Abstract
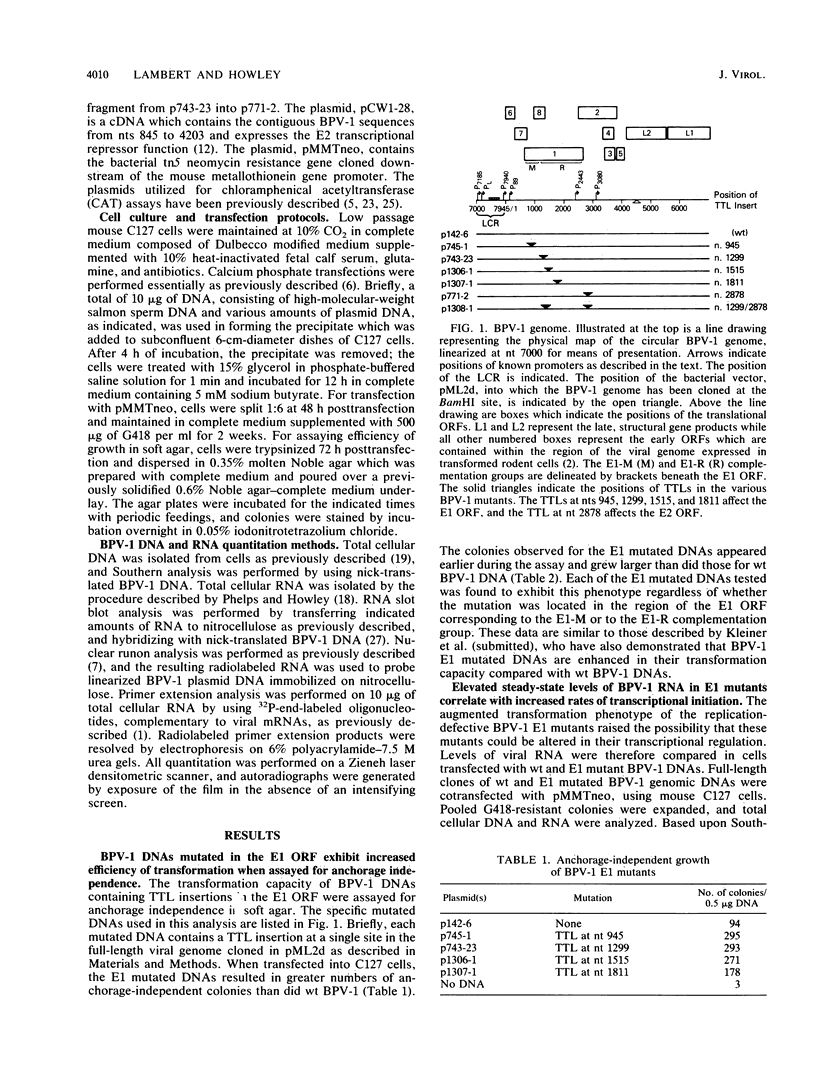
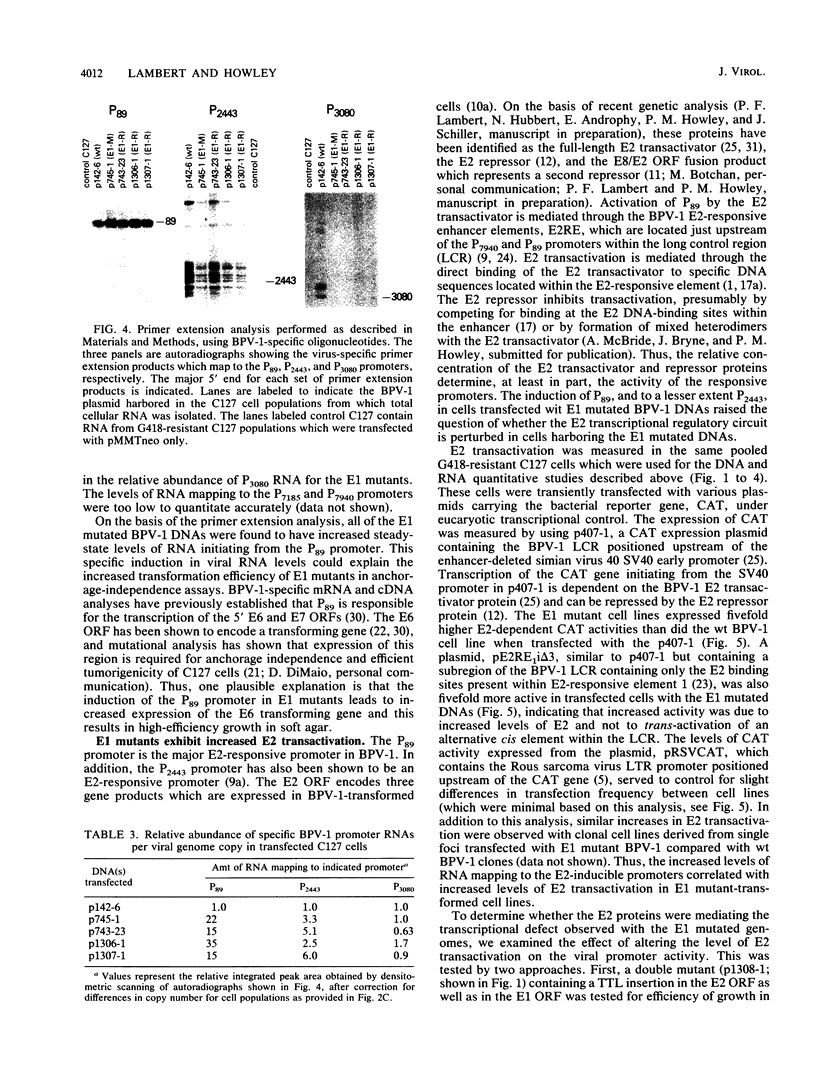
Bovine papillomavirus type 1 (BPV-1) is capable of replicating as a stable, high-copy-number plasmid in transformed rodent cells. The BPV-1 E1 open reading frame (ORF) encodes multiple functions involved in viral DNA replication. Mutations which disrupt the translational integrity of the E1 ORF disable the viral genome from replicating as a stable plasmid and result in the integration of the viral genome into the host chromosome generally at a low copy number. Despite the low copy number of the integrated genomes, BPV-1 E1 mutants transform rodent cells to anchorage independence very efficiently, at levels equal to or greater than that of wild-type (wt) BPV-1. Studies were performed to provide insight into why these low-copy-number, replication-defective mutants are capable of expressing an equal or greater transformation potential than wt BPV-1. Analysis of viral RNA revealed higher rates of transcription per viral genome in cells harboring E1 mutated BPV-1 DNA than in cells containing wt BPV-1 DNA. Furthermore, the levels of viral RNA mapping the P89 promoter were found to be 15- to 35-fold higher in cells transformed by E1 mutated DNAs compared with wt BPV-1 transformants. This promoter controls expression of the BPV-1 E6 transforming gene and is regulated by the viral E2 gene products. The studies presented in this report determined that the E1 mutants were perturbed in their E2 transcriptional regulation, suggesting a possible explanation for the observed P89 induction. Mutations throughout the E1 ORF, in either of the two regions previously identified as encoding distinct replication functions, were altered in viral transcription.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Androphy E. J., Lowy D. R., Schiller J. T. Bovine papillomavirus E2 trans-activating gene product binds to specific sites in papillomavirus DNA. Nature. 1987 Jan 1;325(6099):70–73. doi: 10.1038/325070a0. [DOI] [PubMed] [Google Scholar]
- Baker C. C., Howley P. M. Differential promoter utilization by the bovine papillomavirus in transformed cells and productively infected wart tissues. EMBO J. 1987 Apr;6(4):1027–1035. doi: 10.1002/j.1460-2075.1987.tb04855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Howley P. M., Levinson A. D., Seeburg P. H. The primary structure and genetic organization of the bovine papillomavirus type 1 genome. Nature. 1982 Oct 7;299(5883):529–534. doi: 10.1038/299529a0. [DOI] [PubMed] [Google Scholar]
- DiMaio D. Nonsense mutation in open reading frame E2 of bovine papillomavirus DNA. J Virol. 1986 Feb;57(2):475–480. doi: 10.1128/jvi.57.2.475-480.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaio D., Settleman J. Bovine papillomavirus mutant temperature sensitive for transformation, replication and transactivation. EMBO J. 1988 Apr;7(4):1197–1204. doi: 10.1002/j.1460-2075.1988.tb02931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Groff D. E., Lancaster W. D. Molecular cloning and nucleotide sequence of deer papillomavirus. J Virol. 1985 Oct;56(1):85–91. doi: 10.1128/jvi.56.1.85-91.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen T. H., Cripe T. P., Ginder G. D., Karin M., Turek L. P. Trans-activation of an upstream early gene promoter of bovine papilloma virus-1 by a product of the viral E2 gene. EMBO J. 1987 Jan;6(1):145–152. doi: 10.1002/j.1460-2075.1987.tb04732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermonat P. L., Spalholz B. A., Howley P. M. The bovine papillomavirus P2443 promoter is E2 trans-responsive: evidence for E2 autoregulation. EMBO J. 1988 Sep;7(9):2815–2822. doi: 10.1002/j.1460-2075.1988.tb03137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howley P. M. The molecular biology of papillomavirus transformation. Warner-Lambert Parke-Davis Award Lecture. Am J Pathol. 1983 Dec;113(3):414–421. [PMC free article] [PubMed] [Google Scholar]
- Hubbert N. L., Schiller J. T., Lowy D. R., Androphy E. J. Bovine papilloma virus-transformed cells contain multiple E2 proteins. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5864–5868. doi: 10.1073/pnas.85.16.5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert P. F., Spalholz B. A., Howley P. M. A transcriptional repressor encoded by BPV-1 shares a common carboxy-terminal domain with the E2 transactivator. Cell. 1987 Jul 3;50(1):69–78. doi: 10.1016/0092-8674(87)90663-5. [DOI] [PubMed] [Google Scholar]
- Law M. F., Lowy D. R., Dvoretzky I., Howley P. M. Mouse cells transformed by bovine papillomavirus contain only extrachromosomal viral DNA sequences. Proc Natl Acad Sci U S A. 1981 May;78(5):2727–2731. doi: 10.1073/pnas.78.5.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusky M., Botchan M. R. A bovine papillomavirus type 1-encoded modulator function is dispensable for transient viral replication but is required for establishment of the stable plasmid state. J Virol. 1986 Nov;60(2):729–742. doi: 10.1128/jvi.60.2.729-742.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusky M., Botchan M. R. Characterization of the bovine papilloma virus plasmid maintenance sequences. Cell. 1984 Feb;36(2):391–401. doi: 10.1016/0092-8674(84)90232-0. [DOI] [PubMed] [Google Scholar]
- Lusky M., Botchan M. R. Genetic analysis of bovine papillomavirus type 1 trans-acting replication factors. J Virol. 1985 Mar;53(3):955–965. doi: 10.1128/jvi.53.3.955-965.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride A. A., Schlegel R., Howley P. M. The carboxy-terminal domain shared by the bovine papillomavirus E2 transactivator and repressor proteins contains a specific DNA binding activity. EMBO J. 1988 Feb;7(2):533–539. doi: 10.1002/j.1460-2075.1988.tb02842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskaluk C., Bastia D. The E2 "gene" of bovine papillomavirus encodes an enhancer-binding protein. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1215–1218. doi: 10.1073/pnas.84.5.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps W. C., Howley P. M. Transcriptional trans-activation by the human papillomavirus type 16 E2 gene product. J Virol. 1987 May;61(5):1630–1638. doi: 10.1128/jvi.61.5.1630-1638.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabson M. S., Yee C., Yang Y. C., Howley P. M. Bovine papillomavirus type 1 3' early region transformation and plasmid maintenance functions. J Virol. 1986 Nov;60(2):626–634. doi: 10.1128/jvi.60.2.626-634.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarver N., Byrne J. C., Howley P. M. Transformation and replication in mouse cells of a bovine papillomavirus--pML2 plasmid vector that can be rescued in bacteria. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7147–7151. doi: 10.1073/pnas.79.23.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarver N., Rabson M. S., Yang Y. C., Byrne J. C., Howley P. M. Localization and analysis of bovine papillomavirus type 1 transforming functions. J Virol. 1984 Nov;52(2):377–388. doi: 10.1128/jvi.52.2.377-388.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller J. T., Vass W. C., Lowy D. R. Identification of a second transforming region in bovine papillomavirus DNA. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7880–7884. doi: 10.1073/pnas.81.24.7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalholz B. A., Byrne J. C., Howley P. M. Evidence for cooperativity between E2 binding sites in E2 trans-regulation of bovine papillomavirus type 1. J Virol. 1988 Sep;62(9):3143–3150. doi: 10.1128/jvi.62.9.3143-3150.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalholz B. A., Lambert P. F., Yee C. L., Howley P. M. Bovine papillomavirus transcriptional regulation: localization of the E2-responsive elements of the long control region. J Virol. 1987 Jul;61(7):2128–2137. doi: 10.1128/jvi.61.7.2128-2137.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalholz B. A., Yang Y. C., Howley P. M. Transactivation of a bovine papilloma virus transcriptional regulatory element by the E2 gene product. Cell. 1985 Aug;42(1):183–191. doi: 10.1016/s0092-8674(85)80114-8. [DOI] [PubMed] [Google Scholar]
- Stenlund A., Bream G. L., Botchan M. R. A promoter with an internal regulatory domain is part of the origin of replication in BPV-1. Science. 1987 Jun 26;236(4809):1666–1671. doi: 10.1126/science.3037693. [DOI] [PubMed] [Google Scholar]
- Thiele C. J., Reynolds C. P., Israel M. A. Decreased expression of N-myc precedes retinoic acid-induced morphological differentiation of human neuroblastoma. 1985 Jan 31-Feb 6Nature. 313(6001):404–406. doi: 10.1038/313404a0. [DOI] [PubMed] [Google Scholar]
- Thorner L., Bucay N., Choe J., Botchan M. The product of the bovine papillomavirus type 1 modulator gene (M) is a phosphoprotein. J Virol. 1988 Jul;62(7):2474–2482. doi: 10.1128/jvi.62.7.2474-2482.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldeck W., Rösl F., Zentgraf H. Origin of replication in episomal bovine papilloma virus type 1 DNA isolated from transformed cells. EMBO J. 1984 Sep;3(9):2173–2178. doi: 10.1002/j.1460-2075.1984.tb02109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. C., Okayama H., Howley P. M. Bovine papillomavirus contains multiple transforming genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1030–1034. doi: 10.1073/pnas.82.4.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. C., Spalholz B. A., Rabson M. S., Howley P. M. Dissociation of transforming and trans-activation functions for bovine papillomavirus type 1. Nature. 1985 Dec 12;318(6046):575–577. doi: 10.1038/318575a0. [DOI] [PubMed] [Google Scholar]