Abstract
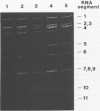
The gene encoding outer capsid protein VP3 of subpopulations of two animal rotaviruses, simian SA11 and Nebraska calf diarrhea virus (NCDV), was analyzed. Two laboratory strains of simian SA11 rotavirus (SA11-SEM and SA11-FEM) differed with respect to VP3. This dimorphism was indicated by a difference in electrophoretic mobility and a difference in reactivity with anti-VP3 monoclonal antibodies. The overall VP3 amino acid homology between the two SA11 VP3 proteins was 82.7%, whereas the VP3 protein of SA11-FEM was 98.5% homologous in amino acid sequence to NCDV VP3, suggesting that SA11-FEM VP3 was derived by gene reassortment in the laboratory during contamination with a bovine rotavirus. A comparison of the deduced amino acid sequence of the VP3 of two virulent NCDV strains and an attenuated NCDV strain (RIT 4237), revealed only five amino acid differences which were scattered throughout the protein but did not involve the trypsin cleavage sites. Of interest, the VP3 of the standard strain of NCDV which is virulent for cows differed in only one amino acid (position 23, Gln to Lys) from the VP3 of an NCDV mutant which was attenuated both for cows and for children.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias C. F., López S., Espejo R. T. Heterogeneity in base sequence among different DNA clones containing equivalent sequences of rotavirus double-stranded RNA. J Virol. 1986 Mar;57(3):1207–1209. doi: 10.1128/jvi.57.3.1207-1209.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delem A., Lobmann M., Zygraich N. A bovine rotavirus developed as a candidate vaccine for use in humans. J Biol Stand. 1984 Oct;12(4):443–445. doi: 10.1016/s0092-1157(84)80068-2. [DOI] [PubMed] [Google Scholar]
- Flores J., Greenberg H. B., Myslinski J., Kalica A. R., Wyatt R. G., Kapikian A. Z., Chanock R. M. Use of transcription probes for genotyping rotavirus reassortants. Virology. 1982 Sep;121(2):288–295. doi: 10.1016/0042-6822(82)90168-4. [DOI] [PubMed] [Google Scholar]
- Gorziglia M., Hoshino Y., Buckler-White A., Blumentals I., Glass R., Flores J., Kapikian A. Z., Chanock R. M. Conservation of amino acid sequence of VP8 and cleavage region of 84-kDa outer capsid protein among rotaviruses recovered from asymptomatic neonatal infection. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7039–7043. doi: 10.1073/pnas.83.18.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M. M., Midthun K., Flores J., Kapikian A. Z., Chanock R. M. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8701–8704. doi: 10.1073/pnas.82.24.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Greenberg H. B., Flores J., Kapikian A. Z. Serotypic similarity and diversity of rotaviruses of mammalian and avian origin as studied by plaque-reduction neutralization. J Infect Dis. 1984 May;149(5):694–702. doi: 10.1093/infdis/149.5.694. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., Flores J., Greenberg H. B. Identification of the rotaviral gene that codes for hemagglutination and protease-enhanced plaque formation. Virology. 1983 Feb;125(1):194–205. doi: 10.1016/0042-6822(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Kantharidis P., Dyall-Smith M. L., Holmes I. H. Marked sequence variation between segment 4 genes of human RV-5 and simian SA 11 rotaviruses. Arch Virol. 1987;93(1-2):111–121. doi: 10.1007/BF01313897. [DOI] [PubMed] [Google Scholar]
- Kawaoka Y., Webster R. G. Sequence requirements for cleavage activation of influenza virus hemagglutinin expressed in mammalian cells. Proc Natl Acad Sci U S A. 1988 Jan;85(2):324–328. doi: 10.1073/pnas.85.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton D. R., Ward R. L. Effect of mutation in immunodominant neutralization epitopes on the antigenicity of rotavirus SA-11. J Gen Virol. 1985 Nov;66(Pt 11):2375–2381. doi: 10.1099/0022-1317-66-11-2375. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- López S., Arias C. F., Bell J. R., Strauss J. H., Espejo R. T. Primary structure of the cleavage site associated with trypsin enhancement of rotavirus SA11 infectivity. Virology. 1985 Jul 15;144(1):11–19. doi: 10.1016/0042-6822(85)90300-9. [DOI] [PubMed] [Google Scholar]
- López S., Arias C. F., Méndez E., Espejo R. T. Conservation in rotaviruses of the protein region containing the two sites associated with trypsin enhancement of infectivity. Virology. 1986 Oct 15;154(1):224–227. doi: 10.1016/0042-6822(86)90445-9. [DOI] [PubMed] [Google Scholar]
- López S., Arias C. F. The nucleotide sequence of the 5' and 3' ends of rotavirus SA11 gene 4. Nucleic Acids Res. 1987 Jun 11;15(11):4691–4691. doi: 10.1093/nar/15.11.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackow E. R., Shaw R. D., Matsui S. M., Vo P. T., Dang M. N., Greenberg H. B. The rhesus rotavirus gene encoding protein VP3: location of amino acids involved in homologous and heterologous rotavirus neutralization and identification of a putative fusion region. Proc Natl Acad Sci U S A. 1988 Feb;85(3):645–649. doi: 10.1073/pnas.85.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malherbe H. H., Strickland-Cholmley M. Simian virus SA11 and the related O agent. Arch Gesamte Virusforsch. 1967;22(1):235–245. doi: 10.1007/BF01240518. [DOI] [PubMed] [Google Scholar]
- Mebus C. A., Kono M., Underdahl N. R., Twiehaus M. J. Cell culture propagation of neonatal calf diarrhea (scours) virus. Can Vet J. 1971 Mar;12(3):69–72. [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Blavat G., Greenberg H. B., Clark H. F. Molecular basis of rotavirus virulence: role of gene segment 4. J Virol. 1986 Jan;57(1):46–49. doi: 10.1128/jvi.57.1.46-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Blavat G. Identification of the two rotavirus genes determining neutralization specificities. J Virol. 1986 Jan;57(1):376–378. doi: 10.1128/jvi.57.1.376-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira H. G., Azeredo R. S., Fialho A. M., Vidal M. N. Genomic heterogeneity of simian rotavirus SA11. J Gen Virol. 1984 Apr;65(Pt 4):815–818. doi: 10.1099/0022-1317-65-4-815. [DOI] [PubMed] [Google Scholar]
- Potter A. A., Cox G., Parker M., Babiuk L. A. The complete nucleotide sequence of bovine rotavirus C486 gene 4 cDNA. Nucleic Acids Res. 1987 May 26;15(10):4361–4361. doi: 10.1093/nar/15.10.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabara M., Deregt D., Babiuk L. A., Misra V. Genetic heterogeneity within individual bovine rotavirus isolates. J Virol. 1982 Dec;44(3):813–822. doi: 10.1128/jvi.44.3.813-822.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer E. G., Avendaño L. F., García B. I. Analysis of human rotavirus mixed electropherotypes. Infect Immun. 1983 Feb;39(2):569–574. doi: 10.1128/iai.39.2.569-574.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Morita Y., Urasawa T., Urasawa S. Cross-reactive neutralization epitopes on VP3 of human rotavirus: analysis with monoclonal antibodies and antigenic variants. J Virol. 1987 May;61(5):1726–1730. doi: 10.1128/jvi.61.5.1726-1730.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Morita Y., Greenberg H. B., Urasawa S. Direct serotyping of human rotavirus in stools by an enzyme-linked immunosorbent assay using serotype 1-, 2-, 3-, and 4-specific monoclonal antibodies to VP7. J Infect Dis. 1987 Jun;155(6):1159–1166. doi: 10.1093/infdis/155.6.1159. [DOI] [PubMed] [Google Scholar]
- Toyoda T., Sakaguchi T., Imai K., Inocencio N. M., Gotoh B., Hamaguchi M., Nagai Y. Structural comparison of the cleavage-activation site of the fusion glycoprotein between virulent and avirulent strains of Newcastle disease virus. Virology. 1987 May;158(1):242–247. doi: 10.1016/0042-6822(87)90261-3. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Isolauri E., D'Hondt E., Delem A., André F. E., Zissis G. Protection of infants against rotavirus diarrhoea by RIT 4237 attenuated bovine rotavirus strain vaccine. Lancet. 1984 May 5;1(8384):977–981. doi: 10.1016/s0140-6736(84)92323-7. [DOI] [PubMed] [Google Scholar]