Abstract
Synaptobrevins/vesicle-associated membrane proteins (VAMPs) together with syntaxins and a synaptosome-associated protein of 25 kDa (SNAP-25) are the main components of a protein complex involved in the docking and/or fusion of synaptic vesicles with the presynaptic membrane. We report here the molecular, biochemical, and cell biological characterization of a novel member of the synaptobrevin/VAMP family. The amino acid sequence of endobrevin has 32, 33, and 31% identity to those of synaptobrevin/VAMP-1, synaptobrevin/VAMP-2, and cellubrevin, respectively. Membrane fractionation studies demonstrate that endobrevin is enriched in membrane fractions that are also enriched in the asialoglycoprotein receptor. Indirect immunofluorescence microscopy establishes that endobrevin is primarily associated with the perinuclear vesicular structures of the early endocytic compartment. The preferential association of endobrevin with the early endosome was further established by electron microscopy (EM) immunogold labeling. In vitro binding assays show that endobrevin interacts with immobilized recombinant α-SNAP fused to glutathione S-transferase (GST). Our results highlight the general importance of members of the synaptobrevin/VAMP protein family in membrane traffic and provide new avenues for future functional and mechanistic studies of this protein as well as the endocytotic pathway.
INTRODUCTION
Protein trafficking along the exocytotic and endocytotic pathways is a vital and fundamental cellular process. Proteins destined for the exocytotic pathway are initially targeted to the endoplasmic reticulum and transported through the Golgi apparatus. At the trans-Golgi network (TGN), proteins are sorted to distinct post-Golgi structures such as the plasma membrane (or its subdomains) and the endosomal and lysosomal compartments (Palade, 1975; Mellman and Simons, 1992; Hong and Tang, 1993; Rothman and Wieland, 1996; Schekman and Orci, 1996). The endosomal compartment plays a central role in cellular physiology (Gruenberg and Maxfield, 1995; Mellman, 1996; Robinson et al., 1996). Endocytosed proteins are internalized from the plasma membrane via coated vesicles and then delivered to the early endosomal compartment, from which proteins can be either recycled to the plasma membrane or delivered to the late endosomal compartment and subsequently to the lysosome or the TGN.
Intracellular trafficking is primarily mediated by various types of transport vesicles that bud from a donor membrane and then fuse with a specific cognate target membrane. To achieve such specificity, the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) hypothesis proposes that specific docking and fusion of vesicles with the cognate membrane compartment is mediated by specific interaction between the vesicle-associated SNAREs with the cognate target SNAREs on the target membrane (Rothman and Warren, 1994; Bennett, 1995; Whiteheart and Kubalek, 1995; Pfeffer, 1996). The majority of the known SNAREs (except for a synaptosome-associated protein of 25 kDa [SNAP-25] and Ykt6p) are anchored to their respective membranes by their C-terminal hydrophobic domain (Sogaard et al., 1994; Pfeffer, 1996; McNew et al., 1997). Synaptobrevins/vesicle-associated membrane proteins (VAMPs) are vesicle-associated SNAREs associated with the synaptic vesicles, whereas syntaxin 1 and SNAP-25 are target SNAREs associated with the presynaptic membrane. The specific pairing of synaptobrevins/VAMPs with the syntaxin 1–SNAP-25 complex plays a key role in the docking and fusion of synaptic vesicles with the presynaptic membrane (Sollner et al., 1993; Rothman and Warren, 1994; Scheller, 1995; Südhof, 1995).
Three members of the synaptobrevin/VAMP family (synaptobrevin/VAMP-1, synaptobrevin/VAMP-2, and cellubrevin) have been identified and characterized in mammalian cells (McMahon et al., 1993; Galli et al., 1994; Jahn and Sudhof, 1994). Synaptobrevin/VAMP-1 and synaptobrevin/VAMP-2 are highly homologous proteins that are expressed in neurons and endocrine cells, whereas cellubrevin is ubiquitously expressed and associated with the endosomal compartment. In this report, we describe the molecular, biochemical, and cell biological characterization of a novel mammalian protein that shares significant amino acid identity to synaptobrevin/VAMPs and cellubrevin. Antibodies raised against the recombinant protein recognize a 15-kDa protein that is preferentially localized to the early endosomal compartment.
MATERIALS AND METHODS
Materials
NRL (normal rat liver), A431 (human epidermoid carcinoma), NIH3T3 (mouse embryonic fibroblast), VERO (African green monkey kidney), C6 (rat glial), CV1 (monkey kidney), HeLa, and OKT9 and HB21 (mouse hybridomas expressing monoclonal antibodies against the human transferrin receptor) cells were obtained from American Type Culture Collection (Rockville, MD). MDCK II (Madin-Darby canine kidney strain II) was a generous gift from Dr. Kai Simons (European Molecular Biology Laboratory, Heidelberg, Germany). Synthetic oligonucleotides were from Oligos Etc (Wilsonville, OR). The Pyrococcus furiosus DNA polymerase was a product of Stratagene (La Jolla, CA). The Taq DNA polymerase and Hybond C-extra nitrocellulose filters were obtained from Amersham (Little Chalford, Buckinghamshire, United Kingdom). Glutathione Sepharose 4B was from Pharmacia (Upsala, Sweden). Fluorescein isothiocyanate-conjugated goat anti-mouse IgG, rhodamine-conjugated goat anti-rabbit IgG, and restriction enzymes were from Boehringer Mannheim (Mannheim, Germany). Brefeldin A (BFA) was obtained from Epicentre Technologies (Madison, WI). Wortmannin was purchased from Sigma (St. Louis, MO). Local New Zealand White rabbits were purchased from the Sembawang Laboratory Animals Centre (Singapore). Freund’s adjuvants (complete and incomplete) were from Life Technologies–BRL (Bethesda, MD).
cDNA Cloning and Sequencing
Three human expressed sequence tag (EST) clones (accession numbers T49805, R01789, and T63214) encoding overlapping sequences similar to those of known members of the VAMP family were identified with the use of the BLAST program. The complete coding sequence of endobrevin was confirmed by sequencing EST clone R01789.
Expression of Recombinant Proteins in Bacteria
For the production of glutathione S-transferase (GST) fusion proteins, a DNA fragment encoding residues 1–75 derived from PCR with the use of primer 1 (5′-GGGAATTCTAACCATGGAGGAAGCCAGTGAAGGTG) and primer 2 (5′-GGGTCTAGATCACTTCACGTTCTTCCACCAGAATT) was digested with EcoRI and XbaI restriction enzymes and subcloned into the EcoRI and XbaI sites of the bacterial expression vector pGEX-KG (Guan and Dixon, 1991). The ligated DNA was transformed into DH5α cells, and ampicillin-resistant colonies expressing the GST fusion proteins were screened as described (Sambrook et al., 1989). For the production and purification of GST fusion proteins, 100 ml of Luría-Bertaní (LB) broth containing 100 μg/ml ampicillin were inoculated with 100 μl of an overnight culture of a expressing clone and grown at 37°C with shaking. The next day, 25 ml of the culture were used to inoculate 1000 ml of LB broth containing 100 μg/ml ampicillin in a 5000 ml flask and were incubated at 37°C with shaking. When the OD 600 of the culture reached 0.4, IPTG was added to a final concentration of 1 mM, and growing continued for another 4 h. Cells were pelleted and resuspended in 50 ml of lysis buffer (phosphate buffered-saline [PBS] containing 50 mM Tris [pH 8], 0.1% Triton X-100, 0.5 mM MgCl2, 1 mg/ml lysozyme, 5 mM DTT, and a cocktail of protease inhibitors [0.5 mM phenylmethylsulfonyl fluoride and antipain, aprotinin, and leupeptin at 10 μg/ml]). Lysis was performed by incubation on ice for 1 h followed by sonication. Lysates were clarified by centrifugation and applied to a glutathione Sepharose 4B column (Pharmacia). After the column was washed with GST purification buffer (PBS containing 50 mM Tris [pH 8.0], 0.1% Triton X-100, 0.5 mM MgCl2), GST fusion protein was eluted with 15 mM reduced glutathione in Tris (pH 8.0) (containing 5% glycerol) and stored at −20°C. For the preparation of hexahistidine (HisX6)-tagged cellubrevin, 1000 ml of LB broth (with 100 μg/ml ampicillin) was inoculated with 20 ml of an overnight culture [BL21(DE3) cells containing the HisX6-tagged cellubrevin in the pET23d vector from Novagen (Madison, WI)]. Cells were grown until an OD 600 of 0.8, and IPTG was added to a final concentration of 1 mM. After growing overnight at room temperature, the cells were harvested by centrifugation at 4000 × g for 20 min, and the pellet was resuspended in cracking buffer (100 mM HEPES [pH 7.3], 500 mM KCl, 5 mM MgCl2, 2 mM β-mercaptoethanol, 1 mM phenylmethylsulfonyl fluoride, 0.1% Triton X-100) at four to five volumes per gram of wet weight. Lysozyme was added to a final concentration of 1 mg/ml, and the cells were sonicated on ice (1 min burst) for a total of 3 min. Lysate was then centrifuged at 10,000 × g for 30 min at 4°C, and the supernatant was added to 8 ml of a 50% slurry of Ni-NTA resin [preequilibrated with buffer A (20 mM HEPES [pH 7.3], 200 mM KCl, 10% glycerol, 2 mM β-mercaptoethanol) containing 25 mM imidazole] and then incubated at 4°C for 1 h with agitation. Resin was then loaded into a column and washed with 10 column volumes of buffer A containing 50 mM imidazole before eluting with 15 ml of buffer A containing 250 mM imidazole. Fractions of 1 ml each were collected and analyzed by SDS-PAGE. Fractions containing proteins were pooled and dialyzed against PBS. For the preparation of HisX6-N-ethylmaleimide-sensitive factor (NSF) fusion protein, 0.2 mM ATP was added to all the buffers to maintain the active structure of NSF.
Preparation of Polyclonal Antibodies
GST–endobrevin (300 μg) emulsified in Freund’s adjuvant was injected subcutaneously into local New Zealand rabbits. The subsequent injections (boosters) containing a similar amount of antigen were then performed every 2 wk. Rabbit serums were collected 10 d after the second and subsequent booster injections. For affinity purification, serum was diluted twice with PBS and then sequentially incubated with cyanogen bromide (CNBr)-activated sepharose beads coupled with GST and then GST–endobrevin (3 mg/ml CNBr sepharose beads) for 2 h at RT. The GST–endobrevin-coupled beads were washed extensively with 10 volumes of PBS and then eluted with 10 ml of immunopure IgG elution buffer (Pierce, Rockford, IL).
Immunofluorescence Microscopy
Immunofluorescence microscopy was performed as described previously (Wong et al., 1992, 1998; Wong and Hong, 1993; Lowe et al., 1996; Xu et al., 1997). Briefly, cells grown on coverslips were washed twice with PBS with 1 mM CaCl2 and 1 mM MgCl2 (PBSCM) and then fixed with 3% paraformaldehyde. After extensive washing, cells were permeabilized with PBSCM with 0.2% saponin (PBSCMS) for 20 min and then sequentially incubated with the primary (rabbit) and secondary (rhodamine-conjugated goat anti-rabbit IgG) antibodies for 1 h at room temperature. Cells were then washed extensively with PBSCMS, mounted in a drop of Vectastain (Vector Laboratories, Burlingame, CA), observed with the axiophot microscope (Carl Zeiss, Thornwood, NY), and then photographed with Kodak Tri-X 400 film. For the treatment of cells with BFA or wortmannin, A431 cells grown on coverslips were incubated with OKT9 and HB21 monoclonal antibodies against human transferrin receptor for 30 min, washed with DMEM media (with 10% FBS), and then treated with BFA (10 μg/ml) or wortmannin (500 nM). After incubating for 60 min at 37°C, cells were washed twice with PBSCM, fixed in 3% paraformaldehyde, permeabilized, and then incubated with antibodies against endobrevin (polyclonal) for 60 min at room temperature. After extensive washing with PBSCMS, cells were incubated with rhodamine-conjugated goat anti-rabbit IgG (10 μg/ml) and FITC-conjugated sheep anti-mouse IgG (10 μg/ml) for 60 min at room temperature. Coverslips were then mounted as described above after extensive washing with PBSCMS.
Immunogold Labeling
Cryosections and electron microscopy (EM) immunogold double labeling were performed as described previously (Slot et al., 1991; Griffiths, 1993; Griffiths et al., 1994).
Preparation of Golgi-Enriched Membranes
Preparation and subfractionation of membranes were performed as described previously (Subramaniam et al., 1992; Wong et al., 1998). Briefly, livers from Harlan Sprague Dawley (Indianapolis, IN) rats were homogenized in three volumes (g/ml) of the homogenization buffer (25 mM HEPES [pH 7.3], 5 mM MgCl2, 1 mM PMSF) containing 0.25 M sucrose with a teflon pestle and centrifuged at 10,000 × g for 10 min to remove unbroken cells, nuclei, and mitochondria. The supernatants were then recentrifuged at 100,000 × g in a Beckman Ty45Ti rotor for 1 h. The supernatant of this centrifugation that consists mainly of cytosol was collected, and the total membrane pellet was resuspended in a minimal volume of homogenization buffer containing 0.25 M sucrose. The membrane suspension was then adjusted to a final concentration of 1.25 M sucrose, overlaid with step gradients of 10 ml of 1.1 M sucrose, 10 ml of 1.0 M sucrose, and 5.0 ml of 0.5 M sucrose in homogenization buffer, and then centrifuged at 28,000 rpm for 3 h in a Beckman SW 28 rotor. The G1 (at the 0.5 M/1.0 M sucrose interphase), G2 (at the 1.0 M/1.1 M sucrose interphase), and the pellet that are enriched in Golgi, endosomes/Golgi, and microsome membranes, respectively, were collected and used for the subsequent experiments.
Western Blotting (Immunoblotting) Analysis
Proteins separated by SDS-PAGE were transferred to a Hybond C-extra nitrocellulose filter and blocked and incubated sequentially with primary antibodies (10 μg/ml) and 125I-protein A (0.1 μCi/ml). Incubation of the filter with primary antibodies and 125I-protein A and washing of the filter was done in blocking buffer (PBS containing 5% skim milk and 0.05% Tween 20). Filters were washed with PBS and PBS containing 0.05% Tween 20 and then processed for autoradiography. Some immunoblots were analyzed by using the Supersignal Chemiluminescent Kit (Pierce) according to the protocol recommended by the manufacturer.
Treatments of Membranes with Salts and Detergents
Preparation and subfractionation of membranes were performed as described previously (Subramaniam et al., 1992; Wong et al., 1998), Endobrevin-enriched membrane (500 μg) was extracted on ice for 1 h in 100 μl of either PBS, 2.5 M urea, 0.15 M sodium bicarbonate (pH 11.0), 2 M KCl, 1% Triton X-100 or 1% NP-40 and then centrifuged at 100,000 × g for 1 h at 4°C. The supernatant was collected, and the pellet was resuspended in 100 μl of 1× SDS sample buffer. Aliquots (20 μl) from both the supernatant as well as the pellet were separated by SDS-PAGE and analyzed by immunoblotting.
In Vitro Binding Assays
In vitro binding assays were performed as described previously (Wong et al., 1998). Endobrevin-enriched membranes (3 mg) were extracted for 1 h (at 4°C) in 500 μl of incubation buffer (100 mM KCl, 20 mM HEPES [pH 7.3], 2 mM EDTA, 2 mM DTT, 0.2 mM ATP) containing 1% Triton X-100. The extracted membranes were diluted with 500 μl of incubation buffer without Triton X-100 and then centrifuged at 100,000 × g at 4°C for 1 h. The extracted proteins in the supernatant were used for the subsequent binding assays. GST–α-SNAP beads (2–5 μg) were washed twice with incubation buffer containing 0.5% Triton X-100 (1 ml each) and then incubated with the different amounts of membrane extract (0, 10, 20, 40, 80, 100, 150, and 200 μg) in a total volume of 100 μl at 4°C for 3 h with agitation. GST–α-SNAP beads were then washed twice with incubation buffer containing 0.5% Triton X-100, once with incubation buffer containing 0.1% Triton X-100, and twice with incubation buffer without Triton X-100 before separation on SDS-PAGE and immunoblotting analysis.
In the complex dissociation experiment, the incubation of membrane extract (200 μg) with GST–α-SNAP was performed in the presence of increasing indicated amounts of NSF (0, 1, 2.5, 5, and 10 μg) under buffer conditions that either promote complex assembly (incubation buffer with 1 mM ATP) or disassembly (incubation buffer with 1 mM ATP and MgCl2).
RESULTS
Endobrevin Is a New Member of the Synaptobrevin/VAMP Protein Family
Three human EST clones (accession numbers T49805, R01789, and T63214) encoding overlapping protein sequences similar to those of known members of the synaptobrevin/VAMP family were identified during database searches. Compilation of these sequences yielded the full-length nucleotide sequence for endobrevin. One of the EST clones (R01789) was sequenced completely from both ends to confirm the assembled sequence. Within the 393 bp DNA sequence, a single open reading frame from nucleotide number 25 to 327 was identified that predicts a protein of ∼12 kDa (Figure 1A). This open reading frame is flanked in-frame with a strong initiation Met codon (Kozak, 1984) at the 5′-end and a stop codon at the 3′-end. In addition, there is an in-frame stop codon upstream from the initiation Met at nucleotide 19. Alignment of the deduced 100 amino acid sequence of this protein with several members of the synaptobrevin/VAMP family is shown in Figure 1B. This protein is ∼32, 33, and 31% identical to synaptobrevin/VAMP-1, synaptobrevin/VAMP-2, and cellubrevin, respectively, and because it is localized to the endosomes (see below), it was thus named endobrevin (endosome-associated synaptobrevin-like protein; Figure 1B). The amino acid homology of endobrevin to these proteins spans the entire polypeptide. However, in contrast to the high degree of homology between cellubrevin and synaptobrevin/VAMPs (70–75%), endobrevin is more distantly related to synaptobrevins/VAMPs and cellubrevin.
Figure 1.
(A) The nucleotide (upper line) and deduced amino acid (lower line) sequence of endobrevin. The C-terminal hydrophobic domain is boxed. (B) The alignment of amino acid sequences of endobrevin, synaptobrevins/VAMPs, and cellubrevin. The identical residues are shaded. The complete nucleotide sequence of endobrevin cDNA has been submitted to GenBank under the accession number AF053233.
Endobrevin Is a 15-kDa Protein Present in the Total Membrane Fraction of MDCK II, Normal Rat Kidney (NRK), and HeLa Cells
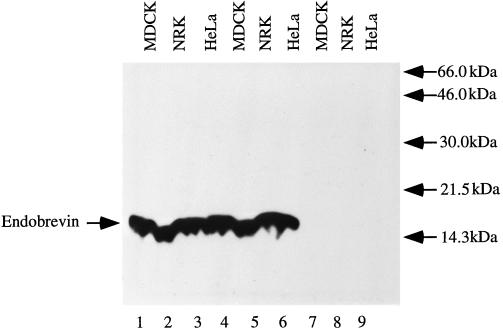
The predicted cytoplasmic domain (residues 1–75) was expressed as a fusion protein to GST (GST–endobrevin) and used to raise polyclonal antibodies against endobrevin. Immunoblot analysis was used to detect endobrevin in total membrane preparations from three different cell lines (MDCK II, NRK, and HeLa). As shown in Figure 2 (lanes 1–3), endobrevin-specific antibodies detected a 15-kDa polypeptide in all three cell lines. Detection of this polypeptide by endobrevin antibodies was blocked by preincubation of antibodies with GST–endobrevin (lanes 7–9) but not by a mixture of HisX6-tagged cellubrevin and GST (lanes 4–6), demonstrating the specificity of the antibodies.
Figure 2.
Detection of endobrevin in the total membrane fraction prepared from MDCK II (lanes 1, 4, and 7), NRK (lanes 2, 5, and 8), and HeLa (lanes 3, 6, and 9) cells by Western blot analysis using affinity-purified antibodies against endobrevin. A 15-kDa protein was specifically detected (lanes 1–3), and the detection of this protein was blocked by preincubation of antibody with GST–endobrevin (lanes 7–9) but not by a mixture of HisX6-cellubrevin and GST (lanes 4–6).
Endobrevin Is an Integral Membrane Protein Enriched in Membrane Fractions that Are Also Enriched for the Asialoglycoprotein Receptor
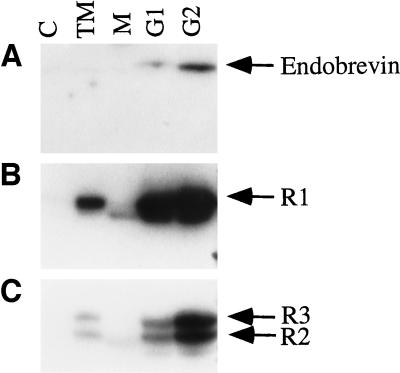
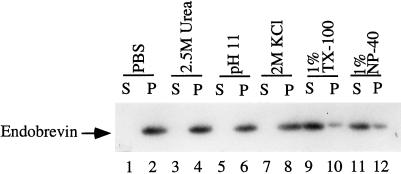
Rat liver membrane preparations were fractionated by a discontinuous sucrose gradient, and the fractions were analyzed for the presence of endobrevin by immunoblot. As shown in Figure 3A, endobrevin was found to be enriched in G1 (membrane fraction at the 0.5–1.0 M sucrose interface) and even more in G2 (membrane fraction at the 1.0–1.1 M sucrose interface) membrane fractions. The G1 and G2 membrane fractions were also enriched in the asialoglycoprotein receptor subunits R1 (46 kDa) and R2/3 (50 and 58 kDa) that are localized to the early endosomal compartment and the plasma membrane (Graeve et al., 1990; Spies, 1990; Lodish, 1991) (Figure 3, B and C). Similar to endobrevin, the asialoglycoprotein receptor subunits are enriched more in the G2 membrane fraction. Under similar conditions, the Golgi marker α-2,6-sialyltransferase is enriched more in the G1 than in the G2 fraction (Wong, Zhang, Xu, Subramaniam, Griffiths, and Hong, unpublished observations). The enrichment of endobrevin in these membrane fractions and the deduced amino acid sequence suggest that endobrevin is an integral membrane protein. To confirm this, G2 membrane fractions were extracted with PBS, 2.5 M urea, 0.15 M sodium bicarbonate (pH 11.0), 2 M KCl, 1% Triton X-100, and 1% NP-40. Figure 4 shows that endobrevin is not solubilized in PBS, in 2 M KCl, in 2.5 M urea, and in 0.15 M sodium bicarbonate (pH 11.0) but is solubilized effectively by 1% Triton X-100 and by 1% NP-40, confirming that endobrevin is indeed an integral membrane protein.
Figure 3.
Endobrevin is enriched in the asialoglycoprotein receptor subunits R1- and R2/3-containing membrane fractions. Cytosol (C), total membrane (TM), microsome-enriched membrane fraction (M), G1 membrane fraction, and G2 membrane fraction (100 μg each) were used in a Western blot analysis using (A) endobrevin antibodies, (B) polyclonal antibodies against asialoglycoprotein receptor subunit R1, and (C) antibodies against the asialoglycoprotein receptor subunits R2/3.
Figure 4.
Endobrevin is an integral membrane protein. G2 membrane fraction was extracted with a range of different reagents as indicated and separated by high-speed centrifugation into pellet (P) and supernatant (S) fractions. Aliquots (100 μg of proteins) of these fractions were analyzed by SDS-PAGE and immunoblot using endobrevin-specific antibodies.
Endobrevin Interacts with α-SNAP
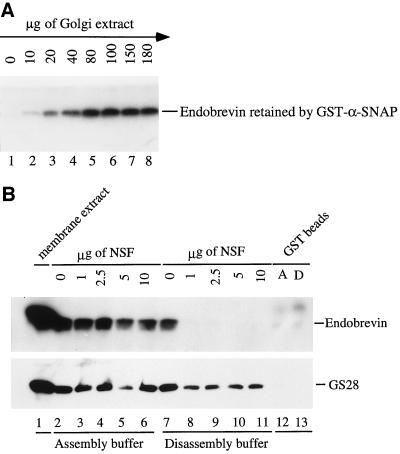
An in vitro binding assay was performed to determine if endobrevin interacts with the general docking and fusion component α-SNAP. α-SNAP was expressed in E. coli bacteria as a GST fusion protein (the entire polypeptide of the α-SNAP protein fused to the C terminus of the GST protein) and immobilized on glutathione-agarose beads. Fixed amounts (3 μg) of GST–α-SNAP and GST proteins coupled to beads were incubated with increasing amounts of soluble membrane extracts of the G2 fraction. Endobrevin bound to the beads was detected by immunoblotting. As shown in Figure 5A, endobrevin binds to α-SNAP in a dose-dependent manner. Saturation of endobrevin binding could be seen after incubation with 80 μg or more of membrane extracts (lanes 5–8). Under the same conditions, endobrevin did not bind GST-coupled beads (Figure 5B). These results suggest that endobrevin in the G2 membrane extract can interact with α-SNAP and that endobrevin is a SNARE (SNAP receptor). McMahon and Sudhof (1995) have shown previously that the binding of synaptobrevin/VAMP to syntaxin is essential for efficient α-SNAP binding. This binding was achieved either by forming a composite receptor surface for α-SNAP or by inducing a conformational change in syntaxin that results in high-affinity binding. Therefore, the interaction of endobrevin with α-SNAP is most likely mediated by an endobrevin-containing SNARE complex. To demonstrate this point, we incubated a fixed amount of membrane extract (stripped by KCl) with GST–α-SNAP beads in the presence of increasing amounts of recombinant NSF under buffer conditions that either promote SNARE-complex assembly or disassembly. As shown in Figure 5B, under conditions that promote SNARE-complex assembly (lanes 2–6), substantial amounts of endobrevin were retained on the GST–α-SNAP beads. As a positive control, GS28 (a Golgi SNARE) was similarly retained (Subramaniam et al., 1996, 1997). However, the association of endobrevin, but not GS28, with the immobilized GST–α-SNAP is essentially abolished by NSF under conditions that promote SNARE-complex disassembly (lanes 8–11). In both the SNARE-complex assembly and disassembly conditions, only background levels of endobrevin and GS28 were retained by the GST-coupled beads (lanes 12 and 13). These results show that the dissociated GS28 (Subramaniam et al., 1997) but not endobrevin remains capable of interacting with α-SNAP under conditions that promote SNARE-complex disassembly. Thus, these results not only demonstrate that the interaction of endobrevin with α-SNAP is specific but also further suggest that endobrevin does not interact with α-SNAP directly but rather through an endobrevin-containing SNARE complex.
Figure 5.
(A) Endobrevin in the membrane extracts can interact with immobilized α-SNAP. GST–α-SNAP (3 μg) immobilized on beads was incubated with increasing amounts of G2 membrane extract as indicated. After the beads were washed extensively, the amounts of endobrevin retained by the beads were detected by immunoblot. (B) The interaction between endobrevin and α-SNAP is mediated by an endobrevin-containing SNARE complex. Membrane extracts (200 μg) were incubated with GST–α-SNAP-coupled beads (3 μg) in the presence of the indicated amounts of NSF in either the SNARE-complex assembly (lanes 2–6) or the SNARE-complex disassembly (lanes 7–11) buffer. After extensive washing, the amounts of endobrevin (upper) and GS28 (lower) retained by the beads were detected by immunoblot. As a binding control, membrane extracts were incubated with GST-coupled beads in SNARE-complex assembly (A, lane 12) and disassembly (D, lane 13) buffer. Lane 1 contains 50 μg of membrane extract.
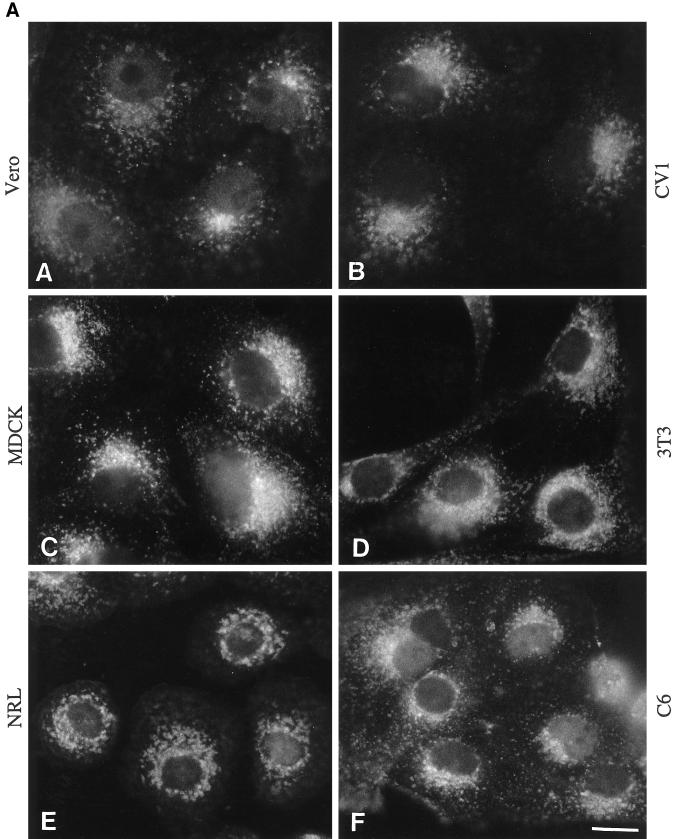
Endobrevin Is Associated with Vesicular Structures in Several Cell Lines
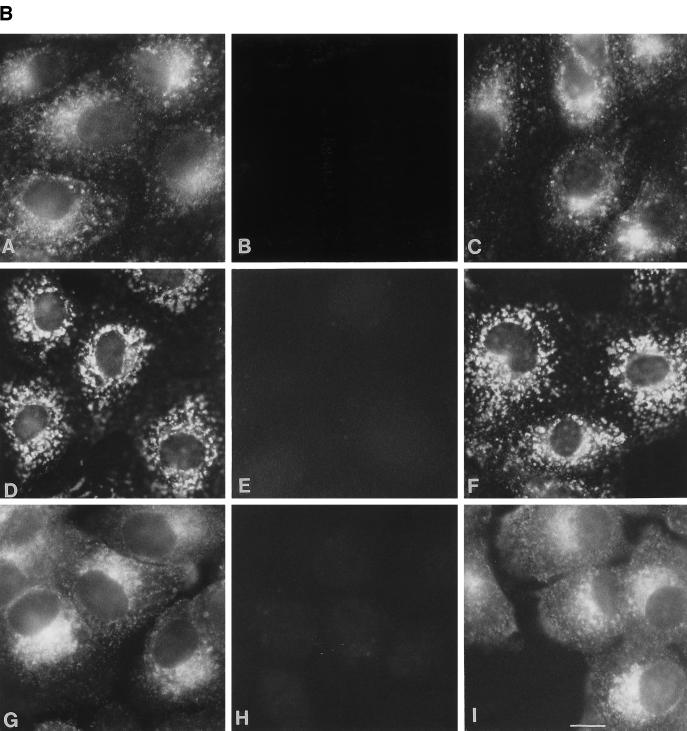
As a first step to study the function of endobrevin, we used polyclonal antibodies against endobrevin to investigate the subcellular localization of endobrevin in several mammalian cell lines. Indirect immunofluorescence microscopy revealed labeling of perinuclear and punctate vesicular structures (throughout the cytoplasm) of endobrevin in six different cell lines (CV1, NIH3T3, C6, NRL, VERO, and MDCK II cells) derived from five different species (Figure 6A). These vesicular structures were more concentrated at the perinuclear region. Figure 6B shows that the labeling of these structures (panels A, D, and G) was selectively abolished by preincubating the anti-endobrevin antibodies with GST–endobrevin (panels B, E, and H) but not with a mixture of HisX6-cellubrevin and GST (panels C, F, and I) in VERO (panels A-C), NRL (panels D-F), and A431 (G-I) cells. The selective abolishment of endobrevin labeling in these cell lines by GST-endobrevin but not by a mixture of HisX6-cellubrevin and GST further demonstrates the specificity of the antibodies.
Figure 6.
(A) Perinuclear and punctate labeling for endobrevin is detected in all of the cell lines tested. Cells (as indicated) grown on cover slips were processed for indirect immunofluorosence microscopy, as described in detail in Materials and Methods, to detect endobrevin. (B) The labeling of endobrevin (panels A, D, and G) was abolished by preincubating the antibodies with GST–endobrevin (panels B, E, and H) but not with a mixture of His-tagged cellubrevin and GST (panels C, F, and I) in VERO (panels A–C), NRL (panels D–F), and A431 (panels G–I). Bar, 10 μm.
Endobrevin Is Associated with the Endocytotic Compartment Marked by Cell Surface-Internalized Transferrin Receptor
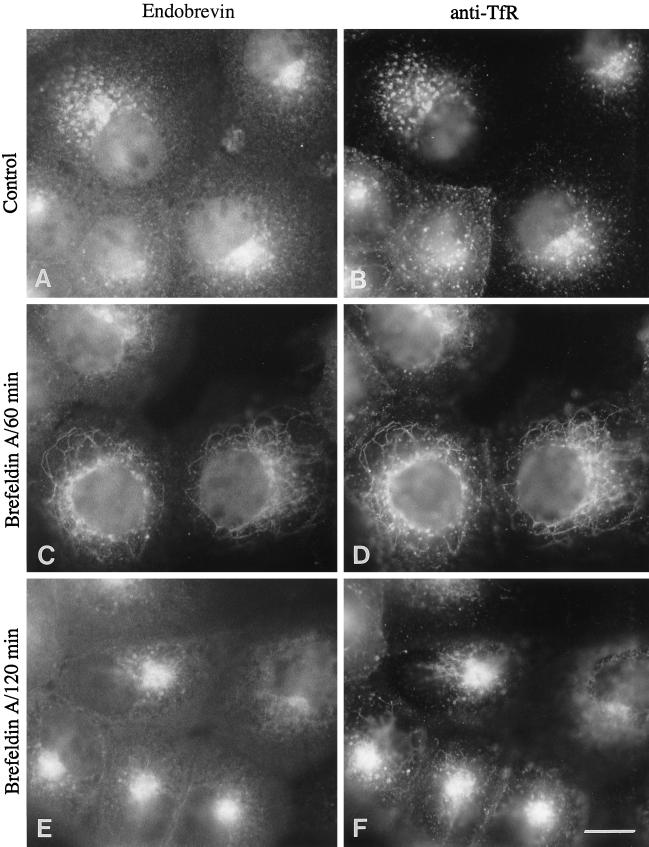
The enrichment of endobrevin in the asialoglycoprotein receptor-enriched membrane fractions indicates that endobrevin may be localized to the endocytic compartments in conjunction with its intracellular vesicular localization. To pinpoint the exact subcellular localization of endobrevin, we performed colocalization studies using surface-internalized monoclonal antibody against transferrin receptor to mark the early endosomes in A431 cells. As shown in Figure 7, the labeling of endobrevin colocalized well with the transferrin receptor, particularly in the perinuclear vesicular structures (Figure 7, A and B). After treatment with BFA for 60 min, endobrevin was observed to colocalize with internalized transferrin receptor in a tubular network (Figure 7, C and D). Prolonged treatment with BFA (120 min) caused the tubular network to concentrate to the perinuclear region that is likely to be the microtubule-organizing center (MTOC) (Figure 7, E and F). The redistribution of internalized transferrin receptor by BFA to the tubular network and subsequent structures around the MTOC have been reported previously (Klausner et al., 1992; Wood and Brown, 1992; Whitney et al., 1995). The similar response of endobrevin to BFA suggests that endobrevin is associated with the early endosome.
Figure 7.
Endobrevin is associated with the endocytotic compartment. A431 cells (after internalized monoclonal antibodies against transferrin receptor) were either untreated (A and B) or treated with 10 μg/ml BFA for 60 min (C and D) and 120 min (E and F) before processing for indirect immunofluorescence microscopy. Cells were double labeled for endobrevin (A, C, and E) and transferrin receptor (B, D, and F). Bar, 10 μm.
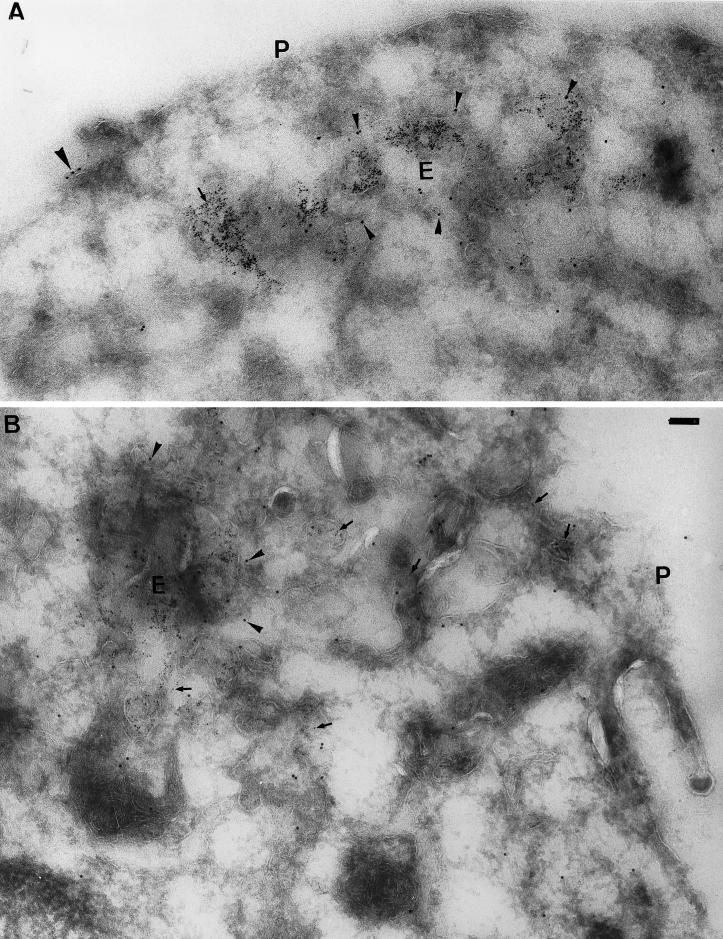
To define the precise location of endobrevin further, EM immunogold labeling was performed in cryosections of J774 cells. As shown in Figure 8, A and B, endobrevin is indeed enriched in the tubular-vesicular structures representing the early endosomes marked by the presence of cell surface-internalized BSA-gold particles in J774 cells. The labeling of endobrevin to a much lesser extent was also observed in the plasma membrane (Figure 8, A and B, indicated “P”) and the late endosomes (Wong, Zhang, Xu, Subramaniam, Griffiths, and Hong, unpublished observations). These results clearly establish that endobrevin is preferentially associated with the early endosome.
Figure 8.
Endobrevin is enriched in the early endosome. (A and B) Cryosections of J774 cells after internalizing BSA-gold (5 nm gold) for 5 min from the cell surface were processed for EM immunogold labeling to detect endobrevin (10 nm gold). Endobrevin (small arrowheads) is enriched in the early endosome (E) marked by the internalized BSA-gold (arrows). Much lesser amounts of endobrevin can also be detected on the plasma membrane (P) (large arrowheads in A). Bar, 100 nm.
Endobrevin Is Localized to the Later Subcompartment of the Early Endosome
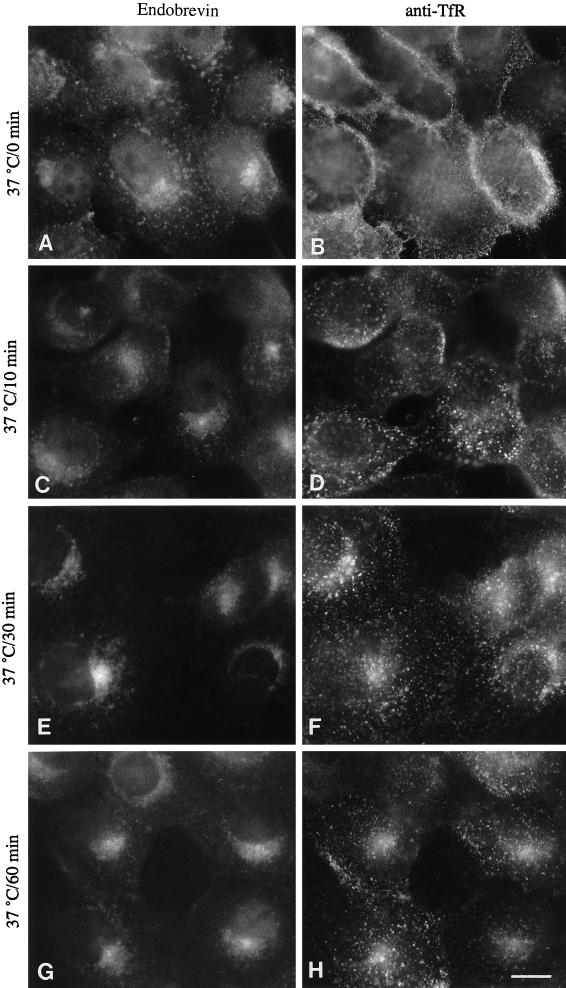
Colocalization of endobrevin with internalized transferrin receptor in control and BFA-treated cells, in conjunction with the electron microscopy data, strongly suggests that endobrevin is present primarily in the early endosomes. To determine which part of the early endosomal compartment does endobrevin colocalize with the internalized transferrin receptor, we determined the kinetics of the cell surface-internalized transferrin receptor through the endocytotic pathway. Monoclonal antibody (OKT9) against the ectodomain of the human transferrin receptor was added to the A431 cells and incubated on ice for 30 min. Cells were then washed twice with DMEM media and then incubated at 37°C for different periods of time. At the end of each time point, cells were fixed, labeled with rabbit antibodies against endobrevin and then with secondary antibodies (FITC-conjugated anti-mouse IgG and rhodamine-conjugated anti-rabbit IgG) before viewing under the immunofluorescence microscope. As shown in Figure 9, surface-bound monoclonal antibody was only detected on the surface (B) and then internalized into small peripheral vesicular structures (D). These peripheral structures become concentrated at the perinuclear region and colocalize with endobrevin (Figure 9, F and H). These results suggest that internalized transferrin receptor is associated initially with peripheral structures that are slightly enriched for endobrevin. After longer periods of time, the internalized transferrin receptor moves to the endobrevin-enriched perinuclear structures, suggesting that endobrevin is enriched in the later subcompartment of the early endosomes.
Figure 9.
Endobrevin is localized mainly to the later compartment of the early endosomes. A431 cells were incubated with monoclonal antibodies against transferrin receptor on ice, shifted to 37°C, and incubated for different periods of time at 37°C before processing for indirect immunofluorescence microscopy. Cells were double labeled for endobrevin (A, C, E, and G) and transferrin receptor (B, D, F, and H). Bar, 10 μm.
Wortmannin Redistributes Endobrevin to Vacuole-Like Structures
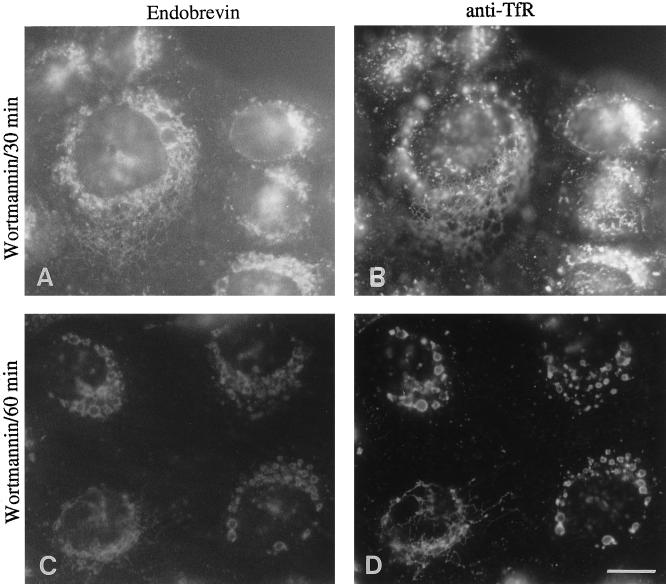
It has been suggested previously that wortmannin does not affect the peripheral early endosomes but causes the later recycling early endosomes to distribute to the swollen vacuole-like structures (Reaves et al., 1996). Because endobrevin is localized to the later perinuclear early endosome, we examined the effect of wortmannin on the distribution of endobrevin and internalized transferrin receptor in A431 cells. After treatment of A431 cells with wortmannin for 30 min, a tubular network is clearly visible that is positive for both endobrevin and internalized transferrin receptor (Figure 10, A and B). A longer treatment (1 h) with wortmannin caused endobrevin and the internalized transferrin receptor to redistribute to the swollen vacuole-like structures (Figure 10, C and D). Taken together, these results further suggest that endobrevin is associated with a later subcompartment of the early endosomes.
Figure 10.
The distribution of endobrevin is affected by wortmannin, a phosphatidylinositol 3-kinase inhibitor. After internalization of transferrin receptor antibodies for 30 min at 37°C, A431 cells were treated with 500 nM wortmannin for 30 min (A and B) and 60 min (C and D) before processing for indirect immunofluorescence microscopy. Bar, 10 μm.
DISCUSSION
Searching the EST database led to the identification of endobrevin that is ∼31–33% identical to synaptobrevin/VAMP-1, synaptobrevin/VAMP-2, and cellubrevin. This identity is relatively low compared with the identities observed among synaptobrevin/VAMP-1, synaptobrevin/VAMP-2, and cellubrevin that are in the range of 70–75%. Endobrevin is thus a distantly related member of the synaptobrevin/VAMP family. In addition, the size of endobrevin is smaller (calculated size is 12 kDa with an apparent size of 15 kDa), compared with 18 kDa or more for synaptobrevin/VAMP-1, synaptobrevin/VAMP-2, and cellubrevin. The Clostridium tetani and Clostridium botulinum bacteria produce several neurotoxins that are known to be potent inhibitors of the exocytotic release of neurotransmitters from synaptic vesicles at nerve terminals. To date, seven serologically distinct botulinal neurotoxins (BoNT/A, B, C1, D, E, F, and G) are known. These neurotoxins act on peripheral motoneurons in which they cause a blockade of acetylcholine release and thus produce the clinical manifestation of botulism. In the case of tetanus toxin (TeTx), it blocks the release of inhibitory neurotransmitters in the nervous system and thus results in the clinical manifestation of tetanus. There are specific cleavage sites for these toxins in syntaxins, synaptobrevins/VAMPs (including cellubrevin), and SNAP-25 (Link et al., 1992; Schiavo et al., 1992, 1993; Blasi et al., 1993a,b; McMahon et al., 1993; Binz et al., 1994; Galli et al., 1994). Consensus sequences of toxin cleavage sites LERDQKLSE for BoNT/F and BoNT/D and S(Q/V)F for TeTx were revealed from the compilation of cleavage sites of synaptobrevins/VAMPs and cellubrevin (Yamasaki et al., 1994). Interestingly, endobrevin does not contain these conserved toxin cleavage sites that are present in the other members of the synaptobrevin/VAMP family. Galli et al. (1994) has attempted previously to determine the function of cellubrevin in the endocytotic pathway in Chinese hamster ovary cells by cleaving the endogenous cellubrevin by TeTx toxin. However, the impairment of the recycling of internalized transferrin receptor back to the cell surface was only ∼50% after treatment with TeTx. The partial effect of TeTx may be due to the existence of other synaptobrevin/VAMP-like proteins (with similar functions) that are insensitive to TeTx cleavage. Endobrevin could be one of these TeTx-insensitive proteins.
Cell surface transferrin receptor binds to iron-saturated transferrin and then internalizes via coated vesicles before delivery to the early endosome. In this early endosomal compartment, iron is released, and the receptor with the iron-free transferrin is primarily returned to the cell surface via recycling from the later-recycling early endosomal subcompartment. It has been reported previously that the early endosomes fuse with the TGN after treatment of cells with BFA (Lippincott-Schwartz et al., 1991; Wood et al., 1991; Whitney et al., 1995). In addition, during the course of the BFA treatment, a tubular network is formed that becomes concentrated in the MTOC. In this study, antibodies bound to the transferrin receptor on the surface of A431 cells were internalized for 30 min to label the early endosomal compartment including the recycling early endosomes. Under this condition, endobrevin colocalizes well with the internalized transferrin receptor especially at the perinuclear region. When cells were treated with BFA, both the transferrin receptor and endobrevin were detected on the tubular network (after BFA treatment for 60 min) that then collapsed around the MTOC (after BFA treatment for 2 h). Therefore, these data, in conjunction with the EM immunogold labeling in J774 cells strongly suggest that endobrevin is primarily localized to the early endosome. Kinetics of the transferrin receptor internalization reveals that endobrevin is associated poorly with early peripheral early endosomes. The majority of the endobrevin is associated with the later perinuclear early endosome marked by its colocalization with the cell surface-internalized transferrin receptor (30 and 60 min internalizations). Colocalization of endobrevin with the internalized transferrin receptor to the vacuole-like structures after treatment with wortmannin, a phosphatidylinositol 3-kinase inhibitor, further supports the conclusion that endobrevin is associated with the later subcompartment of early endosomes. It has been reported previously that wortmannin inhibits several membrane traffic pathways. The membrane-trafficking pathways that are inhibited by wortmannin include the recycling from the mannose-6-phosphate receptor-positive late endosomes to the TGN (Reaves et al., 1996), recycling to the lysosome from the Igp120-positive late endosomal compartment (Reaves et al., 1996), transport of activated platelet-derived growth factor receptor to the degradative compartment of the endocytic pathway (Joly et al., 1994), fluid phase endocytosis and early endosome fusion (Clague et al., 1995; Jones and Clague, 1995; Li et al., 1995), insulin-stimulated glucose transporter GLUT4 translocation (Kanai et al., 1993), transcytosis (Hansen et al., 1995), and the recruitment of transferrin receptor to the plasma membrane in 3T3-L1 adipocytes after stimulation by insulin (Shepherd et al., 1995). In the case of transferrin receptor, it has been reported that its steady-state distribution was altered to swollen vacuole-like structures that are partially colocalized with the mannose-6-phosphate receptor in the presence of wortmannin (Reaves et al., 1996). Interestingly, it has also been suggested that wortmannin’s point of inhibition on the distribution of transferrin receptor is at the recycling compartment but not the peripheral early endosomes (Mayor et al., 1993; Reaves et al., 1996). Because wortmannin causes complete distribution of endobrevin to the swollen vacuole-like compartment, we suggest that endobrevin is most likely to reside in the later-recycling compartment of the early endosome.
The existence of a similar EST clone (accession no. AA049140) encoding endobrevin was also noticed by Scheller and colleagues (Bock and Scheller, 1997). They have named this protein VAMP-8, entirely based on the partial amino acid sequence and its homology to synaptobrevins/VAMPs and cellubrevin. Our present studies provide more detailed molecular, biochemical, and cell biological characterization of this protein. We feel that the name endobrevin is more descriptive and would like to propose that this protein be termed endobrevin.
ACKNOWLEDGMENTS
We thank Anje Habermann for technical assistance, members of Hong Wanjin’s laboratory for critical reading of the manuscript, and Dr. Y.H. Tan for his support. This work was funded by the Institute of Molecular and Cell Biology (to W.H.).
REFERENCES
- Bennett MK. SNAREs and the specificity of transport vesicle targeting. Curr Opin Cell Biol. 1995;7:582–586. doi: 10.1016/0955-0674(95)80016-6. [DOI] [PubMed] [Google Scholar]
- Binz T, Blasi J, Yamasaki S, Baumeister A, Link E, Sudhof TC, Jahn R, Niemann H. Proteolysis of SNAP-25 by types E and A botulinal neurotoxins. J Biol Chem. 1994;269:1617–1620. [PubMed] [Google Scholar]
- Blasi J, Chapman ER, Link E, Binz T, Yamasaki S, De Camilli P, Sudhof TC, Niemann H, Jahn R. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 1993a;365:160–163. doi: 10.1038/365160a0. [DOI] [PubMed] [Google Scholar]
- Blasi J, Chapman ER, Yamasaki S, Binz T, Niemann H, Jahn R. Botulinum neurotoxin C1 blocks neurotransmitter release by means of cleaving HPC-1/syntaxin. EMBO J. 1993b;12:4821–4828. doi: 10.1002/j.1460-2075.1993.tb06171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock JB, Scheller RH. Protein transport. A fusion of new ideas. Nature. 1997;387:133–135. doi: 10.1038/387133a0. [DOI] [PubMed] [Google Scholar]
- Clague M, Thorpe C, Jones AT. Phosphatidylinositol 3-kinase regulation of fluid phase endocytosis. FEBS Lett. 1995;367:272–274. doi: 10.1016/0014-5793(95)00576-u. [DOI] [PubMed] [Google Scholar]
- Galli T, Chilcote T, Mundigl O, Binz T, Niemann H, Camilli PD. Tetanus toxin-mediated cleavage of cellubrevin impairs exocytosis of transferrin receptor-containing vesicles in CHO cells. J Cell Biol. 1994;125:1015–1024. doi: 10.1083/jcb.125.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeve L, Patzak A, Drickamer K, Rodriguez-Boulan E. Polarized expression of functional rat liver asialoglycoprotein receptor in transfected Madin-Darby canine kidney cells. J Biol Chem. 1990;265:1216–1224. [PubMed] [Google Scholar]
- Griffiths G. Fine Structure Immunocytochemistry. Berlin: Springer Verlag; 1993. Cryo and replica techniques for immunolabelling; pp. 137–203. [Google Scholar]
- Griffiths G, Ericsson M, Krijnse-Locker J, Nilsson M, Goud B, Soling H, Tang BL, Wong SH, Hong W. Localization of the Lys, Asp, Glu, Leu tetrapeptide receptor to the Golgi complex and the intermediate compartment in mammalian cells. J Cell Biol. 1994;127:1557–1547. doi: 10.1083/jcb.127.6.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J, Maxfield FR. Membrane transport in the endocytic pathway. Curr Opin Cell Biol. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- Guan K, Dixon JE. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Hansen SH, Olsson A, Casanova JE. Wortmannin, an inhibitor of phosphoinositide 3-kinase, inhibits transcytosis in polarized epithelial cells. J Biol Chem. 1995;270:28425–28432. doi: 10.1074/jbc.270.47.28425. [DOI] [PubMed] [Google Scholar]
- Hong W, Tang BL. Protein trafficking along the exocytotic pathway. Bioessays. 1993;15:231–238. doi: 10.1002/bies.950150403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Sudhof TC. Synaptic vesicles and exocytosis. Annu Rev Neurosci. 1994;17:219–246. doi: 10.1146/annurev.ne.17.030194.001251. [DOI] [PubMed] [Google Scholar]
- Joly M, Kazlauskas A, Fay FS, Corvera S. Disruption of PDFG receptor trafficking by mutation of its PI-3 kinase binding sites. Science. 1994;263:684–687. doi: 10.1126/science.8303278. [DOI] [PubMed] [Google Scholar]
- Jones AT, Clague MJ. Phosphatidylinositol 3-kinase activity is required for early endosome fusion. Biochem J. 1995;311:31–34. doi: 10.1042/bj3110031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai F, Ito K, Todaka M, Hayashi H, Kamohara S, Ishi K, Okada T, Hazeki O, Ui M, Ebina Y. Insulin-stimulated GLUT4 translocation is relevant to the phosphorylation of IRS-1 and the activity of PI-3-kinase. Biochem Biophys Res Commun. 1993;195:762–768. doi: 10.1006/bbrc.1993.2111. [DOI] [PubMed] [Google Scholar]
- Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984;12:857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, D’Souza-Schorey C, Barbieri MA, Roberts RL, Klippel A, Williams LT, Stahl PD. Evidence for phosphatidylinositol 3-kinase as a regulator of endocytosis via activation of Rab5. Proc Natl Acad Sci USA. 1995;92:10207–10211. doi: 10.1073/pnas.92.22.10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link E, Edelmann L, Chou JH, Binz T, Yamasaki S, Eisel U, Baumert M, Sudhof TC, Niemann H, Jahn R. Tetanus toxin action: inhibition of neurotransmitter release linked to synaptobrevin proteolysis. Biochem Biophys Res Commun. 1992;189:1017–1023. doi: 10.1016/0006-291x(92)92305-h. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Yuan LC, Tipper C, Amherdt M, Orci L, Klausner RD. Brefeldin A’s effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell. 1991;67:601–617. doi: 10.1016/0092-8674(91)90534-6. [DOI] [PubMed] [Google Scholar]
- Lodish HF. Recognition of complex oligosaccharides by the multisubunit asialoglycoprotein receptor. Trends Biochem Sci. 1991;16:374–377. doi: 10.1016/0968-0004(91)90154-n. [DOI] [PubMed] [Google Scholar]
- Lowe SL, Wong SH, Hong W. The mammalian ARF-like protein 1 (Arl1) is associated with the Golgi complex. J Cell Sci. 1996;109:209–220. doi: 10.1242/jcs.109.1.209. [DOI] [PubMed] [Google Scholar]
- Mayor S, Presley JF, Maxfield FR. Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J Cell Biol. 1993;121:1257–1269. doi: 10.1083/jcb.121.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, Sudhof TC. Synaptic core complex of synaptobrevin, syntaxin, and SNAP25 forms high affinity α-SNAP binding site. J Biol Chem. 1995;270:2213–2217. doi: 10.1074/jbc.270.5.2213. [DOI] [PubMed] [Google Scholar]
- McMahon HT, Ushkaryov YA, Edelman L, Link E, Binz T, Niemann H, Jahn R, Sudhof TC. Cellubrevin is a ubiquitous tetanus-toxin substrate homologous to a putative synaptic vesicle fusion protein. Nature. 1993;364:346–349. doi: 10.1038/364346a0. [DOI] [PubMed] [Google Scholar]
- McNew JA, Sogaard M, Lampen NM, Machida S, Ye RR, Lacomis L, Tempst P, Rothman JE, Sollner TH. Ykt6p, a prenylated SNARE essential for endoplasma reticulum-Golgi transport. J Biol Chem. 1997;272:17776–17783. doi: 10.1074/jbc.272.28.17776. [DOI] [PubMed] [Google Scholar]
- Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- Mellman I, Simons K. The Golgi complex: in vitro veritas. Cell. 1992;68:829–840. doi: 10.1016/0092-8674(92)90027-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975;189:347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Pfeffer SR. Transport vesicle docking: SNAREs and associates. Annu Rev Cell Biol. 1996;12:441–461. doi: 10.1146/annurev.cellbio.12.1.441. [DOI] [PubMed] [Google Scholar]
- Reaves BJ, Bright NA, Mullock BM, Luzio JP. The effect of wortmannin on the localization of lysosomal type I integral membrane glycoproteins suggests a role for phosphoinositide 3-kinase activity in regulating membrane traffic late in the endocytic pathway. J Cell Sci. 1996;109:749–762. doi: 10.1242/jcs.109.4.749. [DOI] [PubMed] [Google Scholar]
- Robinson MS, Watts C, Zerial M. Membrane dynamics in endocytosis. Cell. 1996;84:13–21. doi: 10.1016/s0092-8674(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Warren G. Implications of the SNARE hypothesis for intracellular membrane topology and dynamics. Curr Biol. 1994;4:220–233. doi: 10.1016/s0960-9822(00)00051-8. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Wieland FT. Protein sorting by transport vesicles. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schekman R, Orci L. Coat proteins and vesicle budding. Science. 1996;271:1526–1532. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- Scheller RH. Membrane trafficking in the presynaptic nerve terminal. Neuron. 1995;14:893–897. doi: 10.1016/0896-6273(95)90328-3. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Benfenati F, Poulain B, Rossetto O, Polverino de Laureto P, DasGupta BR, Montecucco C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359:832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Santucci A, DasGupta BR, Mehta BB, Jontes J, Benfenati F, Wilson MC, Montecucco C. Botulinum neurotoxins serotypes A and E cleave SNAP-25 at distinct COOH-terminal peptide bonds. FEBS Lett. 1993;335:99–103. doi: 10.1016/0014-5793(93)80448-4. [DOI] [PubMed] [Google Scholar]
- Shepherd PR, Soos MA, Siddle K. Inhibitors of phosphoinositide 3-kinase block exocytosis but not endocytosis of transferrin receptors in 3T3–L1 adipocytes. Biochem Biophys Res Commun. 1995;211:535–539. doi: 10.1006/bbrc.1995.1846. [DOI] [PubMed] [Google Scholar]
- Slot JW, Geuze HJ, Gigengack S, Lienhard GE, James DE. Immuno-localization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J Cell Biol. 1991;113:123–135. doi: 10.1083/jcb.113.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogaard M, Tani K, Ye RR, Geromanos S, Tempst P, Kirchhausen T, Rothman JE, Sollner T. A rab protein is required for the assembly of SNARE complexes in the docking of transport vesicles. Cell. 1994;78:937–948. doi: 10.1016/0092-8674(94)90270-4. [DOI] [PubMed] [Google Scholar]
- Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. Snap receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Spies M. The asialoglycoprotein receptor: a model for endocytic transport receptors. Biochemistry. 1990;29:10018–10022. doi: 10.1021/bi00495a001. [DOI] [PubMed] [Google Scholar]
- Subramaniam VN, Loh E, Hong W. N-Ethylmaleimide-sensitive factor (NSF) and α-soluble NSF attachment proteins (SNAP) mediated dissociation of GS28-syntaxin 5 Golgi SNAP receptors (SNARE) complex. J Biol Chem. 1997;272:25441–25444. doi: 10.1074/jbc.272.41.25441. [DOI] [PubMed] [Google Scholar]
- Subramaniam VN, Peter F, Philip R, Wong SH, Hong W. GS28, a 28-kilodalton Golgi SNARE that participates in ER-Golgi transport. Science. 1996;272:1161–1163. doi: 10.1126/science.272.5265.1161. [DOI] [PubMed] [Google Scholar]
- Subramaniam VN, Yusoff ARBM, Wong SH, Lim GB, Chew M, Hong W. Biochemical fractionations and characterization of proteins from Golgi-enriched membranes. J Biol Chem. 1992;267:12016–12021. [PubMed] [Google Scholar]
- Südhof TC. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- Whiteheart SW, Kubalek EW. SNAPs and NSF: general members of the fusion apparatus. Trends Cell Biol. 1995;5:64–69. doi: 10.1016/s0962-8924(00)88948-5. [DOI] [PubMed] [Google Scholar]
- Whitney JA, Gomez M, Sheff D, Kreis TE, Mellman I. Cytoplasmic coat proteins involved in endosomes function. Cell. 1995;83:703–713. doi: 10.1016/0092-8674(95)90183-3. [DOI] [PubMed] [Google Scholar]
- Wong SH, Hong W. The SXYQRL sequence in the cytoplasmic domain of TGN38 plays a major role in trans-Golgi network localization. J Biol Chem. 1993;268:22853–22862. [PubMed] [Google Scholar]
- Wong SH, Low SH, Hong W. The 17-residue transmembrane domain of β-galactoside α2,6-sialyltransferase is sufficient for Golgi retention. J Cell Biol. 1992;117:245–258. doi: 10.1083/jcb.117.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SH, Xu Y, Zhang T, Hong W. Syntaxin 7, a novel syntaxin member associated with the early endosomal compartment. J Biol Chem. 1998;273:375–380. doi: 10.1074/jbc.273.1.375. [DOI] [PubMed] [Google Scholar]
- Wood SA, Brown WJ. The morphology but not the function of endosomes and lysosomes is altered by brefeldin A. J Cell Biol. 1992;119:273–285. doi: 10.1083/jcb.119.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SA, Park JE, Brown WJ. Brefeldin A causes a microtubule-mediated fusion of the trans Golgi network and early endosomes. Cell. 1991;67:591–600. doi: 10.1016/0092-8674(91)90533-5. [DOI] [PubMed] [Google Scholar]
- Xu Y, Wong SH, Zhang T, Subramaniam VN, Hong W. GS15, a 15-kilodalton Golgi soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) homologous to rbet1. J Biol Chem. 1997;272:20162–20166. doi: 10.1074/jbc.272.32.20162. [DOI] [PubMed] [Google Scholar]
- Yamasaki S, Baumeister A, Binz T, Blasi J, Link E, Cornille F, Roques B, Fykse EM, Sudhof TC, Jahn R, Niemann H. Cleavage of members of the synaptobrevin/VAMP family by types D and F botulinal neurotoxins and tetanus toxin. J Biol Chem. 1994;269:12764–12772. [PubMed] [Google Scholar]