Abstract
The susceptibilities of clinical vancomycin-intermediate Staphylococcus aureus (VISA), heterogenous VISA, and laboratory-generated linezolid-resistant S. aureus strains to the new oxazolidinone AZD2563 were assessed by agar dilution MIC determination. All clinical strains were susceptible to linezolid, and the linezolid MICs for them were equal to or twofold higher than those of AZD2563. Cross-resistance with linezolid was seen in laboratory-generated mutants, and for these strains the MIC of AZD2563 was twofold higher than that of linezolid.
The requirement for effective new agents to treat infections caused by gram-positive organisms is becoming increasingly pressing as resistance to existing agents arises and spreads around the world. The problem is particularly acute for Staphylococcus aureus with the widespread emergence of strains with reduced susceptibility to vancomycin (7) and the recent isolation of a clinical strain with high-level vancomycin resistance conferred by transfer of the vanA gene cassette from a vancomycin-resistant enterococcus (2).
The oxazolidinones are a novel class of antimicrobials with activity against nearly all gram-positive organisms including those resistant to other agents. They inhibit protein synthesis by binding to domain V of the 23S rRNA and thereby blocking formation of the initiation complex (10). Resistance occurs rarely in staphylococci and is caused by mutations within the 23S rRNA, usually G2576U (6, 8). Thus far, linezolid is the only member of this class to be marketed, but there are a number of compounds such as AZD2563 that are undergoing investigation. We report on the activity of AZD2563 against clinical strains of S. aureus with reduced vancomycin susceptibility and laboratory-derived strains resistant to linezolid.
The strains tested were ATCC 25923 (control); 12 clinical vancomycin-intermediate S. aureus (VISA) strains from the United States, France, Japan, the United Kingdom, and Sweden; 122 heterogenous VISA (hVISA) strains defined by population analysis profile-area under curve ratio (9) from the United States, France, Japan, the United Kingdom, Norway, and Sweden; and laboratory-selected linezolid-resistant strains. To produce the latter strains, eight unrelated strains of methicillin-resistant S. aureus were subjected to the selection process. Briefly, the MIC of linezolid was determined by broth macrodilution performed in Mueller-Hinton broth, and after 18 h of incubation the two highest concentrations supporting growth were pooled and used as inoculum for a further MIC determination. This process was repeated daily for 28 days. The isolates from days 0, 1, 7, 14, 21, and 28 were tested in the present study. The mechanism of resistance has been previously reported for these strains (R. A. Howe, A. R. Noel, K. E. Bowker, V. I. Enne, T. R. Walsh, and A. P. MacGowan, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. C1-1608, 2002). All but two strains have mutations in domain V of the 23S rRNA gene, the linezolid target site, including G2576U, which was identified in the only two reported clinical isolates of linezolid-resistant S. aureus (6, 8); G2447U, which has been reported in laboratory-derived linezolid-resistant S. aureus (S. M. Swaney, D. L. Shinabarger, R. D. Schaadt, J. H. Bock, J. L. Slightom, and G. E. Zurenko, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C-104, 1998); and C2192T, which has not previously been associated with linezolid resistance.
Susceptibility testing was performed by agar dilution MIC determination by NCCLS methodology with Mueller-Hinton agar and an inoculum of 104 CFU/spot (5). The antimicrobials tested were AZD2563 (AstraZeneca, Macclesfield, Cheshire, United Kingdom), linezolid (Pharmacia Upjohn, Kalamazoo, Mich.), ciprofloxacin and moxifloxacin (Bayer, Newbury, United Kingdom), erythromycin and rifampin (Sigma Chemicals, Poole, Dorset, United Kingdom), telithromycin and gentamicin (Hoechst Marion Roussel, Uxbridge, United Kingdom), and quinupristin-dalfopristin (Aventis, West Malling, United Kingdom). Published NCCLS interpretive breakpoint criteria were used (4).
Table 1 shows the comparative susceptibility data for the VISA and hVISA strains. The most potent agents were the oxazolidinones; all hVISA and VISA strains were susceptible to linezolid. Quinupristin-dalfopristin was active against more than 90% of strains, and of the other comparator agents tested, only moxifloxacin displayed significant activity, with more than 50% of strains susceptible. Frequency distributions of AZD2563 and linezolid MICs for hVISA strains, laboratory-generated linezolid-resistant mutants, and their linezolid-susceptible parental strains are shown in Fig. 1. For hVISA AZD2563 was marginally more potent than linezolid, with MICs twofold lower than those of linezolid for most strains. The MICs of AZD2563 for linezolid-susceptible parental strains and mutants with low-level linezolid resistance (MIC, 8 mg/liter) were also usually twofold lower than those of linezolid. However, there was a trend for MICs of AZD2563 to be twofold higher for strains with higher levels of resistance to linezolid.
TABLE 1.
Comparative susceptibilities of VISA and hVISA strainsa
| Agent | Breakpoint (S/I/R; μg/ml) | hVISA (n = 122)
|
VISA (n = 12)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Range (μg/ml) | MIC50 (μg/ml) | MIC90 (μg/ml) | % Susceptible | Range (μg/ml) | MIC50 (μg/ml) | MIC90 (μg/ml) | % Susceptible | ||
| AZD2563 | —b | 0.25-2 | 1 | 2 | 1 | 1 | 1 | ||
| Linezolid | ≤4/≥8 | 0.25-2 | 1 | 2 | 100 | 1 | 1 | 1 | 100 |
| Quinupristin-dalfopristin | ≤1/≥4 | 0.25-16 | 1 | 1 | 95 | 0.5-16 | 1 | 1 | 92 |
| Telithromycin | ≤1/2/≥4 | 0.12-64 | 64 | 64 | 22 | 0.25-64 | 32 | 64 | 17 |
| Gentamicin | ≤4/8/≥16 | 0.03-≥128 | 128 | >128 | 19 | 0.25-128 | 64 | 128 | 25 |
| Erythromycin | ≤0.5/1-4/≥8 | 0.12->128 | >128 | >128 | 4 | >128 | >128 | >128 | 0 |
| Ciprofloxacin | ≤1/2/≥4 | 0.12->128 | 64 | 64 | 5 | 32-128 | 64 | 64 | 0 |
| Moxifloxacin | ≥2/4/≥8 | 0.06->128 | 4 | 8 | 54 | 4-8 | 4 | 8 | 58 |
| Rifamplcin | ≤1/2/≥4 | 0.015->128 | >128 | >128 | 31 | <0.008->128 | >128 | >128 | 25 |
Abbreviations: S, susceptible; I, intermediate; R, resistant; MIC50 and MIC90, MICs at which 50 and 90%, respectively, of the isolates tested are inhibited.
—, the breakpoint of AZ02563 has not been established.
FIG. 1.
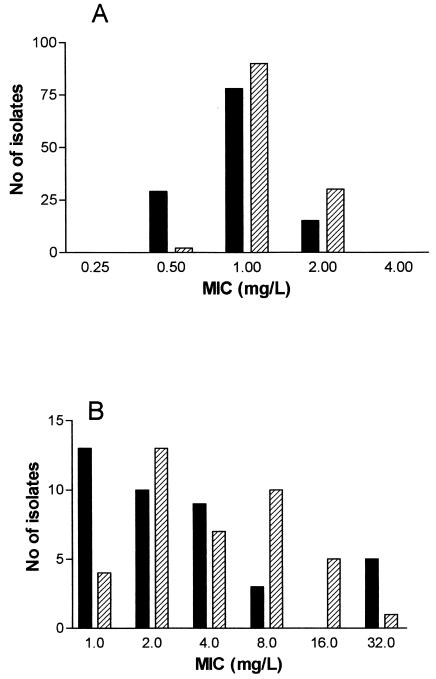
Frequency distribution of MICs for hVISA strains (n = 122) (A) and linezolid-resistant mutants and susceptible parental strains (n = 40) (B). Solid bars, AZD2563; hatched bars, linezolid.
The present study confirms the activity of oxazolidinones against S. aureus, including strains with reduced susceptibility to vancomycin. Cross-resistance was seen with strains resistant to linezolid. The superior activity of AZD2563 over that of linezolid has been reported previously (1, 3). However, the higher AZD2563 MICs seen for linezolid-resistant strains have not been noted before. Little is known regarding differential target site binding of different oxazolidinones, but our results suggest that the binding of AZD2563 is affected to a greater extent than is binding of linezolid in resistant mutants. It will be important to establish if this observation is confirmed when further oxazolidinones enter preclinical evaluation.
REFERENCES
- 1.Anderegg, T. R., D. J. Biedenbach, and R. N. Jones. 2002. In vitro evaluation of AZD2563, a novel oxazolidinone, against 603 recent staphylococcal isolates. Antimicrob. Agents Chemother. 46:2662-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2002. Staphylococcus aureus resistant to vancomycin—United States, 2002. Morb. Mortal. Wkly. Rep. 51:565-567. [PubMed] [Google Scholar]
- 3.Johnson, A. P., M. Warner, and D. M. Livermore. 2002. In vitro activity of a novel oxazolidinone, AZD2563, against randomly selected and multiresistant Gram-positive cocci. J. Antimicrob. Chemother. 50:89-93. [DOI] [PubMed] [Google Scholar]
- 4.National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial susceptibility testing. Document M100-S12. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 5.National Committee for Clinical Laboratory Standards. 2000. Performance standards for antimicrobial susceptibility testing. Document M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 6.Tsiodras, S., H. S. Gold, G. Sakoulas, G. M. Eliopoulos, C. Wennersten, L. Venkataraman, R. C. Moellering, and M. J. Ferraro. 2001. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet 358:207-208. [DOI] [PubMed] [Google Scholar]
- 7.Walsh, T. R., and R. A. Howe. 2002. The prevalence and mechanisms of vancomycin resistance in Staphylococcus aureus. Annu. Rev. Microbiol. 56:657-675. [DOI] [PubMed] [Google Scholar]
- 8.Wilson, P., J. A. Andrews, R. Charlesworth, R. Walesby, M. Singer, D. J. Farrell, and M. Robbins. 2003. Linezolid resistance in clinical isolates of Staphylococcus aureus. J. Antimicrob. Chemother. 51:186-188. [DOI] [PubMed] [Google Scholar]
- 9.Wootton, M., R. A. Howe, R. Hillman, T. R. Walsh, P. M. Bennett, and A. P. MacGowan. 2001. A modified population analysis profile (PAP) method to detect hetero-resistance to vancomycin in Staphylococcus aureus in a UK hospital. J. Antimicrob. Chemother. 47:399-403. [DOI] [PubMed] [Google Scholar]
- 10.Xiong, L., P. Kloss, S. Douthwaite, N. M. Andersen, S. Swaney, D. L. Shinabarger, and A. S. Mankin. 2000. Oxazolidinone resistance mutations in 23S rRNA of Escherichia coli reveal the central region of domain V as the primary site of drug action. J. Bacteriol. 182:5325-5331. [DOI] [PMC free article] [PubMed] [Google Scholar]


