Abstract
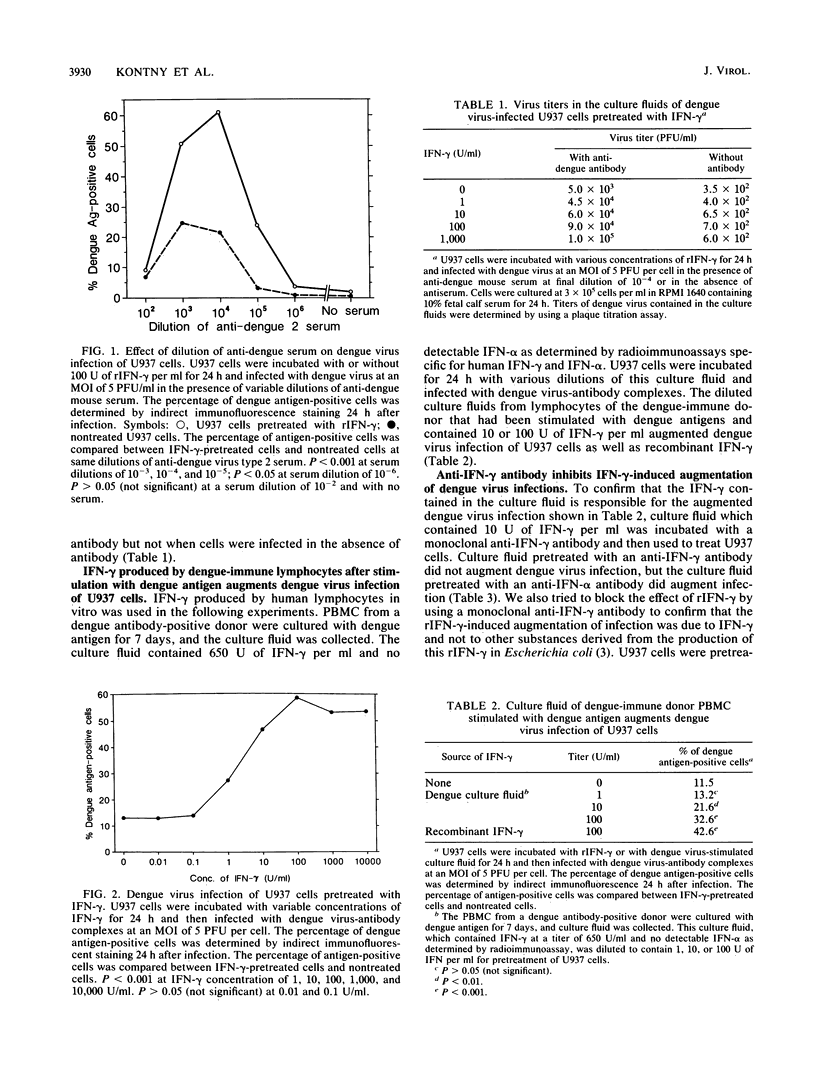
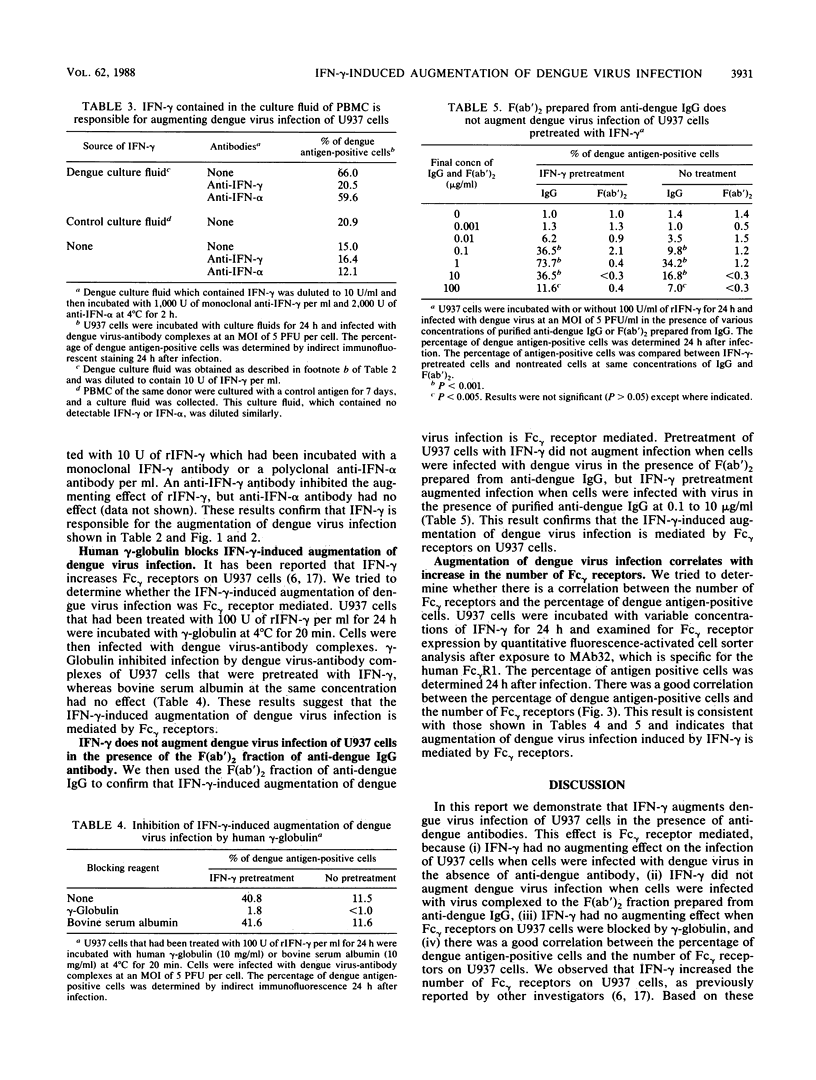
It has been reported that anti-dengue antibodies at subneutralizing concentrations augment dengue virus infection of monocytic cells. This is due to the increased uptake of dengue virus in the form of virus-antibody complexes by cells via Fc gamma receptors. We analyzed the effects of recombinant human gamma interferon (rIFN-gamma) on dengue virus infection of human monocytic cells. U937 cells, a human monocytic cell line, were infected with dengue virus in the form of virus-antibody complexes after rIFN-gamma treatment. Pretreatment of U937 cells with rIFN-gamma resulted in a significant increase in the number of dengue virus-infected cells and in the yield of infectious virus. rIFN-gamma did not augment dengue virus infection when cells were infected with virus in the absence of anti-dengue antibodies. Gamma interferon (IFN-gamma) produced by peripheral blood lymphocytes from dengue-immune donors after in vitro stimulation with dengue antigens also augmented dengue virus infection of U937 cells. IFN-gamma did not augment dengue virus infections when cells were infected with virus in the presence of F(ab')2 prepared from anti-dengue immunoglobulin G. Human immunoglobulin inhibited IFN-gamma-induced augmentation. IFN-gamma increased the number of Fc gamma receptors on U937 cells. The increase in the percentage of dengue antigen-positive cells correlated with the increase in the number of Fc gamma receptors after rIFN-gamma treatment. These results indicate that IFN-gamma-induced augmentation of dengue virus infection is Fc gamma receptor mediated. Based on these results we conclude that IFN-gamma increases the number of Fc gamma receptors and that this leads to an augmented uptake of dengue virus in the form of dengue virus-antibody complexes, which results in augmented dengue virus infection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. L., Guyre P. M., Whitin J. C., Ryan D. H., Looney R. J., Fanger M. W. Monoclonal antibodies to Fc receptors for IgG on human mononuclear phagocytes. Antibody characterization and induction of superoxide production in a monocyte cell line. J Biol Chem. 1986 Sep 25;261(27):12856–12864. [PubMed] [Google Scholar]
- Burke D. S., Nisalak A., Johnson D. E., Scott R. M. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988 Jan;38(1):172–180. doi: 10.4269/ajtmh.1988.38.172. [DOI] [PubMed] [Google Scholar]
- Daughaday C. C., Brandt W. E., McCown J. M., Russell P. K. Evidence for two mechanisms of dengue virus infection of adherent human monocytes: trypsin-sensitive virus receptors and trypsin-resistant immune complex receptors. Infect Immun. 1981 May;32(2):469–473. doi: 10.1128/iai.32.2.469-473.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis F. A., Meager A. Immune interferon produced to high levels by antigenic stimulation of human lymphocytes with influenza virus. J Exp Med. 1981 Nov 1;154(5):1279–1289. doi: 10.1084/jem.154.5.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. W., Leung D. W., Pennica D., Yelverton E., Najarian R., Simonsen C. C., Derynck R., Sherwood P. J., Wallace D. M., Berger S. L. Expression of human immune interferon cDNA in E. coli and monkey cells. Nature. 1982 Feb 11;295(5849):503–508. doi: 10.1038/295503a0. [DOI] [PubMed] [Google Scholar]
- Guyre P. M., Morganelli P. M., Miller R. Recombinant immune interferon increases immunoglobulin G Fc receptors on cultured human mononuclear phagocytes. J Clin Invest. 1983 Jul;72(1):393–397. doi: 10.1172/JCI110980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B. In vivo enhancement of dengue virus infection in rhesus monkeys by passively transferred antibody. J Infect Dis. 1979 Oct;140(4):527–533. doi: 10.1093/infdis/140.4.527. [DOI] [PubMed] [Google Scholar]
- Halstead S. B., Nimmannitya S., Cohen S. N. Observations related to pathogenesis of dengue hemorrhagic fever. IV. Relation of disease severity to antibody response and virus recovered. Yale J Biol Med. 1970 Apr;42(5):311–328. [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B., O'Rourke E. J., Allison A. C. Dengue viruses and mononuclear phagocytes. II. Identity of blood and tissue leukocytes supporting in vitro infection. J Exp Med. 1977 Jul 1;146(1):218–229. doi: 10.1084/jem.146.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B., O'Rourke E. J. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J Exp Med. 1977 Jul 1;146(1):201–217. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B. The Alexander D. Langmuir Lecture. The pathogenesis of dengue. Molecular epidemiology in infectious disease. Am J Epidemiol. 1981 Nov;114(5):632–648. doi: 10.1093/oxfordjournals.aje.a113235. [DOI] [PubMed] [Google Scholar]
- Kurane I., Ennis F. A. Induction of interferon alpha from human lymphocytes by autologous, dengue virus-infected monocytes. J Exp Med. 1987 Oct 1;166(4):999–1010. doi: 10.1084/jem.166.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurane I., Ennis F. A. Production of interferon alpha by dengue virus-infected human monocytes. J Gen Virol. 1988 Feb;69(Pt 2):445–449. doi: 10.1099/0022-1317-69-2-445. [DOI] [PubMed] [Google Scholar]
- Kurane I., Hebblewaite D., Brandt W. E., Ennis F. A. Lysis of dengue virus-infected cells by natural cell-mediated cytotoxicity and antibody-dependent cell-mediated cytotoxicity. J Virol. 1984 Oct;52(1):223–230. doi: 10.1128/jvi.52.1.223-230.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurane I., Meager A., Ennis F. A. Induction of interferon alpha and gamma from human lymphocytes by dengue virus-infected cells. J Gen Virol. 1986 Aug;67(Pt 8):1653–1661. doi: 10.1099/0022-1317-67-8-1653. [DOI] [PubMed] [Google Scholar]
- Perussia B., Dayton E. T., Lazarus R., Fanning V., Trinchieri G. Immune interferon induces the receptor for monomeric IgG1 on human monocytic and myeloid cells. J Exp Med. 1983 Oct 1;158(4):1092–1113. doi: 10.1084/jem.158.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangkawibha N., Rojanasuphot S., Ahandrik S., Viriyapongse S., Jatanasen S., Salitul V., Phanthumachinda B., Halstead S. B. Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreak. Am J Epidemiol. 1984 Nov;120(5):653–669. doi: 10.1093/oxfordjournals.aje.a113932. [DOI] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Yamada Y. K., Meager A., Yamada A., Ennis F. A. Human interferon alpha and gamma production by lymphocytes during the generation of influenza virus-specific cytotoxic T lymphocytes. J Gen Virol. 1986 Nov;67(Pt 11):2325–2334. doi: 10.1099/0022-1317-67-11-2325. [DOI] [PubMed] [Google Scholar]


