Abstract
A study of some of the factors which influenced the rate and amount of cells released from tissue fragments during trypsinization led to a revision of the method described by Youngner for monkey kidney. The revision includes the use of a glass mixing-chamber and magnetic stirrer in place of the Waring blendor. Simpler to use, the revised method has been found to yield, consistently, about 7 × 10 7 cells per g of kidney tissue, or from two to three times more than that obtained by the earlier method.
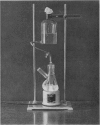
The revised method may be done either manually or automatically. A simple glass apparatus which automatically regulates the continuous addition of trypsin and removal of cell suspension during trypsinization has been developed. It operates reliably over a threefold volume range and a varying flow rate. The yield of cells per gram of tissue treated in the automatic trypsinizer is about 30% greater than when the change of fluids is done manually.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MELNICK J. L., RAPPAPORT C., BANKER D. D., BHATT P. N. Stabilized suspension of monkey kidney cells suitable for intercontinental shipment. Proc Soc Exp Biol Med. 1955 Apr;88(4):676–678. doi: 10.3181/00379727-88-21692. [DOI] [PubMed] [Google Scholar]
- MELNICK J. L., RIORDAN J. T. Poliomyelitis viruses in tissue culture. IV. Protein-free nutrient media in stationary and roller tube cultures. Proc Soc Exp Biol Med. 1952 Oct;81(1):208–213. doi: 10.3181/00379727-81-19823. [DOI] [PubMed] [Google Scholar]
- SANFORD K. K., EARLE W. R., EVANS V. J., WALTZ H. K., SHANNON J. E. The measurement of proliferation in tissue cultures by enumeration of cell nuclei. J Natl Cancer Inst. 1951 Feb;11(4):773–795. [PubMed] [Google Scholar]
- ST. GEORGE S., FRIEDMAN M., BYERS S. O. Mass separation of reticuloendothelial and parenchymal cells of rat's liver. Science. 1954 Sep 17;120(3116):463–465. doi: 10.1126/science.120.3116.463. [DOI] [PubMed] [Google Scholar]
- YOUNGNER J. S. Monolayer tissue cultures. I. Preparation and standardization of suspensions of trypsin-dispersed monkey kidney cells. Proc Soc Exp Biol Med. 1954 Feb;85(2):202–205. doi: 10.3181/00379727-85-20830. [DOI] [PubMed] [Google Scholar]