediate susceptibility or resistance to vancomycin, further concerns have been raised about the use of vancomycin as a prophylactic agent over a long time period (8, 11, 16, 28, 29).
The M-EDTA combination has previously been shown to be synergistic against free-floating gram-negative bacilli and C. albicans in a suspension solution (18, 32, 33). However, the present study, through its in vitro and ex vivo models of colonization, is the first to demonstrate that the M-EDTA combination is highly effective in reducing the colonization of staphylococcal organisms embedded in the biofilm on a catheter surface. In a study of suspended cells, minocycline has been shown to be active against methicillin-sensitive and -resistant staphylococcal organisms that cause catheter-related bacteremia (5). Similarly, EDTA has been shown to have limited broad-spectrum activity against methicillin-resistant staphylococci and even Candida (9, 25). The advantage of EDTA as a chelator is that it also has an anticoagulant activity, which has been shown to be at least equivalent to that of heparin (24). In the present study, the combination has been shown to be at least 10-fold more efficacious in eradicating biofilm-embedded staphylococcal and C. albicans organisms than either minocycline or EDTA alone.
The in vitro MRD model of colonization used in this study is limited by the fact that the biofilms formed are immature and devoid of host factors such as fibrin and fibronectin after exposure of catheter segments to the organisms used. Hence, an ex vivo model of colonization obtained from CVCs that had been indwelling in cancer patients and had been highly colonized by the semiquantitative roll plate catheter culture method was also used to compensate for the limitations of the MRD. The CSLM allows the examination of the fully hydrated, mature biofilm in real time and the quantitation of living, adherent bacterial cells embedded in biofilm (4). The data from the CSLM support the finding of the in vitro model that MH-EDTA as used previously (in a concentration comparable to that which was used in the animal and clinical studies) was significantly more effective in reducing the colonization of bacterial organisms embedded in mature biofilm than other anticoagulant solutions consisting of heparin or EDTA.
There are several limitations to the present study. The first is that catheter segments were exposed only once (for 24 h) to one of the antimicrobials. However, in situ, various agents were flushed through the catheter day after day, which explains why in some studies vancomycin-heparin flushed through on a daily basis reduced the risk of gram-positive catheter-related infections (2, 10, 26). However, in the long term, partial inhibition or microbial reduction could select for resistant organisms. The second limitation relates to the presumed eradication of staphylococci and Candida spp. by MH-EDTA in the in vitro model. This observation is limited by the detection limits of our culture methods for the in vitro model and should be interpreted in the context of the ex vivo model data whereby the MH-EDTA failed to reduce bacterial colonization in 33% of the exposed catheter segments. Hence, MH-EDTA might greatly inhibit microbial growth in biofilm but not necessarily eradicate it.
In conclusion, in experiments performed in an in vitro model of catheter colonization, M-EDTA was highly active in reducing the density of colonization by S. epidermidis, S. aureus, and C. albicans organisms embedded in biofilm. The in vitro activity of ML-EDTA against the biofilm-embedded organisms was significantly greater than that of ML alone, EDTA alone, vancomycin-heparin, or heparin solution. In addition, through an ex vivo model of colonization, M-EDTA with a high concentration of minocycline (3 mg/ml) was significantly more effective than other anticoagulants such as EDTA or heparin. These in vitro and ex vivo data support previous results of animal and clinical studies demonstrating the efficacy of this antimicrobial flush solution in the prevention of catheter-related bloodstream infections (3, 18, 21).
Acknowledgments
We thank Richard H. Veeh, Senior Research Associate at the Center for Biofilm Engineering, Montana State University, Bozeman, Montana, for his assistance with CSLM.
REFERENCES
- 1.Bleyer, A., L. Mason, I. Raad, and R. Sherertz. 2000. A randomized, double-blind trial comparing heparin and minocycline/EDTA as flush solutions for hemodialysis catheters. Infect. Control Hosp. Epidemiol. 21:100-101. (Abstract.)
- 2.Carratala, J., J. Niubo, A. Fernandez-Sevilla, X. Juve, J. Castellsague, J. Berlanga, J. Linares, and F. Gudiol. 1999. Randomized, double-blind trial of an antibiotic-lock technique for prevention of gram-positive central venous catheter-related infection in neutropenic patients with cancer. Antimicrob. Agents Chemother. 43:2200-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chatzinikolaou, I., T. F. Zipf, H. Hanna, J. Umphrey, W. M. Roberts, R. Sherertz, R. Hachem, and I. Raad. 2003. Minocycline and ethylene-diaminetetraacetate (M-EDTA) lock solution for the prevention of implantable port infections in pediatric cancer patients. Clin. Infect. Dis. 36:116-119. [DOI] [PubMed] [Google Scholar]
- 4.Cook, G., J. W. Costerton, and R. O. Darouiche. 2000. Direct confocal microscopy studies of the bacterial colonization in vitro of a silver-coated heart valve sewing cuff. Int. J. Antimicrob. Agents 13:169-173. [DOI] [PubMed] [Google Scholar]
- 5.Darouiche, R. O., I. Raad, G. P. Bodey, and D. M. Musher. 1995. Antibiotic susceptibility of staphylococcal isolates from patients with vascular catheter-related bacteremia: potential role of the combination of minocycline and rifampin. Int. J. Antimicrob. Agents 16:31-36. [DOI] [PubMed] [Google Scholar]
- 6.Evans, R. C., and C. J. Holmes. 1987. Effect of vancomycin hydrochloride on Staphylococcus epidermidis biofilm associated with silicone elastomer. Antimicrob. Agents Chemother. 32:889-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farber, B. F., M. H. Kaplan, and A. G. Clogston. 1990. Staphylococcus epidermidis biofilm associated with silicone elastomer. Antimicrob. Agents Chemother. 161:37-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fridkin, S. K. 2001. Vancomycin-intermediate and -resistant Staphylococcus aureus: what the infectious disease specialist needs to know. Clin. Infect. Dis. 32:108-115. [DOI] [PubMed] [Google Scholar]
- 9.Gil, M. L., M. Casanoa, and J. P. Martinez. 1994. Changes in the cell wall glycoprotein composition of Candida albicans associated to the inhibition of germ tube formation by EDTA. Arch. Microbiol. 161:489-494. [DOI] [PubMed] [Google Scholar]
- 10.Henrickson, K. J., R. A. Axtel, S. M. Hoover, S. M. Kuhn, J. Pritchett, S. C. Kehl, and J. P. Klein. 2002. Prevention of central venous catheter-related infections and the thrombotic events in immunocompromised children by the use of vancomycin/ciprofloxacin/heparin flush solution: a randomized, multicenter, double-blind trial. J. Clin. Oncol. 18:1269-1278. [DOI] [PubMed] [Google Scholar]
- 11.Hiramatsu, K., H. Hanaki, T. Ino, K. Yabuta, T. Oguri, and F. C. Tenover. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40:135-136. [DOI] [PubMed] [Google Scholar]
- 12.Khardori, N., E. Wong, H. Nguyen, C. Jeffery-Wiseman, E. Wallin, R. P. Tewari, and G. P. Bodey. 1991. Effect of subinhibitory concentrations of clindamycin and trospectomycin on the adherence of Staphylococcus epidermidis in an in vitro model of vascular catheter colonization. J. Infect. Dis. 164:108-113. [DOI] [PubMed] [Google Scholar]
- 13.Lloyd, D., and A. J. Hayes. 1995. Vigour, vitality and viability of microorganisms. FEMS Microbial. Lett. 133:107. [Google Scholar]
- 14.Maki, D. G., and L. Mermel. 1998. Infections due to infusion therapy, p. 689-694. In J. V. Bennett and P. S. Brachman (ed.), Hospital infections. Lippincott-Raven, Philadelphia, Pa.
- 15.Mermel, L. A., B. M. Farr, R. J. Sherertz, I. I. Raad, N. O'Grady, J. S. Harris, and D. E. Craven. 2001. Guidelines for the management of intravascular catheter-related infections. Clin. Infect. Dis. 32:1249-1272. [DOI] [PubMed] [Google Scholar]
- 16.Miller, D., V. Urdaneta, and A. Weltman. 2002. Public health dispatch: vancomycin-resistant Staphylococcus aureus—Pennsylvania, 2002. Morb. Mortal. Wkly. Rep. 51:902. [PubMed] [Google Scholar]
- 17.O'Grady, N. P., M. Alexander, E. P. Dellinger, J. L. Gerberding, S. O. Heard, D. G. Maki, H. Masur, R. D. McCormick, L. A. Mermel, M. L. Pearson, I. I. Raad, A. Randolph, and R. A. Weinstein. 2002. Centers for Disease Control and Prevention: guidelines for the prevention of intravascular catheter-related infections. Morb. Mortal. Wkly. Rep. 51(RR-10):1-29. [PubMed] [Google Scholar]
- 18.Raad, I., A. Buzaid, J. Rhyne, R. Hachem, R. Darouiche, H. Safar, M. Albitar, and R. J. Sherertz. 1997. Minocycline and ethylenediaminetetraacetate for the prevention of recurrent vascular catheter infections. Clin. Infect. Dis. 25:149-151. [DOI] [PubMed] [Google Scholar]
- 19.Raad, I., W. Costerton, U. Sabharwal, M. Sacilowski, E. Anaissie, and G. P. Bodey. 1993. Ultrastructural analysis of indwelling vascular catheters: a quantitative relationship between luminal colonization and duration of placement. J. Infect. Dis. 168:400-407. [DOI] [PubMed] [Google Scholar]
- 20.Raad, I., R. Darouiche, R. Hachem, M. Sacilowski, and G. P. Bodey. 1995. Antibiotics and prevention of microbial colonization of catheters. Antimicrob. Agents Chemother. 39:2397-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raad, I., R. Hachem, R. K. Tcholakian, and R. Sherertz. 2002. Efficacy of minocycline and EDTA lock solution in preventing catheter-related bacteremia, septic phlebitis, and endocarditis in rabbits. Antimicrob. Agents Chemother. 46:327-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raad, I. I., and H. A. Hanna. 2002. Intravascular catheter-related infections. New horizons and recent advances. Arch. Intern. Med. 162:871-878. [DOI] [PubMed] [Google Scholar]
- 23.Rackoff, W. R., M. Weiman, R. Jakobowski, R. Hirschi, V. Stallings, J. Bilodeau, P. L. Danz, L. Bell, and B. Lange. 1995. A randomized, controlled trial of the efficacy of a heparin and vancomycin solution in preventing central venous catheter infections in children. J. Pediatr. 127:147-151. [DOI] [PubMed] [Google Scholar]
- 24.Reardon, D. M., B. Warner, and E. A. Trowbridge. 1991. EDTA, the traditional anticoagulant of haematology: with automation is it time for review? Med. Lab. Sci. 48:72-75. [PubMed] [Google Scholar]
- 25.Root, J. L., O. R. McIntyre, N. J. Jacobs, and C. P. Daghlian. 1988. Inhibitory effect of disodium EDTA upon the growth of Staphylococcus epidermidis in vitro: relation to infection prophylaxis of Hickman catheters. Antimicrob. Agents Chemother. 32:1627-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz, C., K. J. Henrickson, K. Roghmann, and K. Powell. 1990. Prevention of bacteremia attributed to luminal colonization of tunneled central venous catheters with vancomycin-susceptible organisms. J. Clin. Oncol. 8:1591-1597. [DOI] [PubMed] [Google Scholar]
- 27.Sherertz, R. J., I. I. Raad, A. Belani, L. Koo, and K. Rand. 1990. Three-year experience with sonicated vascular catheter cultures in a clinical microbiology laboratory. J. Clin. Microbiol. 28:76-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sievert, D. M., M. L. Boulton, G. Stoltman, D. Johnson, M. G. Stobierski, F. P. Downes, P. A. Somsel, J. T. Rudrik, W. Brown, W. Hafeez, T. Lundstrom, E. Flanagan, R. Johnson, J. Mitchell, and S. Chang. 2002. Staphylococcus aureus resistant to vancomycin—United States. Morb. Mortal. Wkly. Rep. 51:565-567. [Google Scholar]
- 29.Smith, T. L., M. K. Pearson, K. R. Wilcox, C. Cruz, M. V. Lancaster, B. Robinson-Dunn, F. C. Tenover, M. J. Zervos, J. D. Bank, E. White, and W. R. Jarvis. 1999. Emergence of vancomycin resistance in Staphylococcus aureus. N. Engl. J. Med. 340:493-501. [DOI] [PubMed] [Google Scholar]
- 30.Spafford, P. S., R. A. Sinkin, C. Cox, L. Reubens, and K. R. Powell. 1994. Recommendations for preventing the spread of vancomycin resistance. Recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC). Morb. Mortal. Wkly. Rep. 44:1-13. [PubMed] [Google Scholar]
- 31.Terzieva, S., J. Donnelly, V. Ulevicius, S. A. Grinshpun, K. Willeke, G. N. Stelma, and K. P. Brenner. 1996. Comparison of methods for detection and enumeration of airborne microorganisms collected by liquid impingement. Appl. Environ. Microbiol. 62:2264-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wooley, R. E., M. Jones, J. P. Gilbert, and E. B. Shotts, Jr. 1983. In vitro action of combinations of antimicrobial agents and EDTA-tromethamine on Pseudomonas aeruginosa. Am. J. Vet. Res. 44:1521-1524. [PubMed] [Google Scholar]
- 33.Wooley, R. E., M. S. Jones, J. P. Gilbert, and E. B. Shotts, Jr. 1983. In vitro action of combinations of antimicrobial agents and EDTA-tromethamine on Escherichia coli. Am. J. Vet. Res. 44:1154-1158. [PubMed] [Google Scholar]


